Abstract
A mesophilic, anaerobic, gram-negative bacterium, strain SB164P1, was enriched and isolated from oxidized marine surface sediment with elemental sulfur as the sole energy substrate in the presence of ferrihydrite. Elemental sulfur was disproportionated to hydrogen sulfide and sulfate. Growth was observed exclusively in the presence of a hydrogen sulfide scavenger, e.g., ferrihydrite. In the absence of a scavenger, sulfide and sulfate production were observed but no growth occurred. Strain SB164P1 grew also by disproportionation of thiosulfate and sulfite. With thiosulfate, the growth efficiency was higher in ferrihydrite-supplemented media than in media without ferrihydrite. Growth coupled to sulfate reduction was not observed. However, a slight sulfide production occurred in cultures incubated with formate and sulfate. Strain SB164P1 is the first bacterium described that grows chemolithoautotrophically exclusively by the disproportionation of inorganic sulfur compounds. Comparative 16S rDNA sequencing analysis placed strain SB164P1 into the delta subclass of the class Proteobacteria. Its closest relative is Desulfocapsa thiozymogenes, and slightly more distantly related are Desulfofustis glycolicus and Desulforhopalus vacuolatus. This phylogenetic cluster of organisms, together with members of the genus Desulfobulbus, forms one of the main lines of descent within the delta subclass of the Proteobacteria. Due to the common phenotypic characteristics and the phylogenetic relatedness to Desulfocapsa thiozymogenes, we propose that strain SB164P1 be designated the type strain of Desulfocapsa sulfoexigens sp. nov.
Since Bak and Cypionka (2) first described an energy metabolism based on the disproportionation of the inorganic sulfur compounds thiosulfate and sulfite into sulfide and sulfate by a sulfate reducer designated Desulfovibrio sulfodismutans (3), this type of process has been recognized in numerous genera of sulfate-reducing bacteria (15). However, most of the sulfate reducers tested were unable to couple the disproportionation of inorganic sulfur compounds to growth. Disproportionation can be described as an inorganic fermentation in which sulfur compounds with an intermediate oxidation state serve as both electron donors and electron acceptors in an energy-generating redox process (27). In recent years, it was demonstrated that thiosulfate disproportionation is an important metabolism in sulfur transformations in aquatic systems (13) and that microorganisms which are able to carry out this type of metabolism are numerically abundant (3, 14).
Recently, Thamdrup et al. (32) discovered that enrichment cultures with elemental sulfur and iron or manganese oxides were able to grow by disproportionation of elemental sulfur. This observation was surprising, because under standard conditions the reaction is endergonic with ΔG′° = 40.9 kJ reaction−1 (2). The authors stressed that the use of oxidized metals as sulfide scavengers makes the process energetically favorable. Disproportionation of elemental sulfur in pure cultures was first reported by Bak (1). The organism, later named Desulfocapsa thiozymogenes, is a freshwater sulfate reducer that grows either heterotrophically by incomplete oxidation of different alcohols or chemolithoautotrophically by disproportionation of thiosulfate, sulfite, or elemental sulfur (12). Apart from the recently described species Desulforhopalus vacuolatus (11) and Desulfofustis glycolicus (9; see below), Desulfocapsa thiozymogenes is most closely related to Desulfobulbus spp. Recently, Lovley and Phillips (18) have demonstrated that Desulfobulbus propionicus 1pr3 is also able to disproportionate elemental sulfur. However, this metabolism was observed only when ferric iron was present in the medium as a sulfide scavenger, and growth was very slow.
In this communication, we report on the enrichment, isolation, and phylogenetic affiliation of a marine microorganism that grows exclusively chemolithoautotrophically by elemental sulfur, thiosulfate, and sulfite disproportionation.
MATERIALS AND METHODS
Sources of inoculum, media, enrichment, and isolation.
Oxidized surface sediment from an eelgrass (Zostera noltii)-covered mud flat in the basin of Arcachon (France) served as the inoculum for a dilution series. Aliquots (9 ml) of carbonate-buffered marine minimal medium supplemented with trace metals and vitamins (for details, see reference 32; the only modification was the use of 20 g of NaCl liter−1 instead of 15 g liter−1) were dispensed into sterile 16-ml glass test tubes. The test tubes had been supplied in advance with 1 ml of a 300 mM heat-sterilized suspension of ferrihydrite [Fe(OH)3] and 200 to 300 mg of sterile sulfur flowers. A 1-cm3 portion of oxidized surface mud was added via a tip-cut plastic syringe to the first test-tube in a series of seven. The medium and the sediment was thoroughly mixed, and 1 ml of the homogenized slurry was transferred to the next test tube. The procedure was repeated until all the tubes had been inoculated. The gas phase of each tube was exchanged for a gas mixture of 90% N2–10% CO2, and the tubes were sealed with black rubber stoppers. The inoculated test tubes were incubated in the dark at 22°C for up to 12 weeks. Positive enrichments were identified by the formation of iron sulfides produced during the disproportionation of elemental sulfur (32). Sulfur-disproportionating bacteria were isolated by deep agar dilutions (23) prepared as described by Finster et al. (7). The isolation tubes contained two separated agar layers, one consisting of 1 ml of ferrihydrite suspension in 3 ml of 3% agar and the second containing inoculum and elemental sulfur in 6 ml of 1% agar. Colonies were withdrawn from the agar deeps with drawn Pasteur pipettes and transferred to liquid medium containing ferrihydrite and elemental sulfur. Tubes containing sulfur-disproportionating bacteria were identified by iron sulfide formation.
The purification procedure was repeated by deep agar dilutions in basal marine medium supplemented with ferrous iron (0.52 g of FeCl2 · 4H2O liter−1) instead of the iron agar layer and thiosulfate (10 mM) instead of elemental sulfur. Growing colonies were directly identifiable as black spots in the agar as a result of iron sulfide precipitation. The colonies were transferred to liquid medium containing thiosulfate in ferrous iron medium, and grown cultures were examined microscopically to compare the morphology with that of the original isolate from elemental sulfur-containing medium. Also, the capability of elemental sulfur disporportionation was tested. Cultures were routinely grown with elemental sulfur in the presence of ferrihydrite.
Purity was tested in medium supplemented with fumarate (5 mM), pyruvate (5 mM), glucose (10 mM), and yeast extract (0.1%) and by regular microscopic checks.
Stoichiometry of the disproportionations.
The transformations of thiosulfate and elemental sulfur were quantified in 250-ml batch cultures in medium with and without ferrihydrite. Cultures were inoculated with 2% (vol/vol) of a pregrown culture, incubated at 28°C in the dark, and sampled regularly for 11 days when thiosulfate was the substrate and for 24 days when elemental sulfur was the substrate. Aliquots for sulfate, dissolved sulfide, and thiosulfate determination were filtered under N2 and preserved in zinc acetate (final concentration, 0.2%), while aliquots for iron sulfides were preserved unfiltered in zinc acetate (final concentration, 5%). Aliquots for cell counts were fixed in glutaraldehyde (final concentration, 2.5%). Aliquots of 100 μl for Fe(II) measurements were extracted in 5 ml of 0.5 N HCl. Immediately after each sampling, the headspace of the incubation bottles was flushed with a gas mixture consisting of 90% N2–10% CO2, and the bottles were sealed with rubber stoppers.
Substrate tests.
The spectrum of electron donors and electron acceptors used by the isolate was determined by using 20-ml screw-cap test tubes filled to the rim. The tubes were filled with only 10 ml of medium when oxygen was supplied as the electron acceptor. The bacteria were precultivated with elemental sulfur and ferrihydrite. The inoculum was 1% (vol/vol) of a pregrown culture. In electron donor tests, either sulfur, thiosulfate (10 mM), sulfate (10 mM), dimethyl sulfoxide (10 mM), nitrate (5 mM), or oxygen (0.02 atm) served as the electron acceptor. Test medium containing elemental sulfur or thiosulfate and ferrihydrite served as positive controls. In electron donor tests when sulfate served as electron acceptor, ferrihydrite was added to visualize the production of hydrogen sulfide. The sulfate-reducing capacity of strain SB164P1 was also assayed in ferric iron-free incubations with sulfate (10 mM), formate (10 mM), acetate, propionate, pyruvate, lactate, fumarate, or hydrogen (90%) as the electron donor (concentrations, 5 mM if not otherwise indicated). The test tubes were incubated at 26°C in the dark. All tests were carried out in duplicate, and the tubes were incubated for 4 weeks and periodically examined for sulfide production and/or growth.
The capacity of dissimilatory sulfate reduction was measured as the amount of radiolabeled hydrogen sulfide produced after an incubation period of 7 days with radiolabeled sulfate. The following substrates were tested, based on our knowledge about compounds metabolized by the different genera of sulfate-reducing bacteria (36) (concentrations in parentheses, 5 mM if not indicated): formate (10 mM), acetate, propionate, butyrate, valerate, pyruvate, lactate, succinate, malate, fumarate, glucose, benzoate (1 mM), nicotinate (1 mM), betaine, choline chloride, methanol (10 mM), ethanol, n-propanol, n-butanol, alanine, glutamate, α-ketoglutarate, oxaloacetate, and citrate. The following incubations served as controls: (i) unsupplemented cultures to determine the background sulfate transformation, and (ii) sulfur- or thiosulfate-supplemented cultures to determine sulfate transformation during sulfur or thiosulfate disproportionation.
The experiment was conducted as follows. Aliquots (2.5 ml) of an outgrown thiosulfate-ferrihydrite culture were transferred to evacuated sterile 5-ml rubber-septum-sealed glass vials with a sterile plastic syringe and needle. The cultures were supplemented with the electron donor and sulfate from sterile stock solutions to give a sulfate concentration of approximately 10 mM and a final volume of approximately 3 ml. Finally, 5 μl of carrier-free radiolabeled sulfate was added, resulting in an activity of 4.5 kBq μM−1 (sulfate). In total, 56 vials were prepared. Two incubations were stopped immediately after addition of radiolabeled sulfate by injection of 0.5 ml of a 20% zinc acetate solution. They were used to check if labeled sulfate is reduced chemically. The other incubations were stopped after 7 days by an injection of zinc acetate. Reduced radiolabeled sulfur was released from the medium by the single-step chromium-reduction method (8) and counted with a liquid scintillation counter.
Growth parameters.
The vitamin requirements, as well as the pH, salt, and temperature tolerance of the isolate, were studied by incubating the isolate in 20-ml screw-cap test tubes with elemental sulfur and ferrihydrite. Activity was monitored by microscopic examination and by observing the formation of iron sulfide.
The temperature range for growth was determined by incubating inoculated test tubes in an insulated aluminum temperature gradient block with a minimum temperature of 0°C and a maximum temperature of 50°C in 5°C steps. All experiments were carried out in duplicate and stopped after a maximum of 6 weeks.
Growth conditions were evaluated by comparing the time passed until iron sulfide formation was observed. The fastest iron sulfide formation was interpreted as optimal conditions.
Molybdate addition.
Thiosulfate-ferrihydrite-grown cultures of strain SB164P1 were inoculated into marine medium with thiosulfate plus ferrihydrite or elemental sulfur plus ferrihydrite and increasing concentrations of molybdate (0, 0.5, 5, 50, 500, or 5,000 μM), a specific inhibitor of sulfate-reducing bacteria. This experiment was stimulated by the recent publication by Krämer and Cypionka (15), proposing that the reversal of sulfate reduction serves as the energy generating pathway in thiosulfate-disproportionating sulfate reducers.
Analytical procedures.
Sulfide was determined spectrophotometrically by the methylene blue method (5). Sulfate and thiosulfate were quantified by nonsuppressed anion-exchange chromatography (pump model 510, IC-Pak anion column, conductivity detector model 430 [Waters]). HCl-extracted Fe2+ was measured spectrophotometrically by monitoring its reaction with Ferrozine without reducing agents (28) (0.1% in 50 mM HEPES [pH 7]), and total HCl-extractable Fe was measured similarly with reducing Ferrozine (Ferrozine with 1% hydroxylamine hydrochloride).
Acid-volatile and chromium-reducible sulfur compounds were separated by a two-step distillation procedure (8). Before distillation, zinc acetate-fixed samples were filtered through fiberglass filters that retained the sulfides. Elemental sulfur, present in excess in elemental sulfur-grown cultures, was removed by giving the filters a wash with acetone, followed by three consecutive washes with CS2 and a final acetone wash. Thus, chromium reducible sulfur only represents pyrite.
Cell counts.
Cell counts were performed on glutaraldehyde-preserved aliquots. Before being counted, iron precipitates were dissolved in a dithionite solution (50 g of sodium dithionite liter−1 in 0.2 M sodium citrate and 0.35 M acetic acid) (17). Then 50 to 200 μl of the preserved sample was added to 2 ml of the extractants. After 5 min, the mixture was filtered through a black 0.2-μm-pore-size polycarbonate filter, which was subsequently stained with 0.01% acridine orange for 1 min. The staining solution was filtered off, and the filter was washed with 90% methanol to remove nonspecifically bound acridine orange. All the solutions were sterilized by filtration (0.2-μm-pore-size filter). The cells were counted by epifluorescence microscopy.
G+C content of DNA.
The G+C content of the DNA was determined by high-pressure liquid chromatography separation and UV detection (30). Calibration and G+C determination were performed as described by Mesbah et al. (20).
Comparative 16S rDNA sequence analysis.
DNA isolation, PCR-mediated amplification of the 16S rRNA gene from positions 28 to 1491 (numbering according to the International Union of Biochemistry nomenclature for Escherichia coli 16S rRNA), and sequencing analysis were done as previously described by Liesack and Finster (16) but with one modification: instead of purification on Centricon 100 columns (Amicon, Witten, Germany), the 16S rDNA PCR products were purified with the Prep-A-Gene kit (Bio-Rad, Munich, Germany) as specified by the manufacturer. The 16S rDNA sequence of strain SB164P1 was added to a database of about 6,000 publicly available bacterial 16S rRNA sequences (19, 25, 33). The database is part of the ARB (from “arbor”, Latin: tree) program package (29). The 16S rDNA sequence from strain SB164P1 was integrated into this database with the automatic alignment tool of the ARB program package (29). This procedure revealed strain SB164P1 as a true member of the delta subclass of the class Proteobacteria. The phylogenetic position of strain SB164P1 was determined in more detail by comparing its 16S rDNA sequence with 69 reference sequences comprising the main lines of descent within the delta subclass of the Proteobacteria. The overall tree topology was evaluated by distance matrix analyses. Evolutionary distances between pairs of microorganisms were calculated for a sequence stretch ranging from positions 28 through 1435 (E. coli 16S rRNA numbering) including only positions present in both sequences of the respective sequence pairs (ARB and PHYLIP [6]). Since the inclusion of highly variable regions of the 16S rRNA gene into the analysis may blur the reconstructed tree due to the high mutational rate of these nucleotide sequence positions and possible alignment errors, these regions were excluded from the phylogenetic analysis. To do that, two different approaches were used (i) manual removal of regions that are known to be highly variable (34) and could not be aligned unambiguously (i.e., positions 69 to 100, 182 to 218, 451 to 479, 833 to 853, 998 to 1042, and 1133 to 1141 in the E. coli 16S rRNA numbering), and (ii) use of only the alignment positions for the phylogenetic analysis which contained identical nucleotides in at least 50% of the 16S rRNA sequences under comparison. The usefulness of this invariance criterion applied in the second approach for the inclusion and exclusion, respectively, of individual alignment positions was shown previously (9). The base frequency of the alignment positions was determined by using the respective tool of the ARB program package. The trees were constructed with the neighbor-joining algorithm (26) (ARB and PHYLIP). In addition, different subsets of about 30 16S rRNA sequences were reanalyzed by maximum-likelihood methods (fastDNAml [19] and ARB) to examine the significance of the phylogenetic position of strain SB164P1 as it has been determined by using the larger data set of 69 reference 16S rRNA sequences in conjunction with the neighbor-joining algorithm.
Nucleotide sequence accession number.
The 16S rDNA sequence of strain SB164P1 has been deposited in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. Y13672.
RESULTS
Enrichment cultures.
Black spots in the reddish brown ferrihydrite, indicating the formation of iron sulfide, were observed in the first dilution step after 4 days and occurred up to the fifth dilution step. Sulfate accumulated concomittantly with iron sulfide. Cultures from the highest dilution with growth (105) were used for further isolation. Microscopic examination revealed two major bacterial morphotypes. The predominant type was rod shaped and slender, and some cells were motile. The other type was coccoid and nonmotile. Both types grew in close proximity to the particle phase, and an increased numbers of cells were seen after particle disruption. Bacteria were seldom observed in supernatant aliquots. In addition to the dominating cell types, small, highly motile vibrioid cells were observed. The culture used for isolation was purified by repeated application of deep agar dilution series.
Isolation.
In low agar dilutions (up to tube 3), large amounts of black iron sulfide developed within 3 weeks of incubation and obscured the bacterial colonies. In the higher dilutions, only a little iron sulfide was produced, allowing the identification of bacterial colonies under the dissection microscope. The colonies were round and whitish and developed in close proximity to sulfur particles. A microscopic examination of an isolated colony revealed only slender rod-shaped cells. After a transfer to liquid medium, five morphologically identical cultures were obtained. The cultures were able to grow with thiosulfate as a substitute for elemental sulfur. An additional purification was conducted with thiosulfate and Fe2+, leaving out the iron agar layer. Thiosulfate-disproportionating colonies were black due to iron precipitates. Well-separated colonies from the highest dilution were transferred to liquid thiosulfate-containing medium. Microscopic examination of the colonies revealed the same structure and cell type as described for the sulfur-ferrihydrite agar shakes. The six isolates were able to grow by elemental sulfur disproportionation. One isolate, designated SB164P1, was chosen for detailed physiological and phylogenetic characterization.
Morphology.
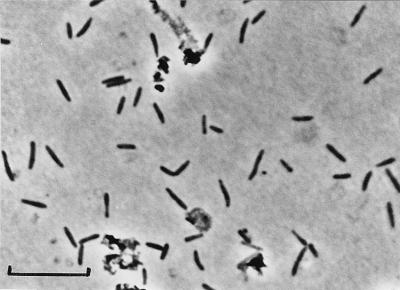
Strain SB164P1 cells were 2 to 4 μm long and 0.5 μm wide with slightly pointed ends (Fig. 1). Motile cells were observed. Cells from outgrown cultures stained gram negative.
FIG. 1.
Phase-contrast photomicrograph of D. sulfoexigens SB164P1 grown with elemental sulfur and ferrihydrite. Most ferrihydrite and iron sulfide precipitates were dissolved by dithionite-citrate-acetic acid extraction. Residual precipitates are seen as highly refractive aggregates. Bar, 10 μm.
Growth and temperature requirements.
Strain SB164P1 did not require organic carbon or vitamins for growth, but NaCl and MgCl2 were necessary for growth. Growth was inhibited below 25 mM NaCl and 2 mM MgCl2 · 6H2O. The cultures developed equally well within concentration ranges of 170 to 330 mM NaCl and 7 to 15 mM MgCl2 · 6H2O, respectively. Higher concentrations were not tested. The pH range for growth was from 6.0 to 8.2 with an optimum between 6.7 and 7.3. In growing cultures, the pH could drop below 6.0 when elemental sulfur served as the substrate. The pH tests were carried out in bicarbonate-buffered medium only. Strain SB164P1 grew within a temperature range from 5 to 35°C. The optimum growth temperature was 30°C.
Metabolic properties.
Apart from sulfur, thiosulfate, and sulfite disproportionation, strain SB164P1 reduced small amounts of sulfate in the presence of formate and hydrogen. Within a period of 6 weeks, 0.09 and 0.11 mM sulfide were produced in the presence of formate and hydrogen, respectively. Sulfate reduction did not sustain growth. No sulfide was detectable (detection limit, 1 μM) in the other incubations. Sulfate reduction with formate was also demonstrated in incubations with radiolabeled sulfate. Radiolabeled sulfide accumulated exclusively with formate as the electron donor. A fermentative type of metabolism with pyruvate, lactate, or glucose was not observed. Neither nitrate, dimethyl sulfoxide, nor oxygen (2%) was used as the electron acceptor.
Disproportionation of sulfur compounds.
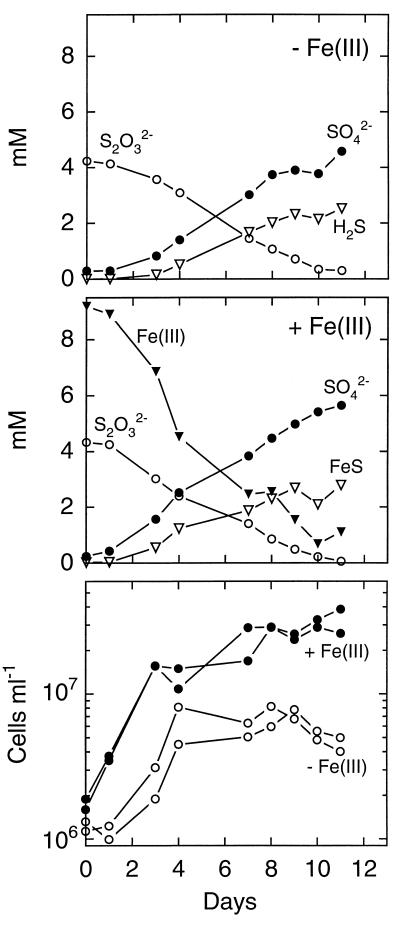
Elemental sulfur and thiosulfate were disproportionated by strain SB164P1 with or without ferric iron present in the growth medium (Fig. 2 and 3). The rate of thiosulfate consumption was similar in the presence and absence of ferrihydrite. In ferric iron-free medium (Fig. 2, top), the ratio of sulfate produced to thiosulfate consumed was 1:1, in agreement with thiosulfate disproportionation:
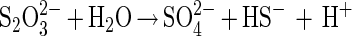 |
Sulfide production was somewhat lower than predicted, which can be explained by degassing of H2S. When ferrihydrite was present as a sulfide scavenger during thiosulfate disproportionation (Fig. 2, middle), the amount of sulfate plus sulfide produced closely matched the amount of thiosulfate consumed. Under these conditions, sulfide accumulated as FeS and dissolved sulfide was not detectable, so that loss through degassing was excluded. Ferrihydrite reacts rapidly with hydrogen sulfide, producing Fe2+. Ferrous iron may further precipitate sulfide as FeS (24), and elemental sulfur formed may be further disproportionated. This results in a sulfide to sulfate accumulation ratio smaller than 1.0.
FIG. 2.
Thiosulfate metabolism of strain SB164P1. (Top) Incubation without ferrihydrite. Results are means of two incubations. (Middle) Incubation with ferrihydrite. Results are means of two incubations. (Bottom) Cell counts from two parallel incubations with and without ferrihydrite. Note the logarithmic scale.
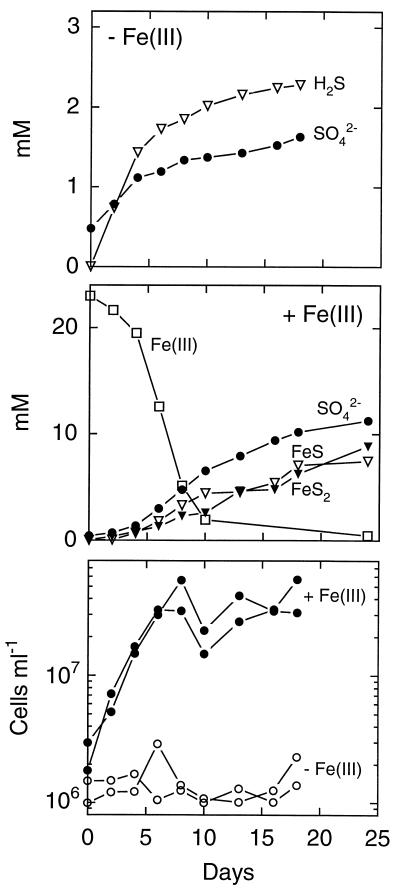
FIG. 3.
Elemental sulfur metabolism of strain SB164P1. Sulfur was added in excess. (Top) Incubation without ferrihydrite. Results are means of two incubations. (Middle) Incubation with ferrihydrite. Results are means of two incubations. FeS2 represents pyrite sulfur. Note the different scales in the top and middle panels. (Bottom) Cell counts from two parallel incubations with and without ferrihydrite. Note the logarithmic scale.
With elemental sulfur, strain SB164P1 produced sulfide and sulfate at a ratio close to 3:1 at the beginning of the incubation (Fig. 3, top), in agreement with sulfur disproportionation:
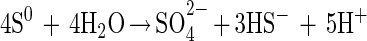 |
After 10 days, the ratio shifted to lower values. Removal of sulfide by degassing during sampling is again the most likely explanation. About 10 times as much sulfate was produced in the presence of ferrihydrite (Fig. 3, middle). Free sulfide was not detected. Sulfide accumulated as both FeS and pyrite, FeS2. Pyrite may form through the following reaction (4):
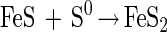 |
The final ratio of Fe(III) consumed to sulfate produced was 2:1, as expected for disproportionation in the presence of ferrihydrite (32).
Strain SB164P1 also grew by sulfite disproportionation in the absence of ferrihydrite (data not shown).
Growth by sulfur and thiosulfate disproportionation.
Strain SB164P1 grew exponentially at the beginning of the incubation period except with elemental sulfur in ferrihydrite-free medium (Fig. 2 and 3, bottom). With elemental sulfur and ferrihydrite, growth ceased after 7 days while sulfur disproportionation continued throughout the entire incubation period. However, the rate of sulfur disproportionation decreased from day 7 until the end of the incubation. Growth cessation coincided with the depletion of Fe(III). The doubling times were 1 and 1.5 days with thiosulfate in the presence and absence of Fe(III), respectively, and 2 days with elemental sulfur and ferrihydrite in the medium. Cultures grew without a lag phase when iron was present in the medium, while the iron-free thiosulfate cultures were delayed by 1 day. In the latter culture, growth ceased after 4 days, when 1.2 mM thiosulfate were consumed. The disproportionation of the remaining 3 mM was not accompanied by growth. Thiosulfate-ferrihydrite cultures grew exponentially at the beginning of the incubation. Afterwards, growth was linear with a slow increase throughout the rest of the incubation. The shift in the growth pattern was paralleled by the time courses of thiosulfate degradation and sulfate and ferrous iron production.
Molybdate addition.
Thiosulfate and elemental sulfur disproportionation were affected by the presence of molybdate. Both thiosulfate and elemental sulfur disproportionation were inhibited completely by 5 mM molybdate. At lower concentrations, the isolate responded differently when grown with thiosulfate and with elemental sulfur. With thiosulfate, the inhibition was relieved at molybdate concentrations below 500 μM. The lag phase in the presence of 500 μM molybdate was significantly longer (8 days) than in molybdate-free incubations (0 to 1 day). At molybdate concentrations lower than 50 μM, no difference was observed compared with the lag phase in molybdate-free cultures. Cultures with elemental sulfur were more sensitive to molybdate. Complete relief of the inhibition was observed only at molybdate concentrations below 5 μM.
G+C content of the DNA.
The average G+C content of strain SB164P1 was 47.2 ± 0.2 mol%.
Phylogeny.
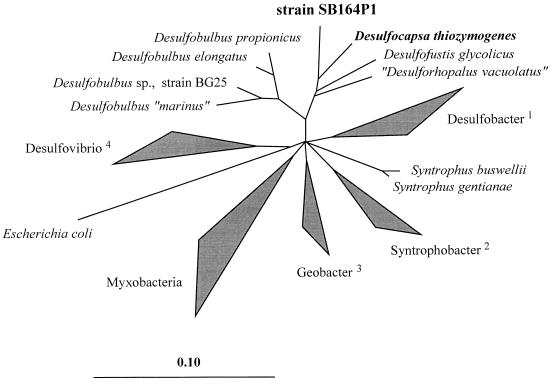
Strain SB164P1 is a member of the delta subclass of the Proteobacteria. It belongs to a coherent group of microorganisms which consists of the recently described species Desulfocapsa thiozymogenes (12), Desulforhopalus vacuolatus (11), and Desulfofustis glycolicus (9) (Fig. 4). Within this phylogenetic cluster, strain SB164P1 is more closely related to Desulfocapsa thiozymogenes than to the other two organisms. This is indicated by the overall similarity values of 91.5, 90.7, and 89.8%, respectively, between the respective 16S rRNA sequence pairs. This group forms a common line of descent with members of the genus Desulfobulbus, with overall similarity values between strain SB164P1 and the four Desulfobulbus species ranging from 86.6 to 88.7% while the corresponding values for all other representatives of the delta subclass of the Proteobacteria are lower, i.e., between 80.2 and 84.6%. The monophyletic character of the Desulfocapsa/Desulfobulbus group was supported in all treeing analyses independent of the algorithms used for tree reconstruction and the procedure applied to exclude highly variable alignment positions. However, except for Desulfovibrio and related organisms, the relative branching order of the major subgroups within the delta subclass of the Proteobacteria could not be determined unambiguously, resulting in a multifurcation of the respective branches in the tree (Fig. 4).
FIG. 4.
Phylogenetic tree based on 16S rRNA sequences showing the relationship between strain SB164P1 and its closest relatives as well as 62 other reference organisms of the delta subclass of the Proteobacteria. The triangles indicate groups of phylogenetically related species or genera: 1, Desulfobacter spp. plus Desulfobacterium, Desulfobacula, and Desulfosarcina spp.; 2, Syntrophobacter spp. plus Desulfoacinum and Desulforhabdus spp.; 3, Geobacter spp. plus Desulfuromonas, Desulfuromusa, and Pelobacter spp.; 4, Desulfovibrio spp. plus Desulfohalobium and Desulfomicrobium spp. The 16S rRNA sequence of E. coli was used as an outgroup reference. The scale bar represents the estimated number of base changes per nucleotide sequence position.
DISCUSSION
Strain SB164P1 was routinely cultured in marine medium containing elemental sulfur as a source of energy and reduction equivalents and ferric iron as a sulfide scavenger. It grew exclusively by disproportionation of elemental sulfur, thiosulfate, or sulfite. With thiosulfate and sulfite, a sulfide scavenger was not required, although growth with thiosulfate was stimulated by the presence of ferrihydrite. Growth by elemental sulfur disproportionation has recently been described for marine enrichment cultures (32) and the freshwater isolates Desulfocapsa thiozymogenes (12) and Desulfobulbus propionicus 1pr3 (18). Strain SB164P1, in contrast to Desulfocapsa thiozymogenes and Desulfobulbus propionicus, did not grow by sulfate reduction. It is thus the first obligate disproportionator of sulfur compounds to be described. Like Desulfocapsa thiozymogenes (12) and marine enrichment cultures (32), strain SB164P1 did not require organic carbon for growth. However, carbon fixation has not been directly demonstrated.
Growth yields and growth efficiencies with elemental sulfur and thiosulfate.
Based on a ratio of cell dry weight to cell volume of 0.305 g/cm3 as reported for E. coli (21), an average dry weight of 1.8 × 10−13 g for individual cells of strain SB164P1 was calculated. With elemental sulfur, approximately 0.24 g of dry cell mass was produced per mol of elemental sulfur disproportionated compared to the 0.1 g reported by Janssen et al. (12) for Desulfocapsa thiozymogenes. With thiosulfate alone, the growth yield at the end of the exponential growth phase was 0.94 g (dry weight) mol of thiosulfate−1. This is less than half the growth yield reported for Desulfocapsa thiozymogenes (12) and Desulfovibrio sulfodismutans (2), which, however, were both grown with acetate as the carbon source. In the presence of ferric iron, the growth yield was 1.3 g (dry weight) mol of thiosulfate−1. Based on the growth yields given above, we can estimate the growth efficiency of strain SB164P1 with thiosulfate or elemental sulfur. Assuming that (i) strain SB164P1 grew autotrophically and CO2 assimilation produced glucose or organic carbon with the same chemical composition and formation energy as those of glucose and that (ii) thiosulfate or elemental sulfur was the only energy and electron source, we calculate a carbon yield of 3.5 g of C mol of thiosulfate−1. The isolate produced 0.94 g (dry weight) mol of thiosulfate−1, corresponding to 0.38 g of C (using a 40% [by weight] C content of glucose), or 10.7% of the theoretically possible amount of carbon. In the presence of elemental sulfur, a growth efficiency of approximately 3% can be determined. These values are in the range of thermodynamic efficiency values of chemolithoautotrophic sulfide-oxidizing bacteria compiled by Nelson and Hagen (22).
Effect of molybdate.
The inhibitory effect of molybdate on sulfate reduction is well understood (31). By formation of adenosine-5-phosphomolybdate, molybdate depletes the internal ATP pool of the cell. Interestingly, molybdate also inhibited elemental sulfur and thiosulfate disproportionation. Recently, Krämer and Cypionka (15) and Fuseler and Cypionka (10) proposed that ATP synthesis by disproportionation results from the inversion of the initial steps of sulfate reduction, including the formation of adenosine-5-phosphosulfate through substrate-level phosphorylation. Molybdate may interfere with the formation of APS and consequently may inhibit disproportionation reactions. We have no explanation why sulfur disproportionation was more sensitive to molybdate than was thiosulfate disproportionation.
Ecological considerations.
Strain SB164P1 is the first marine bacterium found to be able to thrive by disproportionation of intermediately oxidized inorganic sulfur compounds alone. Since it was isolated from the fifth step in a series of 10-fold dilutions, it appears to be abundant in the sediment. Up to 106 cells per cm3 of sulfur-disproportionating bacteria have previously been counted in marine sediments with a medium similar to that used in the present study (32). Most-probable-number counts of sulfate reducers in sediments from Arcachon Bay range from 106 to 107 cells per cm3 (35). The occurrence of obligate sulfur disproportionators in numbers comparable to those of sulfate reducers is a strong indication that disproportionation is a common way of living in these environments. This is further accentuated when the relatively low growth yield of the process is considered. Jørgensen and Bak (13) argued on the basis of most-probable-number counts that thiosulfate disproportionation in marine environments is mainly a cometabolism executed by sulfate reducers but not a major type of metabolism. These authors, however, did not include a sulfide scavenger in their medium. In natural sediments, the scavenging of hydrogen sulfide by iron oxides is a very important process, which often maintains sulfide concentrations at low levels in spite of intense sulfide production through sulfate reduction. We conclude from the results of our studies that the presence of a sulfide scavenger is essential for counting, enrichment, and isolation of SB164P1-like bacteria and, consequently, that life by disproportionation is more common than has so far been assumed.
Phylogenetic position.
The closest relative of strain SB164P1 is Desulfocapsa thiozymogenes. Desulforhopalus vacuolatus and Desulfofustis glycolicus are slightly more distantly related (Fig. 4). From the phylogenetic point of view, the dissimilarity value of 8.5% between the 16S rRNA sequences of Desulfocapsa thiozymogenes and strain SB164P1 would allow us to propose the taxonomic rank of a new genus for the latter organism. However, considering the very similar phenotypic and genotypic properties, including the G+C content of the genomic DNA (47.2% versus 50.7%), we favour the inclusion of strain SB164P1 into the genus Desulfocapsa and suggest the species name D. sulfoexigens.
Description of Desulfocapsa sulfoexigens sp. nov.
Desulfocapsa sulfoexigens (sul.fo.ex′i.gens. L. n. sulfurum, sulfur; L. v. exigo, to demand; M. L. part. adj. sulfoexigens, demanding sulfur for growth). Gram-negative cells that are elongated with slightly pointed ends, 0.5 μm wide and 2 to 4 μm long. Multiplication occurs by binary fission. Under most conditions, only a few cells are motile. Spores are not produced. Growth occurs in mineral medium without vitamins or organic carbon sources with bicarbonate as the sole carbon source. Disproportionation of elemental sulfur, sulfite, and thiosulfate to sulfide and sulfate occurs. Growth by sulfur disproportionation occurs only in the presence of a hydrogen sulfide-scavenging agent, like ferric iron. Growth by disproportionation of thiosulfate occurs with and without iron. No growth occurs by sulfate reduction. The pH range is from 6.0 to 8.2 with an optimum of 6.7 to 7.3. The temperature range is 4 to 35°C with an optimum of 30°C. The habitat is oxidized marine surface sediment. The type strain was isolated from marine sediment covered by the eelgrass, Zostera noltii, in the basin of Arcachon, France.
The G+C content of the genomic DNA is 47.2 ± 0.2 mol%.
The type strain is strain SB164P1, DSM 10523, deposited with the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
ACKNOWLEDGMENTS
K.F. is indebted to Niels Peter Revsbech for support. Sonja Fleissner and Swantje Fleischer are acknowledged for technical assistance. We thank three anonymous reviewers for constructive criticism of the manuscript.
The study was financed by a European Union project on Coastal Lagoon Eutrophication and Anaerobic Processes (C.L.AE.N) (contract EV5V-CT92-008), by the Danish Committee on Biotechnology, by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (contract 21/03111221), and by the Max Planck Society.
REFERENCES
- 1.Bak F. Fermentation of inorganic sulfur compounds by sulfate-reducing bacteria. In: Guerrero R, Pedrós-Alió C, editors. Trends in microbial ecology. Barcelona: Spanish Society for Microbiology; 1993. pp. 75–78. [Google Scholar]
- 2.Bak F, Cypionka H. A novel type of energy metabolism involving fermentation of inorganic sulphur compounds. Nature. 1987;326:891–892. doi: 10.1038/326891a0. [DOI] [PubMed] [Google Scholar]
- 3.Bak F, Pfennig N. Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov. by disproportionation of inorganic sulfur compounds. Arch Microbiol. 1987;147:184–189. [Google Scholar]
- 4.Berner R A. Sedimentary pyrite formation. Am J Sci. 1970;268:1–23. [Google Scholar]
- 5.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 6.Felsenstein J. PHYLIP (Phylogeny Inference Package). Seattle: University of Washington; 1993. [Google Scholar]
- 7.Finster K, Liesack W, Tindall B J. Desulfospira joergensenii, gen. nov., sp. nov., a new sulfate-reducing bacterium isolated from marine surface mud. Syst Appl Microbiol. 1997;20:201–208. [Google Scholar]
- 8.Fossing H, Jørgensen B B. Measurements of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochemistry. 1989;8:205–222. [Google Scholar]
- 9.Friedrich M, Springer N, Ludwig W, Schink B. Phylogenetic positions of Desulfofustis glycolicus gen. nov., sp. nov., and Syntrophobotulus glycolicus gen. nov., sp. nov., two new strict anaerobes growing with glycolic acid. Int J Syst Bacteriol. 1996;46:1065–1069. doi: 10.1099/00207713-46-4-1065. [DOI] [PubMed] [Google Scholar]
- 10.Fueseler K, Cypionka H. Elemental sulfur as an intermediate of sulfide oxidation with oxygen by Desulfobulbus propionicus. Arch Microbiol. 1995;164:104–109. [Google Scholar]
- 11.Isaksen M F, Teske A. Desulforhopalus vacuolatus gen. nov., sp. nov., a new moderately psychrophilic sulfate-reducing bacterium with gas vacuoles isolated from a temperate estuary. Arch Microbiol. 1996;166:160–168. [Google Scholar]
- 12.Janssen P H, Schuhmann A, Bak F, Liesack W. Disproportionation of inorganic sulfur compounds by the sulfate-reducing bacterium Desulfocapsa thiozymogenes gen. nov., sp. nov. Arch Microbiol. 1996;166:184–192. [Google Scholar]
- 13.Jørgensen B B. A thiosulfate shunt in the sulfur cycle of marine sediments. Science. 1990;249:152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen B B, Bak F. Pathways and microbiology of thiosulfate transformations, and sulfate reduction in a marine sediment (Kattegat, Denmark) Appl Environ Microbiol. 1991;57:847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krämer M, Cypionka H. Sulfate formation via ATP sulfurylase in thiosulfate- and sulfite-disproportionating bacteria. Arch Microbiol. 1989;151:232–237. [Google Scholar]
- 16.Liesack W, Finster K. Phylogenetic analysis of five strains of gram-negative, obligately anaerobic, sulfur-reducing bacteria and description of Desulfuromusa gen. nov., including Desulfuromusa kysingii sp. nov., Desulfuromusa bakii sp. nov., and Desulfuromusa succinoxidans sp. nov. Int J Syst Bacteriol. 1994;44:753–758. [Google Scholar]
- 17.Lord C J., III . The chemistry and cycling of iron, manganese, and sulfur in salt marsh sediments. Ph.D. dissertation. University of Delaware; 1980. [Google Scholar]
- 18.Lovley D R, Phillips E J P. Novel processes for anaerobic sulfate production from elemental sulfur by sulfate-reducing bacteria. Appl Environ Microbiol. 1994;60:2394–2399. doi: 10.1128/aem.60.7.2394-2399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The ribosomal database project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesbah M, Premachandran U, Whitman W B. Precise measurements of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 21.Neidhardt F C. Chemical composition of Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 3–6. [Google Scholar]
- 22.Nelson D, Hagen K D. Physiology and biogeochemistry of symbiontic and free-living chemoautotrophic sulfur bacteria. Am Zool. 1995;35:91–101. [Google Scholar]
- 23.Pfennig N. Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol. 1978;28:283–288. [Google Scholar]
- 24.Pyzik A J, Sommer S E. Sedimentary iron monosulfides: kinetics and mechanism of formation. Geochim Cosmochim Acta. 1981;45:687–698. [Google Scholar]
- 25.Rodriguez-Tom P, Stoehr P J, Cameron G N, Flores T P. The European Bioinformatics Institute (EBI) databases. Nucleic Acids Res. 1996;24:6–12. doi: 10.1093/nar/24.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Singleton R., Jr . The sulfate-reducing bacteria: an overview. In: Odom J M, Sinleton R Jr, editors. The sulfate-reducing bacteria: contemporary perspectives. New York, N.Y: Springer Verlag; 1993. pp. 1–20. [Google Scholar]
- 28.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 29.Strunk O, Ludwig W. ARB. Munich, Germany: Technische Universität München; 1996. [Google Scholar]
- 30.Tamaoka J, Komagato K. Determination of DNA base composition by reverse-phase high performance liquid chromatography. FEMS Microbiol Lett. 1984;25:125–128. [Google Scholar]
- 31.Taylor B F, Oremland R S. Depletion of adenosine triphosphate in Desulfovibrio by oxyanions of group VI elements. Curr Microbiol. 1979;3:101–103. [Google Scholar]
- 32.Thamdrup B, Finster K, Hansen J W, Bak F. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl Environ Microbiol. 1993;59:101–108. doi: 10.1128/aem.59.1.101-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van de Peer Y, Nicolaï S, De Rijk P, De Wachter R. Database on the structure of small ribosomal subunit RNA. Nucleic Acids Res. 1996;24:86–91. doi: 10.1093/nar/24.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Peer Y, Chapelle S, De Wachter R. A quantitative map of nucleotide substitution rates in bacterial rRNA. Nucleic Acids Res. 1996;24:3381–3391. doi: 10.1093/nar/24.17.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh D T, Bourguès S, De Wit R, Herbert R A. Seasonal variations in rates of heterotrophic nitrogen fixation (acetylene reduction) in Zostera noltii meadows and uncolonised sediments of the Bassin d’Arcachon, south-west France. Hydrobiologia. 1996;329:161–174. [Google Scholar]
- 36.Widdel F, Hansen T A. The dissimilatory sulfate- and sulfur-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes: a handbook on the biology of bacteria. Ecophysiology, isolation, identification applications. 2nd ed. New York, N.Y: Springer Verlag; 1992. pp. 583–624. [Google Scholar]