Abstract
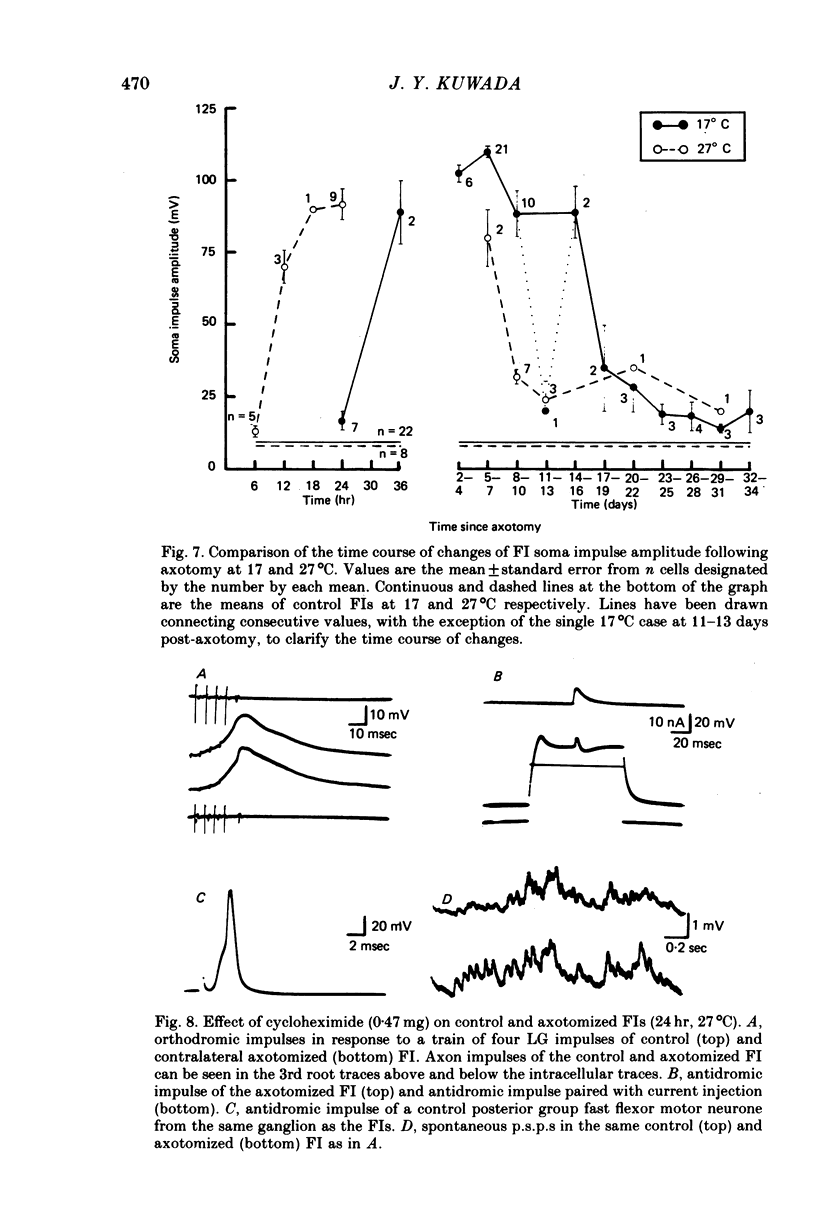
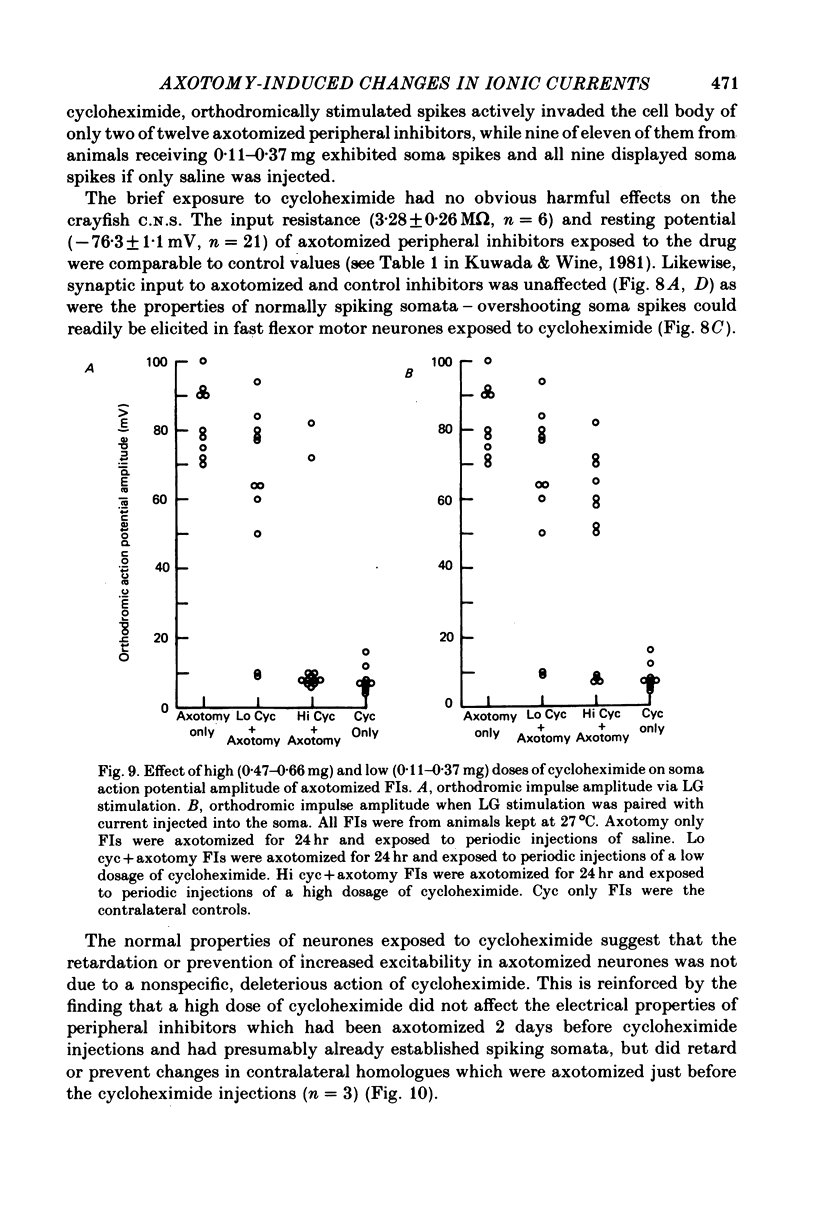
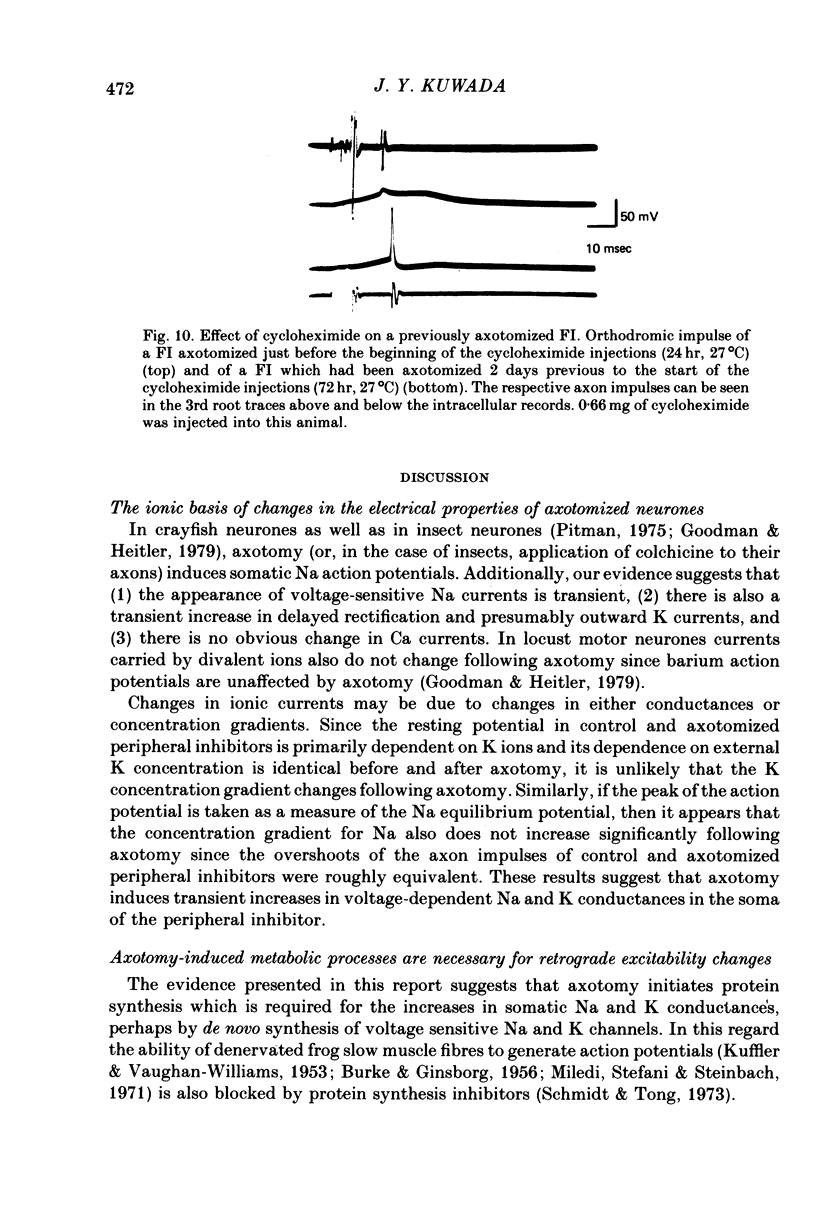
1. Axotomy induces a transient change in the soma membrane from non-spiking to spiking in many neurones of the crayfish abdominal C.N.S. The ionic and metabolic dependence of this phenomenon was investigated in one identified neurone. 2. The inward current of axotomy-induced soma spikes is carried primarily by Na ions. 3. Addition of 50 mM-tetraethylammonium to the external saline unmasks predominantly Ca-mediated soma spikes in control cells. The calcium spikes are not affected by axotomy. 4. Axotomy induces a transient increase in delayed rectification of the soma membrane. 5. Membrane potential is dependent on the external potassium concentration as predicted by the Nernst function while external sodium has essentially no effect. This is not changed following axotomy. 6. The inward current of the axon spike is primarily carried by sodium ions both before and after axotomy. The overshoot of the axon spike is not dramatically changed between the time when axotomy-induced soma spikes are present and when they have disappeared. 7. Following axotomy, both the onset and offset of axotomy-induced soma spikes occur earlier when the temperature is raised by 10 degrees C. 8. Cycloheximide retards or prevents soma spikes normally seen following axotomy. 9. The results indicate that axotomy induces a transient increase in voltage-dependent Na and K conductances but not Ca conductance, and that these changes are dependent on axotomy-inhibited protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN M. J., JACKLET J. W. NEURONS OF INSECTS: RNA CHANGES DURING INJURY AND REGENERATION. Science. 1965 May 28;148(3674):1237–1239. doi: 10.1126/science.148.3674.1237. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Heitler W. J. Electrical properties of insect neurones with spiking and non-spiking somata: normal, axotomized, and colchicine-treated neurones. J Exp Biol. 1979 Dec;83:95–121. doi: 10.1242/jeb.83.1.95. [DOI] [PubMed] [Google Scholar]
- Ishima Y., Yumoto K. Excitability of crayfish giant axons in sodium-free external media containing hydrazinium, hydroxylamine, or guanidinium ions. Jpn J Physiol. 1975;25(2):109–122. doi: 10.2170/jjphysiol.25.109. [DOI] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol. 1953 Aug;121(2):318–340. doi: 10.1113/jphysiol.1953.sp004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada J. Y., Wine J. J. Transient, axotomy-induced changes in the membrane properties of crayfish central neurones. J Physiol. 1981 Aug;317:435–461. doi: 10.1113/jphysiol.1981.sp013835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Steinbach A. B. Induction of the action potential mechanism in slow muscle fibres of the frog. J Physiol. 1971 Sep;217(3):737–754. doi: 10.1113/jphysiol.1971.sp009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Grafstein B. Changes in the morphology and amino acid incorporation of regenerating goldfish optic neurons. Exp Neurol. 1969 Apr;23(4):544–560. doi: 10.1016/0014-4886(69)90124-1. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Pitman R. M. Intracellular citrate or externaly applied tetraethylammonium ions produce calcium-dependent action potentials in an insect motoneurone cell body. J Physiol. 1979 Jun;291:327–337. doi: 10.1113/jphysiol.1979.sp012816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R. M. The ionic dependence of action potentials induced by colchicine in an insect motoneurone cell body. J Physiol. 1975 May;247(2):511–520. doi: 10.1113/jphysiol.1975.sp010944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Electrical responses of insect central neurons: augmentation by nerve section or colchicine. Science. 1972 Nov 3;178(4060):507–509. doi: 10.1126/science.178.4060.507. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Tong E. Y. Inhibition by actinomycin D of the denervation-induced action potential in frog slow muscle fibres. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):91–95. doi: 10.1098/rspb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine J. J., Mistick D. C. Temporal organization of crayfish escape behavior: delayed recruitment of peripheral inhibition. J Neurophysiol. 1977 Jul;40(4):904–925. doi: 10.1152/jn.1977.40.4.904. [DOI] [PubMed] [Google Scholar]



