Abstract
Invasion and intracellular replication of Legionella pneumophila within protozoa in the environment plays a major role in the transmission of Legionnaires’ disease. Intracellular replication of L. pneumophila within protozoa occurs in a rough endoplasmic reticulum (RER)-surrounded phagosome (Y. Abu Kwaik, Appl. Environ. Microbiol. 62:2022–2028, 1996). Since the subsequent fate of many intracellular pathogens is determined by the route of entry, we compared the mechanisms of attachment and subsequent uptake of L. pneumophila by the two protozoa Hartmannella vermiformis and Acanthamoeba polyphaga. Our data provide biochemical and genetic evidence that the mechanisms of attachment and subsequent uptake of L. pneumophila by the two protozoan hosts are, in part, different. First, uptake of L. pneumophila by H. vermiformis is completely blocked by the monovalent sugars galactose and N-acetyl-d-galactosamine, but these sugars partially blocked A. polyphaga. Second, attachment of L. pneumophila to H. vermiformis is associated with a time-dependent and reversible tyrosine dephosphorylation of multiple host proteins. In contrast, only a slight dephosphorylation of a 170-kDa protein of A. polyphaga is detected upon infection. Third, synthesis of H. vermiformis proteins but not of A. polyphaga proteins is required for uptake of L. pneumophila. Fourth, we have identified L. pneumophila mutants that are severely defective in attachment to A. polyphaga but which exhibit minor reductions in attachment to H. vermiformis and, thus, provide a genetic basis for the difference in mechanisms of attachment to both protozoa. The data indicate a remarkable adaptation of L. pneumophila to attach and invade different protozoan hosts by different mechanisms, yet invasion is followed by a remarkably similar intracellular replication within a RER-surrounded phagosome and subsequent killing of the host cell.
The Legionnaires’ disease bacterium, Legionella pneumophila, is a common etiologic agent of bacterial pneumonia (13, 23, 38, 53). Upon transmission to individuals through aerosols generated in the environment, the bacteria invade and replicate within pulmonary macrophages and epithelial cells (20, 31, 39).
In the environment, L. pneumophila is a parasite of at least 13 species of amoebae and ciliated protozoa (reviewed in reference 24). In many outbreaks of Legionnaires’ disease, the bacteria and protozoa have been isolated from the same water source, and the isolated protozoa have been shown to support intracellular multiplication of the bacterial isolate (10, 16, 26, 37). Additionally, in many confirmed cases of Legionnaires’ disease, the bacterium could only be isolated by its capacity to multiply within protozoa (8, 18, 28, 47).
Rowbotham has postulated that the infectious particle involved in transmission of Legionnaires’ disease is L. pneumophila-infected amoebae (44). Several lines of evidence indicate that protozoa play major roles in the continued presence of L. pneumophila in the environment as well as in the infectivity of the bacteria to humans. Intracellular replication of L. pneumophila within protozoa increases bacterial resistance to harsh environmental conditions, which may allow the bacteria to survive extracellularly for prolonged periods of time in the environment (6, 11, 12). Interestingly, it has been recently shown that viability and infectivity of nonculturable L. pneumophila can be “resuscitated” by intracellular replication within protozoa (45). Moreover, intracellular multiplication within protozoa enhances the infectivity of L. pneumophila to human-derived cells (21). These observations may explain why Legionnaires’ disease is not transmitted between individuals and why transmission occurs despite the presence of low numbers of L. pneumophila from the source of aerosol (14, 22, 40).
The hallmark of L. pneumophila infection of humans is the intracellular survival and replication of the bacteria within macrophages in a rough endoplasmic reticulum (RER)-surrounded phagosome (2, 24, 31). The intracellular infection of protozoa is similar to that of macrophages at the ultrastructural and molecular levels (1, 29). In addition, diaminopimelic acid auxotrophs of L. pneumophila are defective in intracellular growth within mammalian and protozoan cells (30a). During intracellular replication, changes in bacterial gene expression are manifested (2–4, 6, 7, 48). Although protein synthesis by the RER is not required for intracellular bacterial replication (2), the role of the RER in intracellular survival is not known.
The mechanisms involved in intracellular trafficking leading to survival and replication of the bacterium within mammalian and protozoan cells are not yet known. Several lines of evidence indicate that the fate of some intracellular pathogens is determined by the route of entry and the specific host cell receptor involved, which may trigger different host cell signal transduction mechanisms (9, 32, 35). We have recently shown that uptake of L. pneumophila by the protozoan Hartmannella vermiformis is mediated by the Gal/GalNAc lectin receptor and is associated with tyrosine dephosphorylation of this receptor (52). In this report, we extended our studies on the host cell processes involved in uptake of L. pneumophila to another protozoan host, Acanthamoeba polyphaga, and compared its host cell response to that of H. vermiformis. The attachment and subsequent cross talk between L. pneumophila and its hosts may play a role in the intracellular fate of the bacterium.
Uptake of L. pneumophila by the protozoan H. vermiformis has been proposed to occur through a microfilament-independent but receptor-mediated mechanism (36), but the processes involved in bacterial uptake by other protozoan hosts have not been reported. This paper describes the investigation of the different mechanisms utilized for the attachment and uptake of L. pneumophila by two protozoa, H. vermiformis and A. polyphaga.
MATERIALS AND METHODS
Bacterial strains.
The virulent AA100 strain of L. pneumophila has been described previously (3, 5). The L. pneumophila mutants used in this study (GF162, GG104, GB112, GM128, GO128, GP65, GQ262, and GT251) were generated by mini Tn10::kan transposon mutagenesis of the AA100 strain (29, 30). Southern hybridizations were used to confirm that these mutants contained distinct insertions in their chromosomes (29). L. pneumophila were grown on buffered charcoal yeast extract (BCYE) agar plates. Growth of the insertion mutants was on BCYE medium with 20 μg of kanamycin (Sigma Chemical Co., St. Louis, Mo.)/ml.
Protozoan culture.
Axenic A. polyphaga was obtained from B. S. Fields (Centers for Disease Control and Prevention, Atlanta, Ga.) and cultured as adherent cells in peptone-yeast-glucose medium (15). H. vermiformis CDC-19 (ATCC 50237) has been cloned and grown in axenic culture as a model for the study of the pathogenesis of L. pneumophila (25). This strain has been isolated from a water source of an outbreak of nosocomial Legionnaires’ disease in a hospital in South Dakota, and its presence in the potable water sites correlated with the presence of the epidemic strain of L. pneumophila (16, 25). H. vermiformis was maintained in ATCC culture medium 1034 (5, 25).
Detection of tyrosine-phosphorylated proteins in H. vermiformis and A. polyphaga upon contact with L. pneumophila.
Amoebae were incubated overnight in culture flasks in serum-free axenic medium or PYG medium for H. vermiformis and A. polyphaga, respectively. The amoebae were harvested by centrifugation and were resuspended in the corresponding fresh medium. Aliquots of ∼2 × 107 amoebae/ml were infected by 109 L. pneumophila. At several time intervals of coincubation at 37°C, amoebal cell lysates were prepared for Western blotting as described below.
Preparation of cell lysates and Western blotting.
After incubation of amoebae with L. pneumophila, infections were stopped by using cold stop buffer (1X PBS, pH 7.2) containing the phosphatase inhibitors NaF (5 mM) and Na3VO4 (1 mM) (Sigma Chemical Co.). Cells were washed three times with cold stop buffer and pelleted by low-speed centrifugation at 735 × g for 2 min. The supernatant containing bacteria was discarded, and amoebae were lysed by using cold 1% Triton X-100 lysis buffer (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin/ml, and 2 μg of aprotinin/ml). The soluble and insoluble fractions were separated by centrifugation at 16,000 × g for 30 min at 4°C in a microcentrifuge. Proteins from soluble fractions were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis under reducing conditions. The transfer of proteins onto Immobilon-P membranes (Millipore, Bedford, Mass.) was performed in a Bio-Rad transfer cell (Bio-Rad, Hercules, Calif.) for 1.5 to 2 h with 0.2 M Tris–0.025 M glycine buffer containing 20% (vol/vol) methanol. After transfer of proteins, membranes were incubated for 30 min in a blocking buffer containing 1.5% bovine serum albumin. Membranes were probed with antiphosphotyrosine antibody conjugated to horseradish peroxidase (RC-20) according to the manufacturer’s recommendations (Transduction Laboratories, Lexington, Ky.). The blots were developed by using an enhanced chemiluminescence kit (DuPont NEN, Boston, Mass.) according to the manufacturer’s instructions. The specificity of the RC-20 antibody for protozoan phosphotyrosine-containing proteins was confirmed by Western blots probed with another antiphosphotyrosine antibody, clone 4G10 (Upstate Biotechnology Inc., Lake Placid, N.Y.) followed by a horseradish peroxidase-P conjugated goat anti-mouse secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) (data not shown).
Inhibition of L. pneumophila uptake by sugars.
Infection of amoebae with L. pneumophila was performed exactly as described previously (5). To analyze the effects of different sugars on invasion of amoebae by L. pneumophila, infections were performed in triplicate in the presence of a 100 mM concentration of the following sugars: galactose (Gal), N-acetyl-d-galactosamine (GalNAc), glucose, lactose, and mannose (Sigma). Sugar solutions were prepared in the medium used to maintain each of the amoebae.
The effects of the sugars on invasion were investigated by gentamicin protection invasion assays. In these assays, A. polyphaga or H. vermiformis was suspended in its respective medium at a concentration of ∼107/ml. Amoebae were incubated in triplicate in the presence of different sugars for 15 min prior to infection. L. pneumophila was added to a final concentration of 108/ml. The samples were incubated at 37°C for 4 h followed by the addition of 50 μg of gentamicin/ml for 1 h to kill extracellular bacteria. Intracellular bacteria are protected from this antibiotic. The amoebae were washed with medium and lysed by the addition of a mild detergent (0.04% Triton X-100). Lysis of the amoebae was monitored microscopically and was complete within 1 min. This treatment had no significant effect on the viability of the bacteria (data not shown). Dilutions were plated on BCYE plates for colony enumeration. The percent invasion was calculated by dividing the number of CFU in the presence of each sugar by the number of CFU in the absence of the sugar.
The effects of the sugars on invasion of amoebae by L. pneumophila were also determined by examination of the growth kinetics of L. pneumophila within H. vermiformis and A. polyphaga in the presence of each sugar. Amoebae were incubated in the presence of each sugar for 15 min prior to infection with L. pneumophila. At several time intervals after infection (1, 3, 5, and 7 days), amoebae were lysed as described above, and samples were diluted and plated on BCYE plates for colony enumeration. The percent invasion was calculated as described above.
Uptake of L. pneumophila by amoebae in the presence of inhibitors of cytoskeletal integrity.
Infection of amoebae with L. pneumophila was performed exactly as described previously (5). To analyze the effects of inhibitors of cytoskeletal function on the uptake of L. pneumophila, amoebae were incubated in the presence of each inhibitor for 1 h prior to infection. The inhibitors used were 2 μM cytochalasin D (microfilaments) and 10 μM colchicine (microtubules) (Sigma). Following a 3-h infection, extracellular bacteria were killed by the addition of 50 μg of gentamicin/ml, and intracellular bacteria were plated for enumeration on BCYE plates.
Infection of A. polyphaga by L. pneumophila in the presence of cycloheximide.
To assess the effect of cycloheximide on the uptake of L. pneumophila by A. polyphaga, ∼107 A. polyphaga was pretreated for 3 h with 200 μg of cycloheximide/ml to inhibit protein synthesis. Amoebae were infected with ∼108 L. pneumophila for 4 h in the presence of cycloheximide. Cycloheximide has no detectable effect on the viability or gene expression of L. pneumophila (5). Subsequently, nonadherent bacteria were removed by extensive washing, and extracellular bacteria were killed by gentamicin, as described above. Intracellular bacteria were recovered after lysis of the amoebae and were plated on BCYE for colony enumeration, as described above.
Attachment of L. pneumophila mutants to H. vermiformis and A. polyphaga.
H. vermiformis was infected with eight different miniTn10::kan insertion mutants that are defective in attachment to human-derived U937 macrophages (29). To assess the ability of the mutant strains to adhere to A. polyphaga and H. vermiformis, 5 × 105 amoebae were infected for 20 min in triplicate with 107 bacteria of each mutant. Infections were carried out in the presence of 10 mM methylamine to prevent internalization of the bacteria (36). The amoebae were washed three times to remove unattached bacteria and were subsequently lysed with a mild detergent (0.04% Triton X-100). Dilutions were plated on BCYE. Adherence of the mutants was measured by comparison to the adherence of the wild-type strain, AA100, to amoebae in the same assay. To ensure that the inhibitors were effective in the inhibition of uptake, control monolayers infected in the presence of the inhibitor were treated with gentamicin to kill extracellular bacteria. The data showed that methylamine inhibited uptake by A. polyphaga and H. vermiformis by approximately 98% (data not shown).
RESULTS
Effects of sugars on the ability of L. pneumophila to invade A. polyphaga and H. vermiformis.
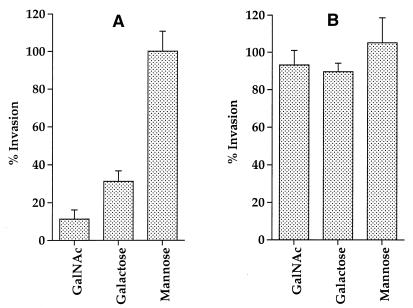
We used two strategies to investigate the roles of several sugars in blocking the attachment and invasion of amoebae by L. pneumophila. First, a gentamicin protection invasion assay was utilized to measure invasion of amoebae in the presence of various sugars (see Materials and Methods). Our data showed that the presence of specific sugars had different effects on bacterial uptake by A. polyphaga and H. vermiformis. Incorporation of 100 mM Gal or GalNAc in the infection assay had a dramatic effect on invasion of H. vermiformis by L. pneumophila (Fig. 1A). These sugar monomers were able to block uptake of L. pneumophila by H. vermiformis by ∼70 and 89%, respectively. Gal and GalNAc are not toxic to amoebae, and their inhibition of uptake of L. pneumophila is specific, dose dependent, and reversible (52). Other sugars (glucose, mannose, and lactose) at a concentration of 100 mM had no detectable effect on the uptake of L. pneumophila by H. vermiformis (Fig. 1A and data not shown).
FIG. 1.
Effects of different sugar monomers on invasion of H. vermiformis (A) and A. polyphaga (B) by L. pneumophila by gentamicin protection assays. Invasion of amoebae by L. pneumophila was performed in the absence or presence of various sugars. The percent invasion was determined by a comparison between the number of intracellular bacteria in the presence of sugars compared to that in untreated cultures. Values are the means of triplicate samples, and error bars represent standard deviations.
In contrast, Gal and GalNAc had slight effects on the uptake of L. pneumophila by A. polyphaga (Fig. 1B). These data showed that uptake of L. pneumophila by H. vermiformis but not by A. polyphaga is dramatically blocked by Gal and GalNAc.
Second, a growth kinetics assay was performed in the presence of the above sugars. The data confirmed our results obtained from the gentamicin protection invasion assays. Over the 7-day infection, 100 mM Gal or GalNAc caused a dramatic reduction or a complete inhibition in the growth kinetics of L. pneumophila within H. vermiformis (Fig. 2A). The inhibition of uptake of L. pneumophila by H. vermiformis in the presence of Gal or GalNAc is specific, reversible, and dose dependent (52). Other sugars (glucose, mannose, and lactose) at a concentration of 100 mM had no detectable effect on the uptake of L. pneumophila by H. vermiformis (Fig. 1A and data not shown).
FIG. 2.
Growth kinetics of L. pneumophila in cocultures with H. vermiformis or A. polyphaga in the absence or presence of different sugars. The bacteria do not replicate extracellularly in the coculture, and thus, the increase in the number of bacteria is due to intracellular replication. Values are the means of triplicate samples, and error bars represent standard deviations.
In contrast, parallel experiments performed with A. polyphaga showed that the presence of Gal or GalNAc caused a slight reduction in the growth kinetics of L. pneumophila (Fig. 2B). These data confirmed the distinct difference in the effects of Gal and GalNAc on the uptake of L. pneumophila by H. vermiformis and A. polyphaga.
Protein tyrosine phosphorylation in A. polyphaga and H. vermiformis upon attachment to L. pneumophila.
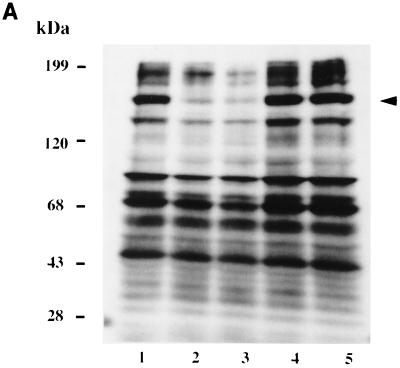
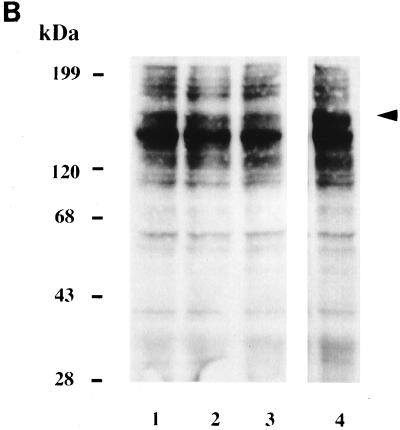
Tyrosine phosphorylation of host proteins has been shown to be important in the uptake of many intracellular pathogens (27, 33, 41–43, 49). We have recently shown protein tyrosine dephosphorylation in the protozoan H. vermiformis upon attachment and invasion by L. pneumophila (52). In this report, we extended our studies to another protozoan host, A. polyphaga, and compared its host cell response to that of H. vermiformis. First, we examined host cell signaling events in H. vermiformis and A. polyphaga upon contact with their bacterial parasite, L. pneumophila. We utilized Western blots of amoebal proteins to examine the status of tyrosine phosphorylation of host proteins in resting amoebae and following contact with L. pneumophila. Examination of H. vermiformis cell lysates showed several proteins that were tyrosine phosphorylated in resting amoebae (Fig. 3A, lane 1) that underwent a time-dependent and reversible dephosphorylation upon contact with L. pneumophila. Dephosphorylation of several H. vermiformis proteins, including those with apparent molecular masses of 190, 170, 130, and 70 kDa, was prominent and evident as early as 5 min (Fig. 3A, lane 2), and was complete by 15 min (Fig. 3A, lane 3), confirming our previous observations (52).
FIG. 3.
Effects of attachment of L. pneumophila to H. vermiformis and A. polyphaga on tyrosine phosphorylation of host proteins. (A) Cell extracts were prepared from uninfected H. vermiformis (lane 1), from H. vermiformis infected with L. pneumophila for 5 min (lane 2) or for 15 min (lane 3), or from uninfected cells incubated for 15 min (lane 4). Lane 5 represents samples prepared from H. vermiformis infected for 15 min followed by removal of extracellular bacteria and further incubation for 15 min. (B) Extracts were prepared from uninfected A. polyphaga (lane 1) or from A. polyphaga infected with L. pneumophila for 5 min (lane 2) or for 15 min (lane 3). Lane 4 represents samples prepared from infection with dead L. pneumophila. Western blots were probed with antiphosphotyrosine antibody (see Materials and Methods). Arrows indicate the position of the 170-kDa protein.
To compare the host cell response in H. vermiformis to that of another protozoan host, we also examined the pattern of protein tyrosine phosphorylation in resting A. polyphaga and upon attachment and invasion by L. pneumophila. Many tyrosine-phosphorylated proteins were detected in resting A. polyphaga (Fig. 3B). The pattern of tyrosine-phosphorylated proteins in A. polyphaga was distinctly different from that in H. vermiformis. However, in contrast to H. vermiformis, there was only a slight tyrosine dephosphorylation of a 170-kDa protein in A. polyphaga upon contact with L. pneumophila. These data demonstrated major differences in triggering the biochemical events involved in the attachment, and possibly the subsequent uptake, of L. pneumophila by the two amoebae.
Effects of inhibitors of cytoskeletal integrity on uptake of L. pneumophila by amoebae.
To further characterize the differences in the mechanisms of uptake of L. pneumophila by H. vermiformis and A. polyphaga, the effects of two inhibitors of cytoskeletal function (cytochalasin D and colchicine) on uptake of L. pneumophila were examined by gentamicin protection invasion assay. After pre-incubating the amoebae with each inhibitor for 3 h, infections were performed for 3 h. Subsequently, extracellular bacteria were killed, and the number of intracellular bacteria was determined. The data showed no detectable effect by either inhibitor on the uptake of L. pneumophila by A. polyphaga (Fig. 4). Similar results were obtained from H. vermiformis infected with L. pneumophila in the presence of these inhibitors (data not shown). These data indicated that the uptake mechanisms of L. pneumophila by the two amoebae are independent of microfilaments and microtubules. Our data are consistent with previous observations that the uptake of L. pneumophila by H. vermiformis is independent of the microfilaments (36).
FIG. 4.
Effects of disruption of A. polyphaga microtubules (by colchicine) and microfilament (by cytochalasin D) and inhibition of protein synthesis (by cycloheximide) on invasion by L. pneumophila. The percent invasion was determined by dividing the number of intracellular bacteria recovered following the invasion period after killing of extracellular bacteria by the number of intracellular bacteria in untreated samples. Values are the means of triplicate samples, and error bars represent standard deviations.
Effect of inhibition of protozoan protein synthesis on uptake of L. pneumophila.
The uptake of L. pneumophila by H. vermiformis and macrophages is known to differ in the requirement for host protein synthesis. Our previous work showed that uptake of L. pneumophila by macrophages is independent of protein synthesis of the host cell (5). In contrast, synthesis of new host proteins is required for uptake of the bacterium by H. vermiformis (5). We extended these observations to the uptake of L. pneumophila by A. polyphaga. Our data showed that inhibition of A. polyphaga protein synthesis had no detectable effect on uptake of L. pneumophila (Fig. 4). In contrast, and consistent with our previous observations, inhibition of H. vermiformis protein synthesis completely inhibited the uptake of L. pneumophila (data not shown) (5). These data further substantiated our observations on the differences in the uptake mechanisms of L. pneumophila by A. polyphaga and H. vermiformis.
Attachment of mutants of L. pneumophila to A. polyphaga and H. vermiformis.
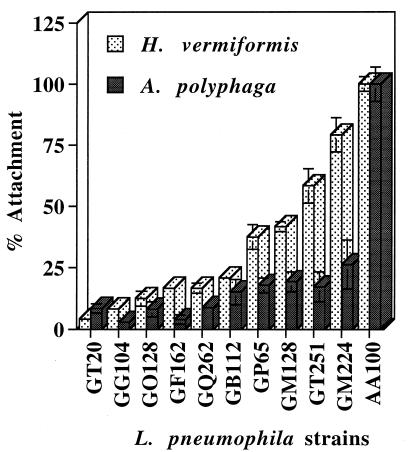
We have recently isolated a group of mutants, generated through transposon mutagenesis, that are defective in attachment to A. polyphaga and to U937 human macrophage-like cells (29, 30). We examined the ability of these mutants to attach to H. vermiformis and compared it to their attachment to A. polyphaga. In these assays, attachments were assessed in the presence of an inhibitor of uptake to prevent internalization of the bacteria (see Materials and Methods). Our data showed that the 10 mutants were severely defective in attachment to A. polyphaga but 4 of them (GP65, GM128, GT251, and GM224) attached at higher levels to H. vermiformis (Fig. 5). Interestingly, six of the mutants were severely defective in attachment to both A. polyphaga and H. vermiformis, suggesting that some of the bacterial ligands involved in attachment to both protozoa may be similar. These data indicated that L. pneumophila possesses multiple ligands involved in distinct attachment to H. vermiformis or A. polyphaga, and some of these may be involved in attachment to both protozoa.
FIG. 5.
Attachment levels of mutants of L. pneumophila to A. polyphaga and H. vermiformis. The number of attached bacteria is represented by the percentage of attached bacteria compared to that of the wild-type, strain AA100, in the presence of an inhibitor of uptake in both amoebae. Values are the means of triplicate measurements, and the error bars represent standard deviations. Some of the error bars cannot be seen due to their small values.
DISCUSSION
The replication of L. pneumophila within free-living amoebae is believed to be an integral component in the transmission of Legionnaires’ disease (24). It has been hypothesized that the intracellular infections of mammalian and protozoan cells by L. pneumophila are mediated by similar mechanisms (1, 19, 24). We have recently shown that 89 distinct insertion mutants of L. pneumophila exhibited similar defective phenotypes (severe to modest) in their cytotoxicity and intracellular replication within U937 macrophages and A. polyphaga (29), thus providing genetic evidence that many of the mechanisms utilized by L. pneumophila to parasitize the two evolutionarily distant hosts are similar. In contrast, processes involved in the uptake of L. pneumophila by mammalian macrophages have been shown to differ from those by H. vermiformis (5, 36, 52), although the mechanisms of uptake by amoebae are not known.
Previous studies have shown that uptake of L. pneumophila by H. vermiformis is not inhibited by cytochalasin D, an inhibitor of microfilament-dependent endocytosis (36). In this report, we provide evidence that the attachment and uptake of L. pneumophila by the two protozoa, A. polyphaga and H. vermiformis, occur by different mechanisms, adding further complexity to the host-parasite interaction process. First, uptake of L. pneumophila by H. vermiformis is completely blocked by the monovalent sugars Gal and GalNAc, but these sugars partially blocked A. polyphaga. Second, attachment of L. pneumophila to H. vermiformis is associated with a time-dependent and reversible tyrosine dephosphorylation of multiple host proteins. In contrast, only a slight dephosphorylation of a 170-kDa protein of A. polyphaga is detected upon infection. Third, synthesis of H. vermiformis proteins but not of A. polyphaga proteins is required for uptake of L. pneumophila. Fourth, we have identified L. pneumophila mutants that are severely defective in attachment to A. polyphaga but which exhibit minor reductions in attachment to H. vermiformis and, thus, provide a genetic basis for the difference in mechanisms of attachment to both protozoa.
The dramatic inhibition of uptake of L. pneumophila by H. vermiformis compared to that by A. polyphaga in the presence of Gal or GalNAc indicates that the receptors utilized by L. pneumophila to attach to the two amoebae may be different or have different affinities. We have recently identified a 170-kDa Gal/GalNAc lectin as one of the tyrosine-phosphorylated proteins in resting H. vermiformis that undergoes dephosphorylation upon attachment and invasion by L. pneumophila (52). Whether the slightly dephosphorylated 170-kDa protein in A. polyphaga, as a result of bacterial infection, is related to the H. vermiformis lectin is still to be determined.
Therefore, A. polyphaga possesses another receptor or a similar receptor but with lower affinity to which L. pneumophila attaches during the initial steps of interaction. The severe defect in attachment of the L. pneumophila mutant strain GM224 to A. polyphaga, but its normal attachment to H. vermiformis, provides strong genetic evidence for the presence of different receptors on both protozoa for attachment of L. pneumophila. Since these mutants are also defective in intracellular replication (29, 30), it is unlikely that expression of the recently described pili of L. pneumophila is defective in any of these mutants (46). Future characterization of the defective ligand in the mutant GM224 will allow us to characterize the host cell receptor involved in attachment.
Attachment of L. pneumophila to H. vermiformis is associated with tyrosine dephosphorylation of multiple host proteins. In contrast, there is only a slight reduction in the level of tyrosine phosphorylation of a 170-kDa protein (which may be related to the Gal/GalNAc lectin of H. vermiformis) in A. polyphaga upon attachment to L. pneumophila. This host cell response may correlate with the relative inhibition of bacterial uptake by Gal and GalNAc sugars, which was less pronounced in A. polyphaga. These data showed a crucial difference in the initial steps involved in attachment and subsequent cross talk between L. pneumophila and two of its protozoan hosts, A. polyphaga and H. vermiformis. Since there are at least 13 species described to be environmental hosts for L. pneumophila (24), it would be interesting to examine the remarkable adaptation and the differential complexity of the interaction of this intracellular parasite with its numerous environmental protozoan hosts.
The fate of some intracellular parasites may be determined by the specific ligand-receptor interaction and subsequent signal transduction involved in uptake by the host cell (9, 32, 35). Our observations of tyrosine dephosphorylation of H. vermiformis proteins, including the Gal/GalNAc receptor, upon contact and uptake of L. pneumophila are rather intriguing since conventional phagocytosis is associated with tyrosine phosphorylation of host cell proteins (9, 52). Interestingly, attachment of many bacterial pathogens to host cell receptors and their exploitation of host cell processes is associated with tyrosine phosphorylation of host cell proteins (27). We speculate that attachment of L. pneumophila to H. vermiformis is associated with disruption of the classical phagocytic process. Additionally, cross talk between L. pneumophila and H. vermiformis activates host cell signaling pathways that will trigger new protein synthesis in H. vermiformis, which is required for subsequent uptake of L. pneumophila (5). In contrast, adherence of L. pneumophila to A. polyphaga induces only minor changes in the host tyrosine phosphorylation, indicating that the initial cross talk does not induce the same set of biochemical signaling events seen in H. vermiformis.
Many facultative intracellular pathogens exploit host signal transduction pathways to their own advantage (27). This exploitation includes induced cytoskeletal rearrangement and subsequent internalization of the bacterium. L. pneumophila invasion of both H. vermiformis and A. polyphaga, however, remains unaffected by inhibitors of the cytoskeleton that are commonly exploited by other bacterial pathogens (27). Both of these inhibitors have been found to perform their functions in protozoa in ways that are similar to those in mammalian cells (34, 36, 50, 51). Therefore, our observations confirm previous reports that the mechanisms of invasion of amoebae by L. pneumophila are different from those utilized by other intracellular pathogens to invade their mammalian host cells (5, 27, 36).
L. pneumophila is transmitted only from environmental sources, where protozoa play a major factor in transmission of Legionnaires’ disease (24). Intracellular replication within protozoa increases the number of L. pneumophila in the environment (24), resuscitates viability and infectivity of nonculturable bacteria (45), increases bacterial resistance to harsh environmental conditions (6, 11, 12), and enhances bacterial infectivity to human cells and A/J mice (17, 21). Thus, understanding the mechanisms of uptake by protozoa will facilitate the design of measures to prevent uptake of L. pneumophila by protozoa, providing effective preventive approaches for controlling the transmission of Legionnaires’ disease.
ACKNOWLEDGMENTS
O.S.H. and C.V. made equal contributions to this work.
Y.A.K. was supported by Public Health Service grant no. 1R29AI38410. O.S.H. was supported by predoctoral national research service award no. TA09509.
REFERENCES
- 1.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 3.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Fields B S, Engleberg N C. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. Infect Immun. 1994;62:1860–1866. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 8.Adeleke A, Pruckler J, Benson R, Rowbotham T, Halablab M, Fields B S. Legionella-like amoebal pathogens—phylogenetic status and possible role in respiratory disease. Emerg Infect Dis. 1996;2:225–229. doi: 10.3201/eid0203.960311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen L H, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaree J M, Fields B S, Feeley J C, Gorman G W, Martin W T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker J, Brown M R W, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker J, Scaife H, Brown M R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates J H, Capmpbell G D, Baron A L. Microbial etiology of acute pneumonia in hospitalized patients. Chest. 1992;101:1005–1012. doi: 10.1378/chest.101.4.1005. [DOI] [PubMed] [Google Scholar]
- 14.Bhopal R S, Fallon R J, Buist E C, Black R J, Urquhart J D. Proximity of the home to a cooling tower and risk of non-outbreak Legionnaires’ disease. Br Med J. 1991;302:378–383. doi: 10.1136/bmj.302.6773.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba catellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiman R F, Fields B S, Sanden G N, Volmer L, Meier A, Spika J S. Association of shower use with Legionnaires’ disease: possible role of amoebae. JAMA. 1990;263:2924–2926. [PubMed] [Google Scholar]
- 17.Brieland J K, Fantone J C, Remick D G, LeGendre M, McClain M, Engleberg N C. The role of Legionella pneumophila-infected Hartmanella vermiformis as an infectious particle in a murine model of Legionnaires’ disease. Infect Immun. 1997;65:5330–5333. doi: 10.1128/iai.65.12.5330-5333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britles R J, Rowbotham T J, Raoult D, Harrison T G. Phylogenetic diversity of intra-amoebal legionellae as revealed by 16S rRNA gene sequence comparison. Microbiology. 1996;142:3525–3530. doi: 10.1099/13500872-142-12-3525. [DOI] [PubMed] [Google Scholar]
- 19.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cianciotto N P, Stamos J K, Kamp D W. Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr Microbiol. 1995;30:247–250. doi: 10.1007/BF00293641. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo J D, Tompkins L S, Falkow S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis P J, Wright A E, Rutter D A, Death J E, Jones B P. Legionella pneumophila in aerosols from shower baths. J Hyg. 1984;93:349–353. doi: 10.1017/s0022172400064901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang G D, Fine M, Orloff J. New and emerging etiologies of community-acquired pneumonia with implications for therapy, a prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 25.Fields B S, Nerad T A, Sawyer T K, King C H, Barbaree J M, Martin W T, Morrill W E, Sanden G N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 26.Fields B S, Sanden G N, Barbaree J M, Morrill W E, Wadowsky R M, White E H, Feeley J C. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr Microbiol. 1989;18:131–137. [Google Scholar]
- 27.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 28.Fry N K, Rowbotham T J, Saunders N A, Embley T M. Direct amplification and sequencing of the 16S ribosomal DNA of an intracellular Legionella species recovered by amoebal enrichment from the sputum of a patient with pneumonia. FEMS Microbiol Lett. 1991;83:165–168. doi: 10.1016/0378-1097(91)90348-e. [DOI] [PubMed] [Google Scholar]
- 29.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant hosts, mammalian and protozoan cells. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao, L.-Y., O. S. Harb, and Y. Abu Kwaik. Submitted for publication.
- 30a.Harb, O. S., J. K. Brieland, and Y. Abu Kwaik. Submitted for publication.
- 31.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial adhesion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg G, Aebi U, Pollard T D. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980;288:455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- 35.Joiner K A, Fuhrman S A, Miettinen H M, Kasper L H, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 36.King C H, Fields B S, Shotts E B, Jr, White E H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurtz J B, Bartlett C L R, Newton U A, White R A, Jones N L. Legionella pneumophila in cooling tower systems. J Hyg. 1982;88:369–381. doi: 10.1017/s0022172400070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marston B J. Epidemiology of community-acquired pneumonia. Infect Dis Clin Pract. 1995;4:S232–S239. [Google Scholar]
- 39.Mody C H, Paine III R, Shahrabadi M S, Simon R H, Pearlman E, Eisenstein B I, Toews G B. Legionella pneumophila replicates within rat alveolar epithelial cells. J Infect Dis. 1993;167:1138–1145. doi: 10.1093/infdis/167.5.1138. [DOI] [PubMed] [Google Scholar]
- 40.O’Brein S J, Bhopal R S. Legionnaires’ disease: the infective dose paradox. Lancet. 1993;342:5–6. doi: 10.1016/0140-6736(93)91877-o. [DOI] [PubMed] [Google Scholar]
- 41.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenshine I, Ruschkowski S, Foubister V, Finlay B B. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect Immun. 1994;62:4969–4974. doi: 10.1128/iai.62.11.4969-4974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 44.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinert M, Emody L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone, B. J., and Y. Abu Kwaik Submitted for publication.
- 47.Stout J E, Joly J, Para M, Plouffe J, Ciesielski C, Blaser M J, Yu V L. Comparison of molecular methods for subtyping patients and epidemiologically linked environmental isolates of Legionella pneumophila. J Infect Dis. 1988;157:486–495. doi: 10.1093/infdis/157.3.486. [DOI] [PubMed] [Google Scholar]
- 48.Susa M, Hacker J, Marre R. De novo synthesis of Legionella pneumophila antigens during intracellular growth in phagocytic cells. Infect Immun. 1996;64:1679–1684. doi: 10.1128/iai.64.5.1679-1684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Udezulu I A, Leitch G J. A membrane-associated neuraminidase in Entamoeba histolytica trophozoites. Infect Immun. 1987;55:181–186. doi: 10.1128/iai.55.1.181-186.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez-Prado J, Meza I. Fibronectin “receptor” in Entamoeba histolytica: purification and association with the cytoskeleton. Arch Med Res. 1992;23:125–128. [PubMed] [Google Scholar]
- 52.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmanella vermiformis as a potential receptor for attachment and invasion by the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodhead M A, Macfarlane J T, McCracken J S, Rose D H, Finch R G. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;i:671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]