Abstract
Vibrio anguillarum serotype O2 strains express a 40-kDa outer membrane porin protein. Immunoblot analysis revealed that antigenic determinants of the V. anguillarum O2 40-kDa porin were conserved within bacterial species of the genus Vibrio. The relative amounts of the V. anguillarum O2 40-kDa porin were enhanced by growth of V. anguillarum O2 in CM9 medium containing 5 to 10% sucrose or 0.1 to 0.5 M NaCl. In contrast, the levels of the porin were significantly reduced when cells were grown at 37°C, and a novel 60-kDa protein was also observed. However, the osmolarity or ionic concentration of the growth medium did not influence expression of the 60-kDa protein. Growth in medium containing greater than 0.6 mM EDTA reduced production of the V. anguillarum O2 40-kDa porin and enhanced levels of a novel 19-kDa protein. Thus, expression of the V. anguillarum O2 40-kDa porin was osmoregulated and possibly coregulated by temperature. The N-terminal amino acid sequence of the V. anguillarum O2 40-kDa protein and the effect of environmental factors on the cellular levels of the porin suggested that the V. anguillarum O2 40-kDa porin was functionally similar to the OmpC porin of Escherichia coli. However, pore conductance assays revealed that the V. anguillarum O2 40-kDa porin was a general diffusion porin with a pore size in the range of that of the OmpF porin of E. coli.
The outer membranes of gram-negative bacteria contain a family of pore-forming proteins, or porins, which form large water-filled channels for passive uptake of small hydrophilic molecules into the periplasm. Other porin proteins facilitate transport of specific nutrients across the outer membranes and are produced when the bacteria are cultured under specific nutrient conditions. Porin proteins share other characteristics, including assembly in vivo into trimeric complexes, close association with the peptidoglycan, and trypsin insensitivity (15, 27). Although several studies have examined the outer membrane profiles of different serotypes of Vibrio anguillarum (8, 29), only the outer membrane proteins involved in iron uptake mechanisms have been fully characterized and have had their genes cloned (1, 39). To date, the porins which have been described for V. anguillarum include a 40-kDa major outer membrane protein (MOMP) of serotype O1 strains (33) and a 35-kDa porin-like protein (Omp35La) (36). Reconstitution of the purified 40-kDa MOMP into model lipid bilayer membranes showed that the protein forms large water-filled channels with weak cationic selectivity and is functionally similar to the Escherichia coli OmpF porin (33). The Omp35La protein was identified as a porin protein by comparison of the N-terminal amino acid sequence to those of known bacterial porins and by identity with OmpF and OmpC of E. coli. It is not known whether the 40-kDa MOMP and Omp35La represent a single porin or two different porins of V. anguillarum; however, antigenic analyses with polyclonal sera suggested that these proteins were conserved in all serotypes of the bacterium (33, 36).
The expression of the OmpF and OmpC porins in E. coli is influenced by a variety of environmental factors, including temperature, osmolarity, toxins, and antibiotics (19, 26, 27). OmpF, which forms the larger channel (1.2 nm), is predominantly enhanced by growth in medium with low osmolarity and low temperature and is repressed by oxidative stress, toxins, and antibiotics. Growth in medium with high osmolarity, high temperature, and antibacterial factors favors expression of the smaller-channel (1.1-nm) OmpC porin, with a concomitant decrease in the overall permeability of the outer membrane (15, 19, 26, 27). The growth conditions which influence the expression of the V. anguillarum porin proteins are not known. V. anguillarum causes vibriosis, a bacteremic infection of marine, feral, and cultured fish species (2, 3, 32, 34), and can become established in freshwater environments (31). Although the major route of infection is not entirely determined, transmission is primarily water borne, and the gastrointestinal tract may be the major site of infection in fish (28). Therefore, V. anguillarum has to adapt both to the marine or freshwater environment and to the gastrointestinal tract and systemic environments of the fish. The bacterium is subject to the nutritional, osmotic, and ionic concentrations inherent in these diverse environments.
It was therefore of interest to examine the influence of culture (medium and environmental) conditions on the relative amounts of the MOMP of V. anguillarum serotype O2 in cell lysates. We report that the apparent amounts of the V. anguillarum O2 40-kDa MOMP were increased by growth in medium with high osmolarity and containing high salt concentrations and were decreased by growth at 37°C and in medium containing the chelator EDTA. Novel proteins of 60 and 19 kDa were observed in cell lysates of V. anguillarum O2 grown at an elevated temperature (37°C) and in EDTA-containing medium, respectively. In an attempt to further characterize the 40-kDa MOMP, the protein was purified, the N-terminal amino acid sequence was obtained, and the porin activity was defined by a model lipid bilayer system. These data suggest that the V. anguillarum O2 40-kDa porin pore is functionally similar to that of the E. coli OmpF porin. However, unlike the OmpF porin, the V. anguillarum O2 MOMP was synthesized in larger amounts at high medium osmolarity and salt concentrations, factors which favor expression of the OmpC porins.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are shown in Table 1. V. anguillarum serotype O2 strain ATCC 19264 (3) was used for preparations of the outer membrane proteins. V. anguillarum O2 was differentiated from the closely related Vibrio ordalii by serotyping (21) and by rate of growth at 25°C (2). In this study Vibrio species were routinely grown in Luria-Bertani (LB) broth or agar medium containing 10 g of NaCl per liter (85.6 mM) at 25°C, while Vibrio salmonicida strains were grown at 15°C. The minimum concentrations of NaCl required for optimum growth of V. anguillarum strains range from 60 to 100 mM (4). Aeromonas, Edwardsiella, and Yersinia strains were cultured at 25°C in LB medium containing 10 g of NaCl per liter. All other bacterial strains were grown in LB medium at 37°C. For analyses of the effects of growth conditions and medium composition on expression of the outer membrane proteins, V. anguillarum O2 was grown in CM9 minimal medium (39) containing various concentration of EDTA, sucrose, or NaCl. The CM9 broths were inoculated with 1% of an exponential-phase culture adjusted to an optical density at 520 nm (OD520) of 1.0. The initial inoculum of the cultures was calculated to be 9 × 104 to 9.5 × 104 CFU/ml. Readings (OD) were taken following a 16-, 24-, or 48-h incubation period. Determinations of CFU per milliliter were performed by plating 100-μl samples from dilutions of the cell culture on LB agar in triplicate.
TABLE 1.
Bacterial strains used in this study
| Species and strain(s) | Serotype | Reference or source |
|---|---|---|
| V. anguillarum | ||
| ATCC 43305 | O1 | 21 |
| ATCC 19264 (NCMB 6, type strain) | O2 | 3 |
| NB 122 | O2 | 2 |
| ATCC 43307 | O3 | L. Griseza |
| ATCC 43308 and 396 (1427/1) | O4 | L. Grisez |
| ATCC 43309 and 399 (1452F/1) | O5 | L. Grisez |
| ATCC 43310 and 402 (3.1.1.40/1) | O6 | L. Grisez |
| ATCC 43311 and 404 (1702/1) | O7 | L. Grisez |
| ATCC 43312 | O8 | L. Grisez |
| V. ordalii MT 601 and ATCC 33509 | O2 | 21 |
| V. salmonicida MT 701, MT NB1, MT NB2, MT NB3, and MT NB4 | W. W. Kayb | |
| V. alginolyticus 88-248 (SB1166) | K. Bernardc | |
| V. cholerae 91-196 (SB1173) | K. Bernard | |
| V. fluvialis 92-112 (SB1169) | K. Bernard | |
| V. parahemolyticus 87-455 (SB1170) | K. Bernard | |
| V. vulnificus 88-842 (SB1008) | K. Bernard | |
| V. damsela | R. M. W. Stevensond | |
| E. coli DH5α, JM109, and LE392 | ||
| Aeromonas hydrophila ATCC 19570 | R. M. W. Stevenson | |
| Aeromonas jandaei ATCC 49568 | R. M. W. Stevenson | |
| Aeromonas salmonicida RS842 | R. M. W. Stevenson | |
| Aeromonas sobria ATCC 43979 | R. M. W. Stevenson | |
| E. tarda RS974 | R. M. W. Stevenson | |
| P. mirabilis 19 | ||
| P. stuarti PR50 | ||
| P. aeruginosa ATCC 33358 | R. M. W. Stevenson | |
| P. fluorescens RS973 | R. M. W. Stevenson | |
| Y. ruckeri ATCC 29473 | R. M. W. Stevenson |
Zoological Institute, Leuven, Belgium.
Department of Microbiology and Biochemistry, University of Victoria, Victoria, Canada.
Health Protection Branch, Laboratory Center for Disease Control, Ottawa, Canada.
Department of Microbiology, University of Guelph, Guelph, Canada.
Preparation of bacterial cell lysates, SDS-PAGE, and Western immunoblot analyses.
Bacterial cells were grown in LB or CM9 broth for 16, 24, or 48 h. The cells were harvested, and the cell pellet was suspended in phosphate-buffered saline (PBS) (10 mM phosphate buffer [pH 7.4], 150 mM NaCl) to an OD520 of 1.0. A 1.0-ml sample of the suspension was centrifuged, and the cell pellet was suspended in 0.1 ml of lysis buffer (10 mM Tris-HCl, 2% sodium dodecyl sulfate [SDS], 10 mM EDTA [pH 9.0]), incubated at 37°C (1 h), neutralized with HCl, and mixed with 0.1 ml of 2× SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2% SDS, 5% β-mercaptoethanol [2-ME], 0.1% bromophenol blue). The cell lysates were heat treated for 10 min, and 20-μl samples were loaded onto gels. SDS-PAGE was performed as described by Laemmli (14) with 12% separating gels. The gels were silver stained or stained with Coomassie brilliant blue R250 to visualize lipopolysaccharide (LPS) molecules (41) and proteins, respectively. Western immunoblot analysis and detection of antigens by polyclonal and monospecific antibodies were performed as previously described (21, 22, 40).
Preparations of the 40-kDa MOMP.
The crude cell envelope fraction was prepared from 6 liters of a 16-h broth culture of V. anguillarum O2 by using French pressure lysis procedures as described by Sprott et al. (35). The crude cell envelope fraction was extracted with 1% (wt/vol) sarcosine (sodium N-lauroylsarcosinate), and the sarcosine-insoluble fraction containing outer membrane proteins (10) was subsequently extracted with 2% SDS in 10 mM Tris-HCl (pH 7.7, 37°C) to obtain a pellet containing the peptidoglycan-associated proteins (25). Further extraction of this pellet with sodium deoxycholate (SDOC) buffer (NaCl-SDOC [0.5 M NaCl, 1% SDOC, 5 mM EDTA, 50 mM Tris-HCl, pH 7.7]) and centrifugation at 100,000 × g (1 h, 25°C) yielded a soluble fraction enriched in the 40-kDa MOMP and containing a few other minor protein bands. A 2-ml sample (0.5 mg of protein/ml) of the NaCl-SDOC-soluble proteins was applied to a column of Sephacryl S-300 filtration matrix (1.6 mm by 60 cm) equilibrated with NaCl-SDOC buffer. The column was eluted with NaCl-SDOC buffer at a flow rate of 0.3 ml/min, and 2.5-ml fractions were collected. A 20-μl sample of each fraction was analyzed for presence of the 40-kDa MOMP by SDS-PAGE analysis. Fractions containing the 40-kDa protein and devoid of LPS were used for chemical and functional analyses of the protein. Protein concentrations were estimated by the bicinchoninic acid method with the Micro BCA protein assay reagent (Pierce, Rockford, Ill.) with bovine serum albumin as a standard.
Monospecific and polyclonal antibodies.
Polyclonal rabbit and rainbow trout sera and monoclonal antibodies against V. anguillarum antigens were generated as previously described (21, 22). Polyclonal serum against the crude (NaCl-SDOC-soluble) 40-kDa MOMP of V. anguillarum O2 was generated by immunizing rabbits with the protein eluted from gels or with homogenized nitrocellulose strips containing the purified antigen and emulsified in Freund’s incomplete adjuvant. These antigen preparations also contained LPS and other minor antigens migrating with the 40-kDa MOMP. All rabbits were given three booster injections at 3-week intervals and bled, and the serum was collected. All polyclonal sera were extensively absorbed with nitrocellulose discs coated with cell lysates of E. coli to remove all cross-reacting antibodies.
For preparation of affinity-purified monospecific antiserum against the MOMP, 0.6 mg of the LPS-free 40-kDa MOMP purified by gel filtration chromatography was coupled to 0.3 g of cyanogen bromide-activated Sepharose 4B beads as described by the manufacturers. Excess protein binding sites on the matrix were blocked by addition of 0.2 M glycine. The affinity matrix, now containing covalently bound protein, was mixed with polyclonal serum against the crude 40-kDa MOMP of V. anguillarum O2, incubated for 16 h at 4°C, and washed extensively with PBS to remove unbound serum proteins. The bound antibodies were eluted with 0.1 M glycine-HCl (pH 2.5) and immediately neutralized with solid Tris base to pH 8.5. These affinity-purified monospecific antibodies against the 40-kDa MOMP of V. anguillarum O2 were designated MOMP-Ab.
Porin conductance measurements.
The pore-forming activities of the 40-kDa MOMP of V. anguillarum O2 purified by gel filtration (LPS free) and by NaCl-SDOC extraction (LPS containing) were assessed in a black-lipid model membrane system with planar lipid bilayers as previously described by Woodruff et al. (43). Lipid bilayers were made from 1.5% (wt/vol) oxidized cholesterol in n-decane. One nanogram of the 40-kDa MOMP was added to the bathing solution on one side of the lipid bilayer. A variety of bathing salts (either 0.1 M KCl, 1.0 M KCl, 3.0 M KCl, or 1.0 M LiCl) and an applied voltage of 50 mV were employed. R.E.W.H. and M. Bains (Department of Microbiology, University of British Columbia, Vancouver, Canada) performed the porin conductance activity assays.
Amino-terminal sequencing and amino acid composition analysis.
The LPS-free 40-kDa MOMP purified by gel filtration chromatography was subjected to SDS-PAGE, transferred to a polyvinylidene difluoride filter, and subjected to N-terminal amino acid sequencing and total amino acid analysis. Amino acid sequencing was performed with a model 473 pulsed liquid protein sequenator (Applied Biosystems Inc., Culver City Calif.) with an attached on-line analyzer (Applied Biosystems model 120A). N-terminal amino acid sequencing and amino acid composition analysis were performed by standard techniques (17) by S. Kielland (Department of Biochemistry and Microbiology, University of Victoria, Victoria, Canada).
Protease digestion of V. anguillarum O2 whole cells and purified proteins.
Bacterial cells were harvested from a 16-h (37°C) broth culture, washed once, and suspended in PBS. The cell suspension and the purified 40-kDa MOMP were adjusted to protein concentrations of 2 and 0.5 mg/ml, respectively, in PBS. Increasing concentrations of trypsin were added to 400-μl samples of the cell suspension and MOMP to obtain a final protease-to-total protein ratio of 1:800 to 1:16 and incubated for 16 h at 37°C. Trypsin digestion was terminated by addition of 15 μg of trypsin inhibitor per ml to the samples. The digests were mixed with an equal volume of 2× SDS-PAGE buffer and heated at 100°C for 10 min, and 20 μg of protein per lane was loaded on gels.
RESULTS
Effects of growth conditions on expression of the 40-kDa MOMP.
V. anguillarum O2 bacterial cells were grown in CM9 minimal salts medium with modifications in the concentrations of EDTA (a chelator for magnesium and calcium ions), NaCl (ionicity), and sucrose (medium osmolarity). The apparent amounts of the 40-kDa MOMP in V. anguillarum O2 cell lysates were examined by SDS-PAGE and Western immunoblot analysis with polyclonal serum and the monospecific MOMP-Ab.
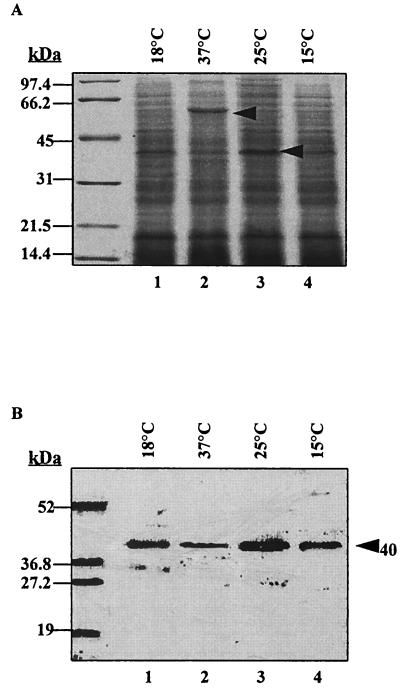
Based on the intensity of staining of the 40-kDa MOMP band in Coomassie blue-stained gels and on Western blots, growth of V. anguillarum at 25°C appeared to favor expression of the MOMP (Fig. 1A, lane 3) in comparison to growth of the bacterium at 18, 37, or 15°C (Fig. 1A, lanes 1, 2, and 4, respectively). Furthermore, when cells were grown at 37°C, the level of expression of the 40-kDa MOMP was greatly reduced, while expression of a new protein with an apparent molecular mass of 60 kDa was observed (Fig. 1A, lane 2). This novel 60-kDa protein did not react on Western immunoblots with the monospecific MOMP-Ab (Fig. 1B, lane 2) or with polyclonal serum against V. anguillarum O2 cells (data not shown). Growth of V. anguillarum O2 cultures was significantly decreased at temperatures of above 25°C (Table 2). V. anguillarum O2 cell cultures grown at 37°C showed only 1.5 × 105 CFU/ml, compared to 3.8 × 106 and 3.2 × 107 CFU/ml for cultures grown at 18 and 25°C, respectively, after 16 h of incubation with or without agitation.
FIG. 1.
Effects of growth temperature on expression of the 40-kDa MOMP. V. anguillarum O2 cultures were grown in CM9 for 16 h at the indicated temperatures, and cell suspensions were adjusted to an OD520 of 1 (1 × 109 to 3 × 109 CFU/ml). The cell pellet from 1 ml of the suspension was lysed in 60 μl of sample buffer (with 2-ME and SDS) and heated for 10 min at 100°C, and 10-μl samples were loaded in each lane. (A) Coomassie blue-stained gel; (B) blot probed with monospecific MOMP-Ab. The arrowheads indicate the positions of a novel 60-kDa protein and the MOMP. Molecular mass standards are indicated on the left.
TABLE 2.
Effects of environmental conditions on the growth and viability of V. anguillarum O2a
| Condition | OD520 | CFU/ml |
|---|---|---|
| Temp (°C) | ||
| 15 | 0.25 | 3.69 × 106 |
| 18 | 0.29 | 3.86 × 106 |
| 25 | 0.52 | 3.2 × 107 |
| 37 | 0.15 | 1.5 × 105 |
| EDTA (mM) | ||
| 0 | 0.72 | 9.5 × 108 |
| 0.1 | 0.80 | 1.9 × 109 |
| 0.3 | 0.70 | 2.49 × 108 |
| 0.6 | 0.69 | 6.0 × 107 |
| 1.0 | 0.67 | 5.5 × 107 |
| 5.0 | 0.06 | 2.38 × 104 |
| NaCl (mM) | ||
| 0 | 0.54 | 4.1 × 107 |
| 50 | 0.71 | 1.77 × 108 |
| 85.6 | 0.81 | 1.06 × 108 |
| 100 | 0.87 | 1.62 × 108 |
| 200 | 0.56 | 7.2 × 107 |
| 400 | 0.57 | 3.6 × 107 |
| 500 | 0.46 | 9.4 × 107 |
| 600 | 0.16 | 1.25 × 105 |
| 800 | 0.015 | NDb |
| Sucrose (%) | ||
| 0.4 | 0.664 | 7.5 × 107 |
| 1.0 | 0.753 | 1.17 × 108 |
| 2.0 | 0.849 | 4.1 × 108 |
| 5.0 | 0.990 | 4.0 × 108 |
| 7.5 | 1.072 | 4.6 × 108 |
| 10.0 | 1.200 | 3.4 × 108 |
All cultures were initially inoculated with 9 × 104 to 9.5 × 104 CFU/ml. OD520 and CFU per milliliter were determined following growth for 16 h (for temperature), 24 h (for sucrose and NaCl), or 48 h (for EDTA).
ND, not determined.
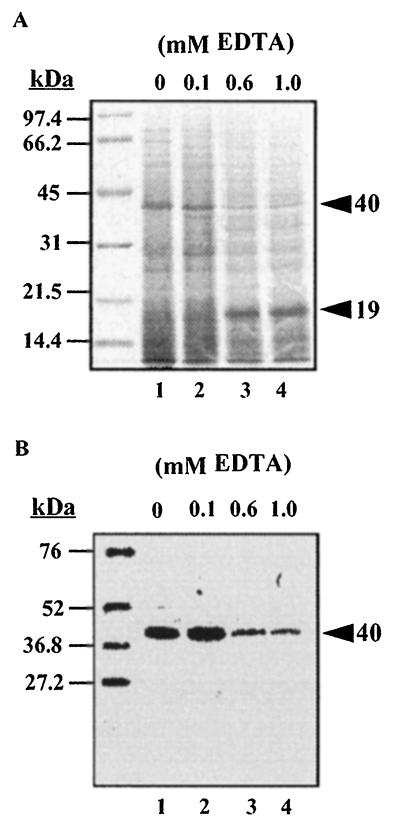
The effects of limitation of magnesium and calcium divalent ions on the expression of the 40-kDa MOMP were examined by growth of V. anguillarum O2 in medium containing increasing amounts (0 to 5.0 mM) of the chelator EDTA. At above 0.6 mM EDTA, a decreased expression of the MOMP was observed concurrent with the appearance of a novel molecule with an approximate molecular mass of 19 kDa (Fig. 2, lanes 3 and 4). Failure of the novel 19-kDa protein to react with the polyclonal V. anguillarum O2 serum (not shown) or the monospecific MOMP-Ab (Fig. 2B, lanes 3 and 4) suggests that the protein was not a proteolytic peptide of an extant cellular protein and was induced by growth of the bacterium in medium containing EDTA. Growth of the bacterium was adversely affected by addition of as little as 0.6 mM chelator to the medium, and at 5 mM, EDTA was bacteriostatic for V. anguillarum O2 cells (Table 2).
FIG. 2.
Protein profiles of V. anguillarum O2 cell lysates after growth in EDTA. Cells were grown at 25°C in CM9 containing the indicated concentrations of EDTA. Cell lysates were prepared for SDS-PAGE analysis as described in the legend to Fig. 1. (A) Coomassie blue-stained gel; (B) blot probed with monospecific MOMP-Ab. The arrowheads indicate the positions of the novel 19-kDa protein and the MOMP. Molecular mass standards are indicated on the left.
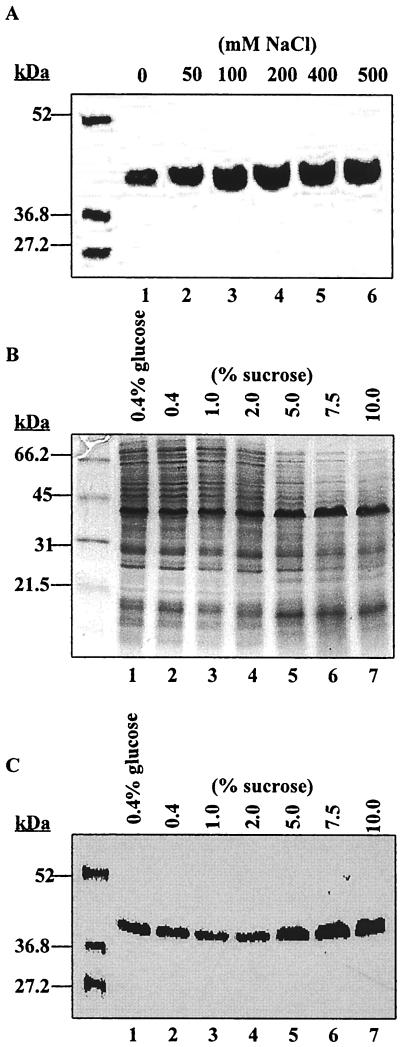
Increasing the ionicity and osmolarity of the growth medium by the addition of 0.1 to 0.5 M NaCl positively affected expression of the 40-kDa MOMP (Fig. 3A, lanes 3 to 6). Optimum growth of V. anguillarum occurred at NaCl concentrations of between 0.05 and 0.1 M, and growth was inhibited by 0.8 M NaCl (Table 2). Interestingly, V. anguillarum strains also showed growth in CM9 without added NaCl. However, CM9 contains magnesium and calcium ions, which may reduce minimum requirements of the bacterium for sodium ions. Thus, NaCl enhanced growth of V. anguillarum O2 but was not essential for viability of the bacterium. Similarly, expression of the 40-kDa MOMP was enhanced when only the osmolarity of the medium was adjusted through addition of increasing amounts (0 to 10%) of sucrose to CM9 (which contains 85.6 mM NaCl). A higher intensity of staining of the 40-kDa band in both Coomassie blue-stained gels and Western immunoblots at sucrose concentrations of 5.0 to 10.0% (Fig. 3B and 3C, lanes 5 to 7, respectively) was observed in comparison to that seen at lower levels of sucrose (lanes 2 to 4). Higher medium osmolarity slightly enhanced growth of V. anguillarum O2 (Table 2).
FIG. 3.
Expression of the 40-kDa MOMP is enhanced by increased medium osmolarity and salt concentration. V. anguillarum O2 cell cultures were grown at 25°C in CM9 medium containing increasing concentrations of NaCl (A) and sucrose (B and C). Cell lysates for SDS-PAGE were prepared as described in the legend to Fig. 1. (A and C) Blots probed with monospecific MOMP-Ab. (B) SDS-PAGE of lysates of cells grown in increasing sucrose concentrations. Molecular mass standards are indicated on the left.
These data revealed that the apparent amounts of the 40-kDa MOMP in V. anguillarum O2 cells were regulated by osmolarity and by growth temperature. Thus, expression of the MOMP was enhanced by increased concentrations of NaCl (ionicity and osmolarity) or by sucrose (osmolarity) in the growth medium. Whether limitation of divalent cations (Ca2+ and Mg2+) in EDTA-containing medium had a direct effect on the levels of the 40-kDa MOMP or whether the decreased amounts of the protein were a consequence of EDTA-induced stress on cell growth and integrity remains unclear.
Purification of the V. anguillarum O2 MOMP.
The majority of the 40-kDa MOMP was retained in the sarcosine-insoluble outer membrane fraction (Fig. 4, lane 1). The MOMP was recovered in the SDOC-insoluble pellet (Fig. 4, lane 3), suggesting that the protein is closely associated with the peptidoglycan. A second extraction with this detergent did not lead to the further removal of components from the SDOC-insoluble pellet (Fig. 4, lanes 4 and 5). SDOC buffer containing a high concentration of NaCl (0.5 M) was employed to solubilize the peptidoglycan-associated proteins (25, 26). The majority of the 40-kDa MOMP and a few minor contaminating proteins were recovered in the NaCl-SDOC-soluble fraction (Fig. 4, lane 7).
FIG. 4.
SDS-PAGE of the 40-kDa MOMP of V. anguillarum O2 at different stages of purification. Lanes: 1, sarcosine-insoluble outer membrane protein pellet; 2, SDOC-soluble extract of the outer membrane protein pellet; 3, SDOC-insoluble outer membrane protein; 4 and 5, supernatant and insoluble pellet, respectively, obtained from a second extraction of the insoluble outer membrane protein with SODC buffer; 6, NaCl-SDOC-insoluble outer membrane protein; 7, NaCl-SDOC-soluble 40-kDa MOMP. Protein samples were treated at 100°C for 10 min in sample buffer, and 20 μg of protein was loaded in lanes 1, 3, 5, 6, and 7. Lanes 2 and 4 contained the maximum volume of sample that could be loaded in each lane. Molecular mass standards are indicated on the left. The MOMP is indicated on the right.
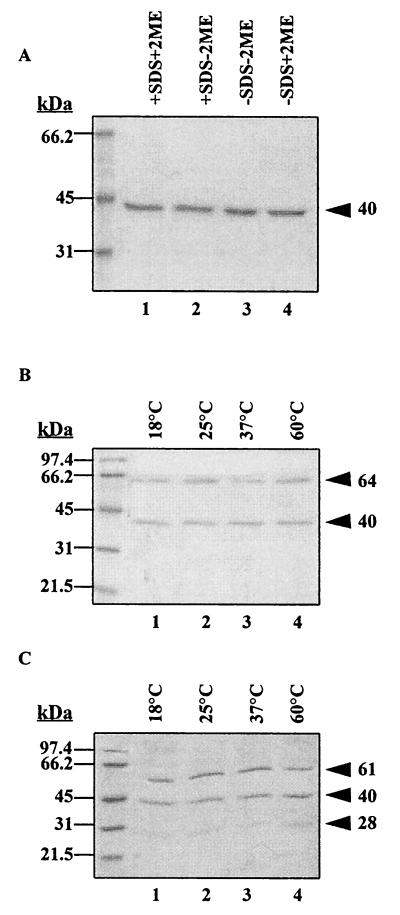
The trimeric (pore-forming) form of the V. anguillarum porin was purified by gel filtration on a Sephacryl S-300 matrix by using the NaCl-SDOC buffer for elution of the protein. Two protein bands at 64 and 40 kDa were observed in fractions 47 to 53 (data not shown). The appearance of the 64-kDa protein was dependent on the temperature of solubilization of the purified V. anguillarum MOMP prior to SDS-PAGE (see Fig. 5). Western immunoblot analysis with polyclonal rabbit serum against the formalin-fixed V. anguillarum O2 cells showed a strong reaction with the 40-kDa antigen band in fractions 47 to 51, and there was weak binding to the 64-kDa antigen band. The polyclonal serum also interacted with LPS molecules, which copurified with the MOMP in fractions 52 and 53 (data not shown). The MOMP eluted in fractions 47 to 51 was apparently devoid of LPS molecules. LPS antigens were not detected in fractions 47 to 51 by silver staining of the gels. Similarly, monoclonal antibody 7B4 (which is specific for V. anguillarum serotype O2 O antigens [21]) also failed to react with fractions 47 to 51 but reacted with O-antigen bands in fractions 52 to 57 (data not shown). Thus, gel filtration chromatography allowed purification of an LPS-free population of the 40-kDa MOMP. Fractions 52 and 53 contained both LPS O antigens and the MOMP, and fractions 55 to 59 contained only LPS antigens.
FIG. 5.
Transition from monomer to trimer of the V. anguillarum O2 MOMP is dependent on temperature and SDS. Purified MOMP (4 μg of protein/lane) was mixed with sample buffer containing the indicated combinations of SDS and 2-ME. SDS-PAGE of MOMP samples treated at 100°C for 10 min (A) and of MOMP samples treated at the indicated temperatures for 10 min in sample buffer containing SDS and 2-ME (B) and 2-ME without added SDS (C) is shown. The apparent molecular masses of the MOMP conformational forms are shown on the right. Molecular mass standards are indicated on the left.
The V. anguillarum MOMP is temperature and SDS modifiable.
When samples of the purified 40-kDa MOMP samples were boiled (100°C) for 10 min in sample buffer with or without SDS or the reducing agent 2-ME, a 40-kDa band was observed (Fig. 5A). In contrast, 64- and 40-kDa protein bands were apparent when samples were solubilized in SDS-containing sample buffer (with or without 2-ME) at temperatures below boiling (Fig. 5B). Similar data were obtained in the absence of 2-ME (not shown). Furthermore, when samples were incubated in sample buffer without SDS (with or without 2-ME), at 18 to 60°C for 10 min, three bands with apparent molecular masses of 28, 40, and 61 kDa were observed (Fig. 5C). The presence or absence of 2-ME in the sample buffer had no apparent effects on the mobilities in SDS-PAGE of these MOMP bands, implying a lack of inter- or intramolecular disulfide linkages. Thus, at temperatures below 100°C, the presence of SDS in the sample buffer abrogated the appearance of the low-molecular-mass (28-kDa) polypeptide (Fig. 5B), and there was a slight decrease (from 64 to 61 kDa) in the apparent mobility of the high-molecular-mass band (Fig. 5B and C).
Therefore, the 40-kDa MOMP was not fully denatured at temperatures below 100°C, and SDS was requisite for denaturation of the polypeptides at low temperatures. Porin proteins occur as trimeric complexes in the outer membranes of gram-negative bacteria, which are dissociated and denatured by boiling in buffers containing SDS (23, 26). In this study, the 40-kDa band represents the completely denatured V. anguillarum porin protein monomer. The 28-kDa band was detected only at low temperatures and low SDS concentrations (as present in the gel and gel running buffer), and it may represent the intermediate or partially denatured conformation of the porin monomer. The 61/64-kDa band may represent the trimeric porin complexes which are stable at below 100°C in sample buffer without SDS.
Susceptibility of the MOMP to proteolytic digestion.
The 40-kDa protein was resistant to trypsin digestion in the intact V. anguillarum O2 cells. In contrast, the majority of other cellular components were depleted at trypsin concentrations of greater than 2.5 μg/ml (data not shown). In contrast to that in the intact cell, the purified 40-kDa MOMP was rapidly degraded by trypsin. Incubation of an MOMP sample of 0.5 mg/ml for only 45 min with as little as 2.5 μg of trypsin/ml (a protease-to-protein ratio of 1:200) led to the complete digestion of the MOMP.
Chemical composition of the porin.
We determined the sequence of the first 20 amino acids at the N-terminal end of the 40-kDa MOMP of V. anguillarum O2 (Table 3). A BlastP search of several databases of reported protein sequences revealed that the N-terminal sequence of the 40-kDa MOMP of V. anguillarum O2 had 93.7% identity to the N-terminal sequence of Omp35La, a recently reported 35-kDa porin-like protein of V. anguillarum (36). However, the amino acid at the N terminus of the Omp35La was glutamate, and that in our 40-kDa MOMP was threonine (Table 3). The first 16 amino acid residues of the 40-kDa MOMP of V. anguillarum O2 had 56% identity to the 40-kDa MOMP porin of Haemophilus somnus (37) and 50 to 44% identity to other bacterial porins. Of interest was the absolute conservation of residues at positions 4 and 5 (Y and N), 8 (G), and 15 (G) within the N-terminal sequences of the bacterial porin proteins (Table 3).
TABLE 3.
Comparison of the N-terminal amino acid sequence of the V. anguillarum 40-kDa MOMP with those of other bacterial porin proteins
| Protein (reference) | Sequencea | % Identityb | % Similarityc |
|---|---|---|---|
| V. anguillarum O2 40-kDa MOMP (this study) | TELYNQDGTS LEMGGR | ||
| V. anguillarum Omp35La (36) | GELYNQDGTS LEMGGRAEAR | 93.7 | 93.7 |
| H. somnus 40-kDa MOMP (37) | TTVYNQNGTKVDVGGR | 56.0 | 87.0 |
| H. influenzae type b P2 (11, 20) | AVVYNNEGTNVE LGGR | 50.0 | 81 |
| E. coli OmpC (18) | AEVYNKDGNKLDLYGK | 44.0 | 69 |
| P. maltocida 32-kDa MOMP (16) | ATVYNQDGTKVDVNGS | 44 | 69 |
Conserved residues are in boldface. The terminal amino acids of the V. anguillarum O2 40-kDa protein and Omp35La are underlined.
Identity of the first 16 amino acids with the V. anguillarum 40-kDa MOMP.
Similarity of the first 16 amino acids with the V. anguillarum 40-kDa MOMP.
We compared the amino acid composition of the 40-kDa MOMP of V. anguillarum O2 with those of porins P2 of Haemophilus influenzae (11, 20), OmpC of E. coli (18), and the 32-kDa MOMP of Pasteurella multocida (16) (data not shown). Several properties were shared by all of the porins, including low proline content, high glycine content, and minimal cysteine content. Additionally, the amino acid composition of the MOMP of V. anguillarum O2 exhibited a high percentage of charged amino acids (34%) and a low percentage of hydrophobic residues (26%). Based on the N-terminal sequence analysis, the 40-kDa MOMP of V. anguillarum O2 is related to the E. coli OmpC-OmpF family of porin proteins.
Porin activity of the MOMP.
Studies by Simón et al. (33) indicated that the MOMP of V. anguillarum O1 was functionally similar to the E. coli OmpF porin, while Suzuki et al. (36) reported an OmpC-like porin protein. We examined the porin pore activities of the LPS-free (purified by gel filtration) and LPS-containing (crude NaCl-SDOC extract) MOMP preparations in a model membrane system. Similar data were obtained for the two MOMP preparations, indicating that LPS did not influence porin pore activity. Stepwise increases in conductance across the planar lipid bilayer membrane showed major peaks at 0.25, 0.65, 1.05, and 1.86 nS for single events in 1 M KCl (data not shown). The 0.25-nS event is probably due to insertion of a trimeric porin complex, while the larger events represent insertion of several porin complexes. The frequency of insertion of channels of each size (size of conductance step) was charted for a number of events and used for determination of the average single-channel conductance for the MOMP. This was done with each salt solution used (either 0.1 M KCl, 1.0 M KCl, 3.0 M. KCl, or 1.0 M LiCl) to obtain the mean single-channel conductances (data not shown). The V. anguillarum O2 MOMP formed large channels of around 1.52-nS conductance in 1 M KCl. A similar conductance, 1.43 nS, was observed in 1 M LiCl. A linear relationship between the salt concentration and conductance was also observed, indicating that the V. anguillarum O2 MOMP forms large, water-filled channels across the membrane, similar to the case for other general-diffusion porins such as E. coli OmpF and OmpC. However, analysis of the conductances observed with LiCl as the mobile salt and with KCl were consistent with the channel being essentially nonselective for cations over anions and vice versa, indicating that the V. anguillarum O2 porin is a general diffusion pore and is functionally similar to OmpF of E. coli.
Antigenic specificity of the monospecific MOMP-Ab determined by immunoblot analyses.
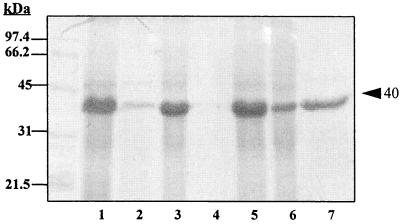
In this study we showed that monospecific MOMP-Ab and fish polyclonal sera reacted with the purified MOMP of V. anguillarum O2, with a single antigen band of between 35 and 42 kDa in cell lysates of V. anguillarum strains of serotypes O1 to O8, with a 40-kDa antigen in all V. ordalii strains, with a 35-kDa antigen in V. salmonicida strains, and with a single antigen band ranging in size from 36 to 42 kDa in strains of Vibrio alginolyticus, Vibrio parahemolyticus, Vibrio fluvialis, Vibrio vulnificus, Vibrio cholerae (non-O1), and Vibrio damsela (data not shown). In contrast to the case for the Vibrio species, there were no cross-reactions of the V. anguillarum O2 MOMP monospecific antibodies with cell lysates of E. coli, Aeromonas species, Edwardsiella tarda, Proteus mirabilis, Providencia stuarti, Pseudomonas aeruginosa, Pseudomonas fluorescens, or Yersinia ruckeri strains (data not shown). Therefore, the antigenic determinants reacting with the monospecific MOMP-Ab against the V. anguillarum O2 40-kDa major porin protein in this study were conserved in all of the Vibrio species. Fish serum antibodies against the V. anguillarum O2 40-kDa porin may be important in nonspecific immune protection against Vibrio species pathogenic to fish. While these studies were in progress in our laboratory, Simón et al. (33) and Suzuki et al. (36) reported a 40-kDa MOMP porin and a 35-kDa porin-like protein, respectively, which were also conserved in all serotypes of V. anguillarum.
DISCUSSION
The results presented in this study establish the porin properties of the 40-kDa MOMP of V. anguillarum serotype O2. Similar to other porin proteins, the V. anguillarum O2 40-kDa porin displayed insensitivity to trypsin when localized within the cell envelope, different mobilities when analyzed by SDS-PAGE at low versus high temperatures, a high density of charged amino acids, and a low percent composition of hydrophobic amino acids (26). N-terminal amino acid sequence analysis of the V. anguillarum O2 40-kDa porin showed similarity with general-diffusion-pore porins found among the γ-subgroup of the purple bacteria, which includes, among others, the Vibrio, Haemophilus, and Escherichia genera. The V. anguillarum O2 40-kDa porin had 93.7, 56, and 44% identity with the 35-kDa porin-like protein of V. anguillarum serotype O1 (36), the 40-kDa MOMP of H. somnus (37), and the E. coli OmpC porin (18), respectively. The absolute conservation of amino acid residues at position 4 (Y), 5 (N), 8 (G), and 15 (G) within the N-terminal amino acid sequences was reported to be characteristic of the trimeric, nonselective porins, excluding those of the genus Neisseria (13). Similarly, the pore conductance of the V. anguillarum O2 40-kDa porin at 1.52 nS (in 1 M KCl) was comparable to that of E. coli OmpF (1.8 nS) (6). Like the E. coli OmpF and V. cholerae OmpUF porins (9), the V. anguillarum O2 40-kDa porin formed a general diffusion pore, with no evident selectivity for cations over anions or vice versa. The 40-kDa MOMP porin of V. anguillarum serotype O1 (33) was also reported to be functionally similar to OmpF, and different voltage-dependent effects on the pore conductance activities of the LPS-containing and LPS-free porin preparations were observed. The porin investigated here was functionally similar to E. coli OmpF, and LPS had no apparent effect on the porin activity.
The 40-kDa porin of V. anguillarum O2 maybe osmoregulated. Similar to the case for the E. coli OmpC porin, the relative amounts of the V. anguillarum O2 40-kDa porin were enhanced by increased osmolarity and ionic strength (NaCl concentration) of the medium. Furthermore, the V. anguillarum O2 40-kDa porin may be coregulated by temperature, as expression of the protein appeared to be favored at 25°C and was suppressed at an elevated temperature (37°C). At 37°C, expression of a novel 60-kDa protein was also observed. Piccininno et al. (30) described a 66-kDa protein in V. anguillarum cells cultured at low osmolarity and high temperature. In our study, the 60-kDa protein was not observed when V. anguillarum O2 cells were grown in medium containing 0 mM NaCl or containing sucrose (as an osmolyte), suggesting that the 60-kDa protein was expressed in response to growth at adverse (stress-inducing) temperatures. Although V. anguillarum strains are isolated primarily from marine environments, the bacterium can become established in freshwater systems (31). V. anguillarum species infecting or associated with migratory fishes are exposed to both marine and freshwater environments. Presumably, the 40-kDa porin is one of the factors which play a role in adaptation of the bacterium to changes in osmolarity and salinity. An examination of the regulatory mechanisms of the porin(s) would provide insights into adaptational features representative of each environment. Since the ambient temperatures of marine environments are below 37°C and temperatures do not fluctuate dramatically, the possible role of thermal control of porin regulation in V. anguillarum O2 is intriguing.
The production of OmpC and OmpF in E. coli is regulated by temperature and osmolarity of the growth medium, such that OmpC is positively regulated and OmpF is negatively regulated (12, 19). The 40-kDa porin of V. anguillarum O2 investigated here revealed features similar to those of both OmpF and OmpC. The V. anguillarum O2 40-kDa porin was similar to the OmpF porins in pore size and pore function. Like for the OmpF porin, elevated temperatures suppressed the 40-kDa porin. However, our studies also showed that the V. anguillarum O2 40-kDa porin, like E. coli OmpC, was positively regulated by osmolarity of the growth medium and salt concentration. The N-terminal sequence of the V. anguillarum O2 40-kDa porin also showed some identity (44%) to that of the E. coli OmpC porin. Further studies on cloning and nucleotide sequence analysis of the gene(s) encoding the V. anguillarum porin protein(s) are necessary to determine the mechanisms of environmental regulation, protein structure, and similarities between OmpF and OmpC porins and the V. anguillarum O2 40-kDa porin and between the V. anguillarum O2 40-kDa porin and the porins identified by Simón et al. (33) and Suzuki et al. (36).
The primary target for EDTA in cell envelopes of gram-negative bacteria is the outer membrane. EDTA chelates and removes Mg2+ and Ca2+ ions which form cationic bridges between phosphate residues of adjacent LPS molecules (6, 7) and removes Mg2+ and Ca2+ ions from porin proteins (9, 42), causing disruption of the structural and functional integrity of the outer membrane (24, 27). However, the EDTA sensitivities of different bacteria are dependent on the structure of the cell membrane (density of the cationic bridges) and composition of the growth medium (7, 27, 38). In this study, EDTA at low concentrations inhibited growth of V. anguillarum O2 cells and at higher concentrations was toxic for the cultures. V. anguillarum O2 cells surviving growth in 0.6 to 5 mM EDTA showed an apparent reduction in cellular levels of most proteins, including the V. anguillarum O2 40-kDa porin. Interestingly, under these growth conditions, there was increased production of a novel 19-kDa protein band. Temple et al. (38) reported enhanced proteolysis of cell envelope proteins when Pseudomonas stutzeri cells were grown in EDTA-containing medium. Resistance to EDTA was correlated with enhanced production of a 20-kDa protein in P. stutzeri (38) and of a cationic 21-kDa protein, OprH, in P. aeruginosa (5, 24). OprH is proposed to stabilize the cell envelope by functionally replacing the cations in EDTA-treated or Mg2+-depleted cells (5, 24). It will be interesting to determine whether the novel 19-kDa protein is functionally similar to OprH. In this study we did not identify whether the levels of the 19-kDa protein and the 40-kDa porin in V. anguillarum O2 cells were modulated directly by EDTA or indirectly by depletion of essential divalent cations from the growth medium.
We have identified environmental and growth medium components which affect the cellular levels of the V. anguillarum serotype O2 40-kDa porin. The V. anguillarum O2 40-kDa porin showed similarity to both the OmpF and OmpC families of porin proteins. Further studies at the molecular level will allow a better understanding of the mechanisms for regulation and expression of the V. anguillarum porin(s) and the role of the protein in pathogenicity of the bacterium and in survival in the marine environment.
ACKNOWLEDGMENTS
This study was funded by a research grant from the Canadian Bacterial Diseases Network to L.M.M.
We thank M. Bain for analysis of the porin conductance activity assays.
REFERENCES
- 1.Actis L A, Tolmasky M E, Crosa L M, Crosa J H. Characterization and regulation of the expression of FatB, an iron transport protein encoded by the pJM1 virulence plasmid. Mol Microbiol. 1995;17:197–204. doi: 10.1111/j.1365-2958.1995.mmi_17010197.x. [DOI] [PubMed] [Google Scholar]
- 2.Airdrie D W, Patterson W D, Stevenson R M W, Flett D E. A different vibriosis problem in New Brunswick salmon rearing. Bull Aquacult Assoc Can. 1989;89:119–121. [Google Scholar]
- 3.Bagge J, Bagge O. Vibrio anguillarum som arsag til ulcussygdom hos torsk (Gardus callarias, Linne) Nord Veterinaemed Bb. 1956;8:481–482. [Google Scholar]
- 4.Baumann P, Schubert R H W. Family II. Vibrionaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The William & Wilkins Co.; 1984. pp. 516–550. [Google Scholar]
- 5.Bell A, Bain M, Hancock R E W. Pseudomonas aeruginosa outer membrane protein OprH: expression from cloned genes and function in EDTA and gentamicin resistance. J Bacteriol. 1991;173:6657–6664. doi: 10.1128/jb.173.21.6657-6664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz R K, Janko, Boos W, Läuger P. Formation of large ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978;511:305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- 7.Brown M R W. The role of the cell envelop in resistance. In: Brown M R W, editor. Resistance of Pseudomonas aeruginosa. London, United Kingdom: John Wiley and Sons; 1975. pp. 71–107. [Google Scholar]
- 8.Buckley J T, Howard S P, Trust T J. Influence of virulence plasmid and geographic origin on outer membrane proteins of Vibrio anguillarum. FEMS Microbiol Lett. 1981;11:41–46. [Google Scholar]
- 9.Chakrabarti S R, Chaudhuri K, Sen K, Das J. Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol. 1996;178:524–530. doi: 10.1128/jb.178.2.524-530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen E J, Hasemann C, Clausell A, Capra J D, Orth K, Moomaw C R, Slaughter C A, Latimer J L, Miller E E. Primary structure of the porin protein of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989;57:1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igo M M, Slauch J M, Silhavy T J. Signal transduction in bacteria: kinases that control gene expression. New Biol. 1990;2:5–9. [PubMed] [Google Scholar]
- 13.Jeanteur D, Lakey J H, Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991;5:2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lugtenberg B, van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other Gram-negative bacteria. Biochim Biophys Acta. 1983;737:51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 16.Marandi M V, Dubreuil J D, Mittal K R. The 32 kDa major outer-membrane protein of Pasteurella multocida capsular serotype D. Microbiol. 1996;142:199–206. doi: 10.1099/13500872-142-1-199. [DOI] [PubMed] [Google Scholar]
- 17.Matsudaira P J. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 18.Mizuno T, Chou M Y, Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane: DNA sequence of the osmoregulated OmpC gene. J Biol Chem. 1983;258:6932–6940. [PubMed] [Google Scholar]
- 19.Mizuno T, Mizushima S. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of porin genes. Mol Microbiol. 1990;4:1077–1082. doi: 10.1111/j.1365-2958.1990.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 20.Munson R S, Tolan R W. Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989;57:88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutharia L M, Amor P A. Monoclonal antibodies against Vibrio anguillarum O2 and Vibrio ordalii identify antigenic differences in lipopolysaccharide O-antigens. FEMS Microbiol Lett. 1994;123:289–298. doi: 10.1111/j.1574-6968.1994.tb07238.x. [DOI] [PubMed] [Google Scholar]
- 22.Mutharia L M, Raymond B T, DeKievit T R, Stevenson R M W. Antibody specificities of polyclonal rabbit and rainbow trout antisera against V. ordalii and serotype O:2 strains of V. anguillarum. Can J Microbiol. 1993;39:492–499. doi: 10.1139/m93-070. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated outer membrane proteins of Escherichia coli K-12. J Biochem. 1976;80:1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- 24.Nicas T I, Hancock R E W. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminotetraacetate, polymixin B, and gentamicin. J Bacteriol. 1980;143:872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- 26.Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 27.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson J C, Jöborn A, Westerdahl A, Bloomberg L, Kjelleberg S, Conway P L. Is the turbot, Sophthalmus maximus (L), intestine a portal of entry for the fish pathogen Vibrio anguillarum. J Fish Dis. 1996;19:225–234. [Google Scholar]
- 29.Pazos F, Santos Y, Majarinos B, Bandin I, Nunez S, Toranzo A E. Phenotypic characteristics and virulence of Vibrio anguillarum-related organisms. Appl Environ Microbiol. 1993;59:2969–2976. doi: 10.1128/aem.59.9.2969-2976.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccininno G, Ciuchini F, Adone R, Ceschia G, Giorgetti G. Morphological, physio-chemical and biological variations in Vibrio anguillarum cultured at low osmolarity. Microbiologica. 1996;19:321–326. [PubMed] [Google Scholar]
- 31.Satoi S. Occurrence of vibriosis for ayu in Lake Biwa. Nippon Suisal Gakkaishi. 1995;61:942–943. [Google Scholar]
- 32.Schiewe M H, Trust T J, Crosa J H. Vibrio ordalii sp. nov.: a causative agent of vibriosis in fish. Curr Microbiol. 1981;6:343–348. [Google Scholar]
- 33.Simón M, Mathes A, Blanch A, Engelhardt H. Characterization of a porin from the outer membrane of Vibrio anguillarum. J Bacteriol. 1996;178:4182–4188. doi: 10.1128/jb.178.14.4182-4188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen U B S, Larsen J L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986;51:593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprott D G, Koval S F, Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 72–103. [Google Scholar]
- 36.Suzuki S, Kuroe K, Yasue K, Kusuda R. Antigenicity and N-terminal amino acid sequence of a 35 kDa porin-like protein of Listonella (Vibrio) anguillarum: comparison among different serotypes and other bacterial species. Lett Appl Microbiol. 1996;23:303–306. doi: 10.1111/j.1472-765x.1996.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 37.Tagawa Y, Ishikawa H, Yuasa N. Purification and partial characterization of the major outer membrane protein of Haemophilus somnus. Infect Immun. 1993;61:91–96. doi: 10.1128/iai.61.1.91-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Temple G S, Ayling P D, Wilkinson S G. Sensitivity of Pseudomonas stutzeri to EDTA: effects of growth parameters and test conditions. Microbios. 1992;72:7–16. [PubMed] [Google Scholar]
- 39.Tolmasky M E, Actis L A, Crosa J H. A histidine decarboxylase gene encoded by the Vibrio anguillarum plasmid pMJ1 is essential for virulence: histamine is a precursor in the biosynthesis of anguibactin. Mol Microbiol. 1995;15:87–95. doi: 10.1111/j.1365-2958.1995.tb02223.x. [DOI] [PubMed] [Google Scholar]
- 40.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels onto nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai C-M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Proc Natl Acad Sci USA. 1982;89:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 42.Weiss M S, Schulz G E. Porin conformation in the absence of calcium: refined structure at 2.5 A resolution. J Mol Biol. 1993;231:817–824. doi: 10.1006/jmbi.1993.1328. [DOI] [PubMed] [Google Scholar]
- 43.Woodruff W A, Parr T R, Hancock R E W, Hanne L F, Nicas T I, Iglewski B H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986;167:473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]