Abstract
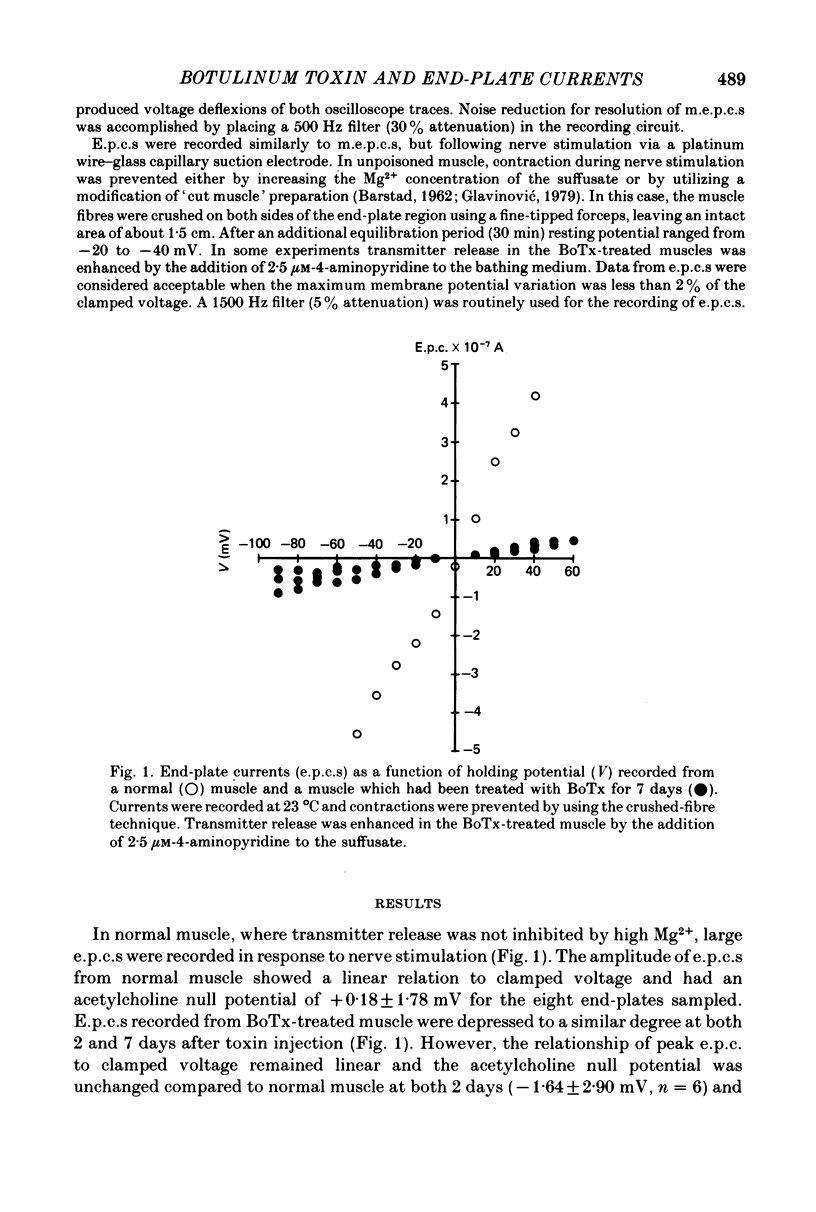
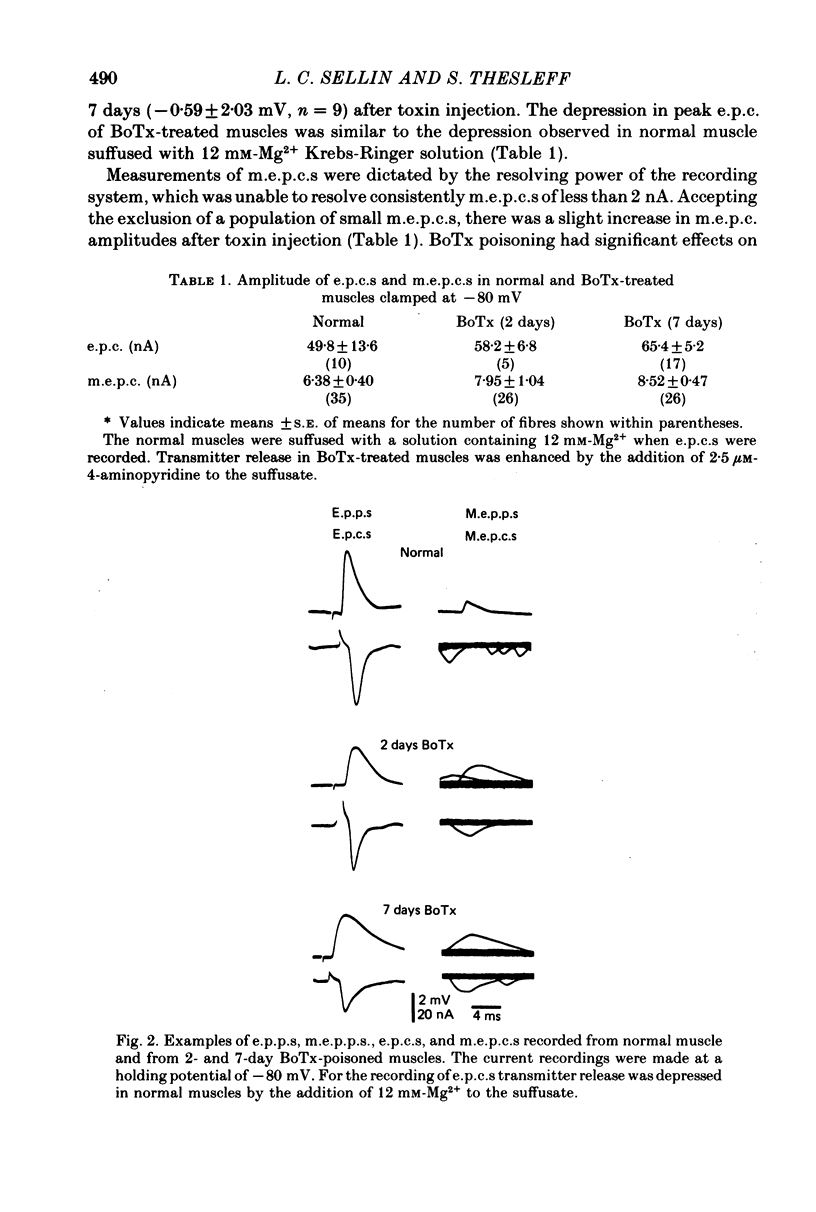
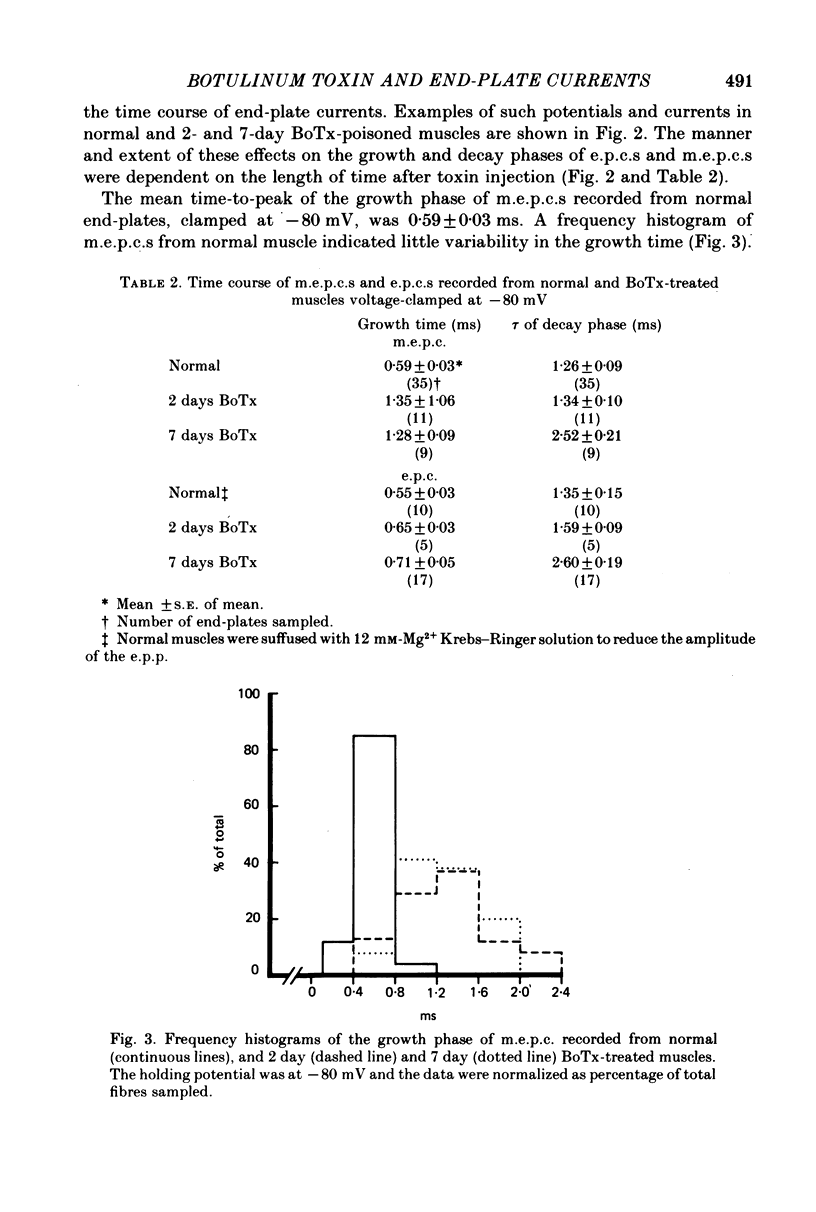
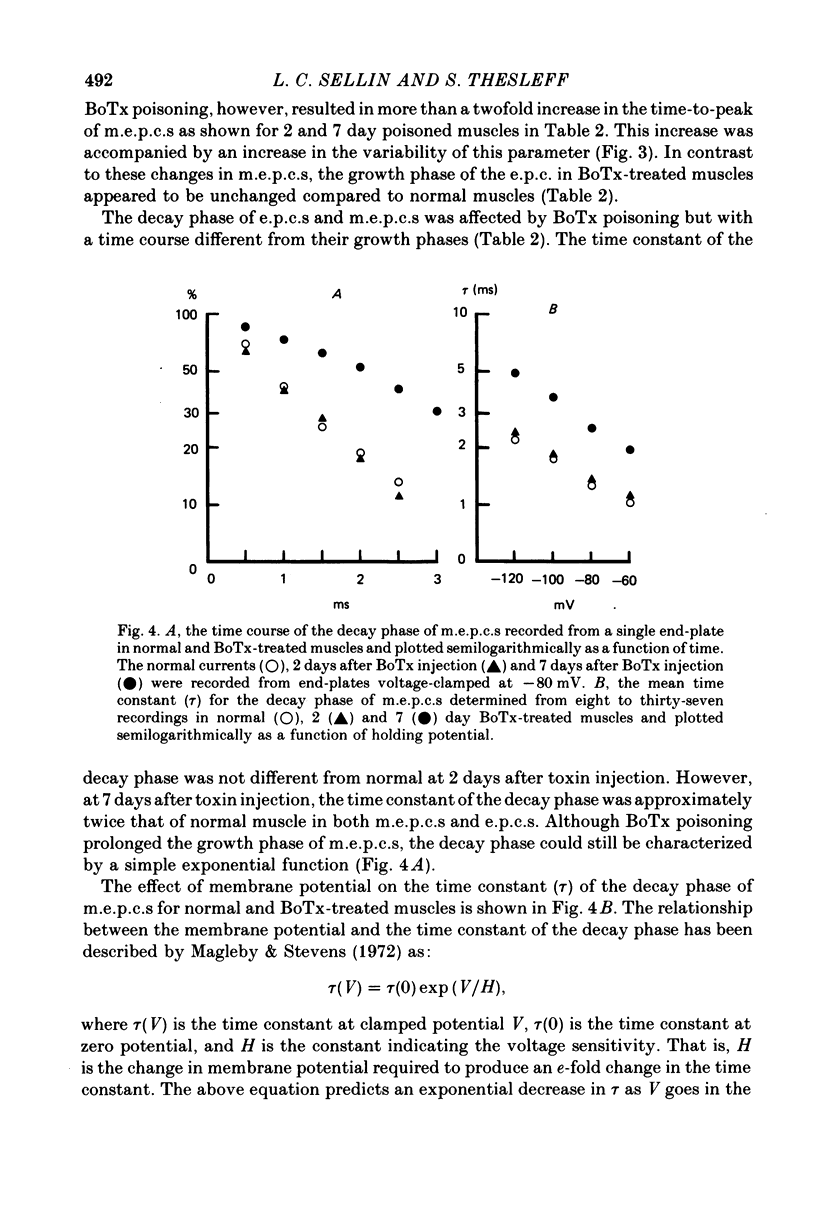
1. Extensor digitorum longus muscles of rats were paralysed with local, non-lethal doses of botulinum toxin Type A (BoTx). At 2 and 7 days after toxin injection, the nerve-muscle preparations were excised and end-plate currents analysed at 23 degrees C by dual-micro-electrode voltage clamp. 2. At 2 days after BoTx injection, the growth time of miniature end-plate currents (m.e.p.c.s) increased from a rather narrow range with a mean of 0.59 to a mean of 1.35 ms with a large variability between m.e.p.c.s. End-plate currents (e.p.c.s) were reduced compared to unpoisoned muscle. The decay phase of m.e.p.c.s and e.p.c.s, the growth phase of e.p.c.s and the voltage sensitivity of m.e.p.c.s were unchanged. 3. At 7 days after BoTx injection, the findings were similar to 2 days except that the time constant of the decay phase of m.e.p.c.s. and e.p.c.s. was about twice a long as normal and that the voltage sensitivity of m.e.p.c.s was increased. 4. The acetylcholine null potential (about 0 mV) was unchanged after treatment with BoTx. 5. The increase in the growth time of m.e.p.c.s compared to e.p.c.s following the injection of BoTx suggests that the poisoning, besides blocking quantal release, affects the time course of spontaneous but not that of evoked release. After BoTx poisoning the trans-synaptic diffusion of a majority of spontaneously released transmitter quanta seems to occur more slowly or from areas more distant from the highest concentration of the post-synaptic receptor than that of evoked release. 6. The increase in the decay phase of m.e.p.c.s and e.p.c.s and its increased voltage sensitivity observed in muscles poisoned for 7 days with BoTx suggest that appearance at the end-plate of a population of new receptors with a prolonged ion channel opening time similar to that previously described for extrajunctional receptors after denervation and for junctional receptors during development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Sakmann B. A comparison of current-voltage relations for full and partial agonists. J Physiol. 1978 Oct;283:621–644. doi: 10.1113/jphysiol.1978.sp012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., del Castillo J., Evoy W. H., Steinhardt R. A. Observations on the action of type A botulinum toxin on frog neuromuscular junctions. J Physiol. 1974 Jul;240(2):227–253. doi: 10.1113/jphysiol.1974.sp010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P. Lifetime and conductance of acetylcholine-activated channels in normal and denervated toad sartorius muscle. J Physiol. 1980 Jan;298:525–538. doi: 10.1113/jphysiol.1980.sp013099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavinović M. I. Voltage clamping of unparalysed cut rat diaphragm for study of transmitter release. J Physiol. 1979 May;290(2):467–480. doi: 10.1113/jphysiol.1979.sp012784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Howard B. D., Gundersen C. B., Jr Effects and mechanisms of polypeptide neurotoxins that act presynaptically. Annu Rev Pharmacol Toxicol. 1980;20:307–336. doi: 10.1146/annurev.pa.20.040180.001515. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt T. A., Loring R. H., Salpeter M. M. Neuronal control of acetylcholine receptor turnover rate at a vertebrate neuromuscular junction. Science. 1980 Oct 31;210(4469):550–551. doi: 10.1126/science.7423205. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Salpeter M. M. Denervation increases turnover rate of junctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2293–2297. doi: 10.1073/pnas.77.4.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]
- Spitzer N. Miniature end-plate potentials at mammalian neuromuscular junctions poisoned by botulinum toxin. Nat New Biol. 1972 May 3;237(70):26–27. doi: 10.1038/newbio237026a0. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]



