Abstract
1. Inward rectification of frog muscle membrane was analysed with the Vaseline gap method. 2. Hyperpolarization, under voltage clamp, produced inward potassium currents, which had a component that activated with a time constant, tau K. 3. The activation time constant tau K of the inward potassium currents was voltage dependent. For a given external potassium concentration, the time constant was maximal for potentials near the potassium equilibrium potential, EK. 4. The potassium chord conductance gK, had a sigmoidal voltage dependency, increasing initially e-fold per 11.6 mV of hyperpolarization. 5. When the internal potassium concentration was fixed, raising external potassium induced a shift of the tau K-V and the gK-V relations in the positive direction along the voltage axis. That shift was comparable to the change in EK. 6. No shift of the tau K-V and the gK-V relations was observed when the internal potassium was reduced from 150 to 50 mM. 7. Changes of internal sodium concentration between 5 and 100 mM did not significantly effect the magnitude of inward rectification.
Full text
PDF

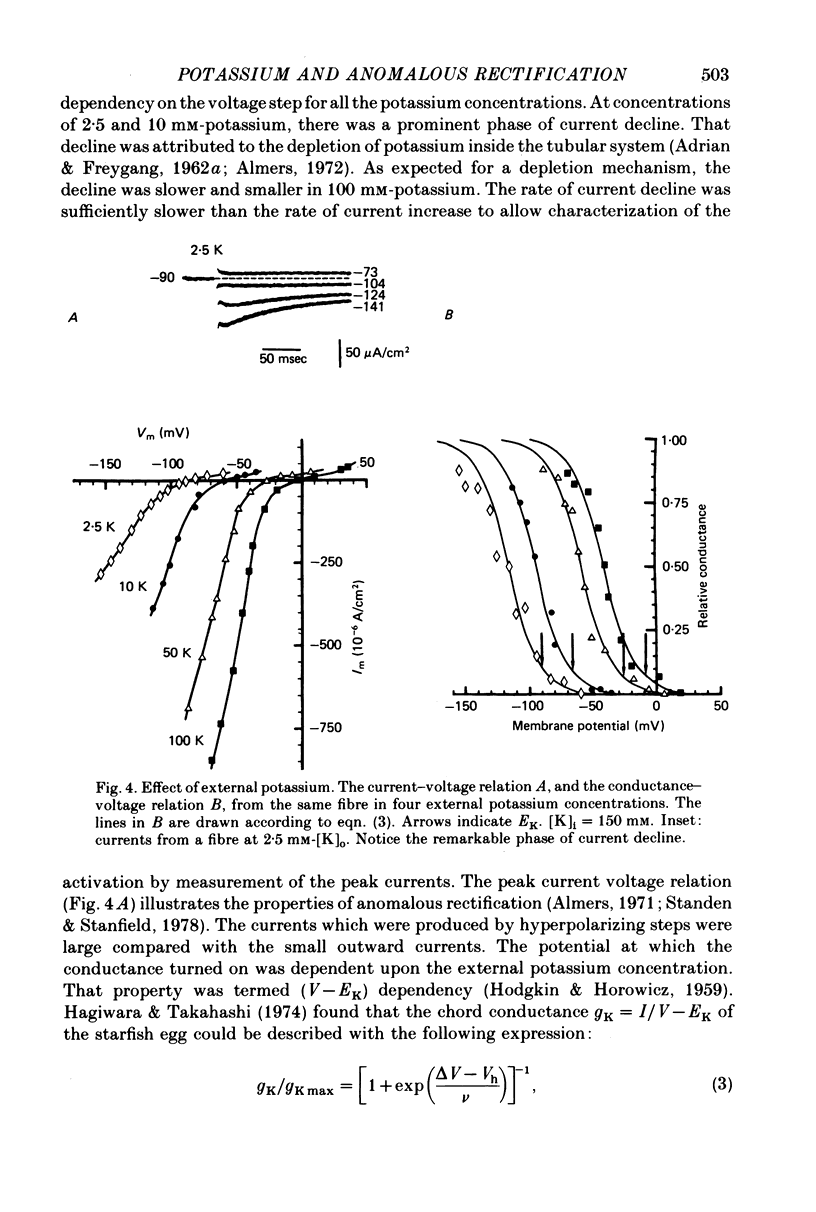
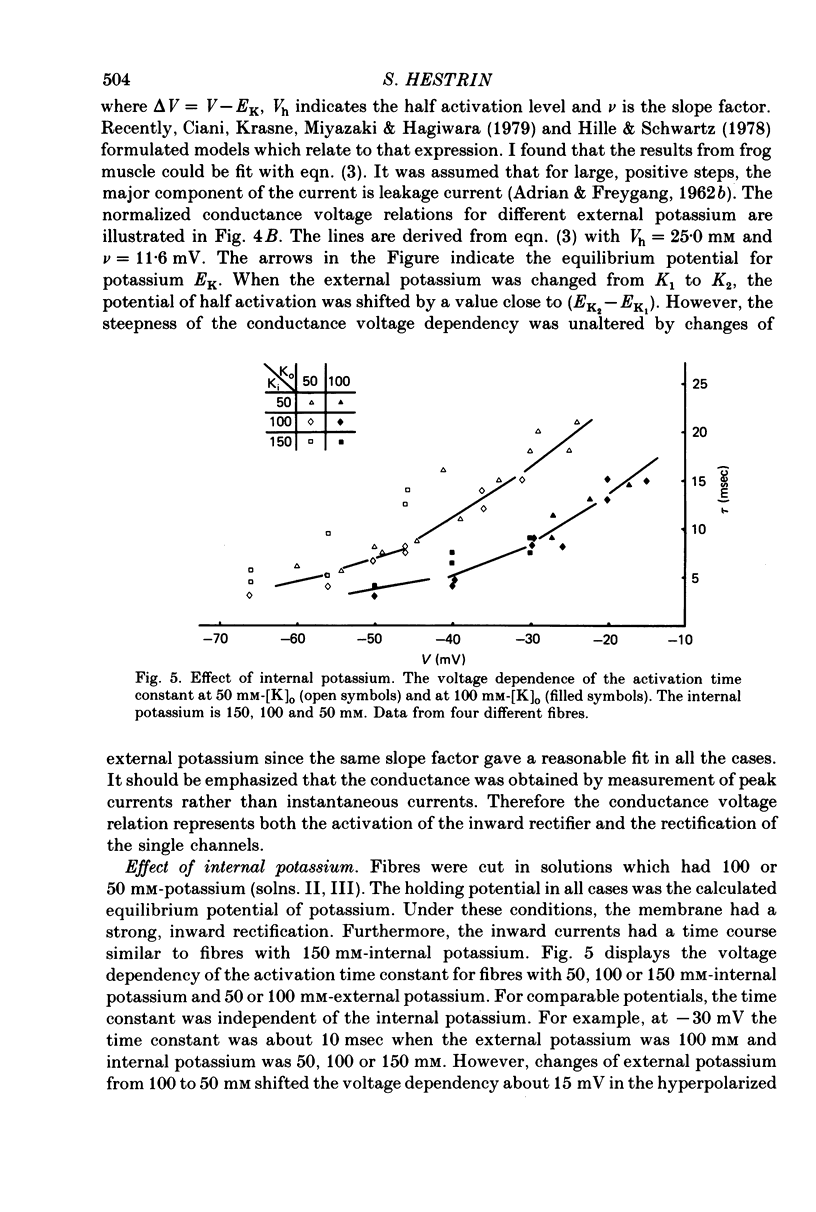
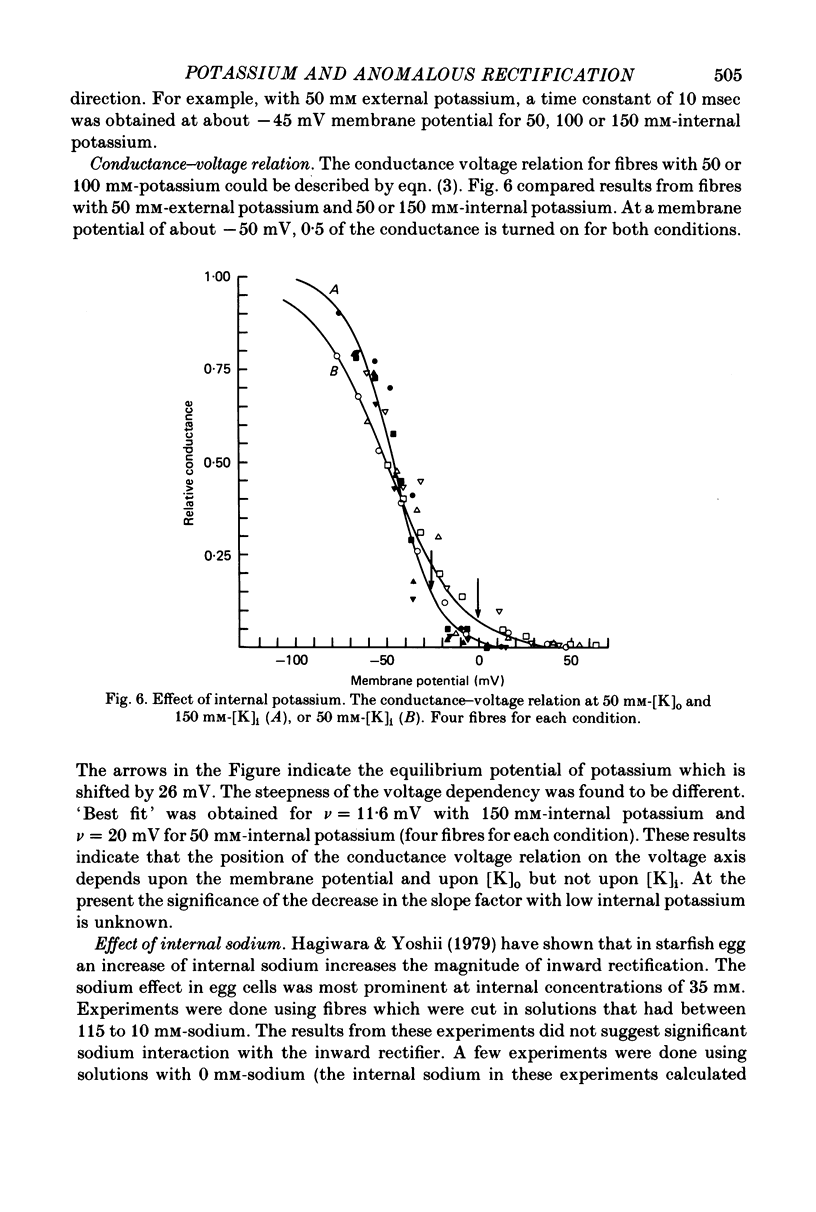



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H. Rectification in muscle membrane. Prog Biophys Mol Biol. 1969;19(2):339–369. [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Potassium pores of nerve and muscle membranes. Membranes. 1975;3:325–358. [PubMed] [Google Scholar]
- Armstrong W. M., Lee C. O. Sodium and potassium activities in normal and "sodium-rich" frog skeletal muscle. Science. 1971 Jan 29;171(3969):413–415. doi: 10.1126/science.171.3969.413. [DOI] [PubMed] [Google Scholar]
- Campbell D. T. Ionic selectivity of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):295–307. doi: 10.1085/jgp.67.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Krasne S., Miyazaki S., Hagiwara S. A model for anomalous rectification: electrochemical-potential-dependent gating of membrane channels. J Membr Biol. 1978 Dec 15;44(2):103–134. doi: 10.1007/BF01976035. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Inward rectification in skeletal muscle: a blocking particle model. Pflugers Arch. 1978 Dec 28;378(2):173–176. doi: 10.1007/BF00584452. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. J Physiol. 1980 Jul;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa R. A., Horowicz P. Effects on sodium efflux of treating frog sartorius muscles with hypertonic glycerol solutions. J Membr Biol. 1973 Dec 6;14(1):33–56. doi: 10.1007/BF01868067. [DOI] [PubMed] [Google Scholar]
- Vergara J., Bezanilla F., Salzberg B. M. Nile blue fluorescence signals from cut single muscle fibers under voltage or current clamp conditions. J Gen Physiol. 1978 Dec;72(6):775–800. doi: 10.1085/jgp.72.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


