Abstract
Resting Mycobacterium aurum MO1 cells were incubated with morpholine, a waste from the chemical industry. The kinetics of biodegradation was monitored by using in situ nuclear magnetic resonance (NMR). The incubation medium was directly analyzed by 1H NMR. This technique allowed the unambiguous identification of two intermediates of the metabolic pathway involved in the biodegradation process, glycolate and 2-(2-aminoethoxy)acetate. The latter compound, which was not commercially available, was synthesized, in three steps, from 2-(2-aminoethoxy)ethanol. Quantitative analysis of the kinetics of degradation of morpholine was performed by integrating the signals of the different metabolites in 1H-NMR spectra. Morpholine was degraded within 10 h. The intermediates increased during the first 10 h and finally disappeared after 20 h incubation. Assays of degradation were also carried out with glycolate and ethanolamine, hypothetical intermediates of the morpholine degradation pathway. They were degraded within 4 and 8 h, respectively. Until now, no tool for direct detection of intermediates or even morpholine has been available, consequently, only hypothetical pathways have been proposed. The approach described here gives both qualitative and quantitative information about the metabolic routes used in morpholine degradation by M. aurum MO1. It could be used to investigate many biodegradative processes.
Recent studies have shown the biodegradative abilities of Mycobacterium species: they are able to metabolize xenobiotics such as polycyclic aromatic hydrocarbons (4, 10, 13, 14, 29), chlorophenols (11), trichloroethylene (33), vinyl chloride (12), amines (6), and isonicotinate (18). Mycobacterium spp. were also found to attack the heterocyclic, secondary amine morpholine (C4H9NO), which had been considered to be persistent for many years. This chemical has great industrial importance and a wide range of applications (22); it is used in the manufacture of rubber additives and also as a very versatile solvent, as an anticorrosive agent, and in the production of various drugs and pesticides. Its high solubility in water and its high potential for N nitrosation, which gives the potent mutagen and carcinogen N-nitrosomorpholine (9, 21, 27), make this xenobiotic of special interest from an environmental point of view. Knapp et al. (15) first discovered two strains of Mycobacterium (MorD and MorG) that were able to utilize morpholine as a sole source of carbon, nitrogen, and energy. A few years later, Dmitrenko et al. (5) isolated a strain of Arthrobacter, and Cech et al. (3) found a strain of Mycobacterium aurum MO1 that had morpholine degradation properties. Knapp’s group studied other Mycobacterium strains isolated from activated sludges (1, 16, 17). In the companion paper (24), we describe another Mycobacterium strain that is able to degrade morpholine.
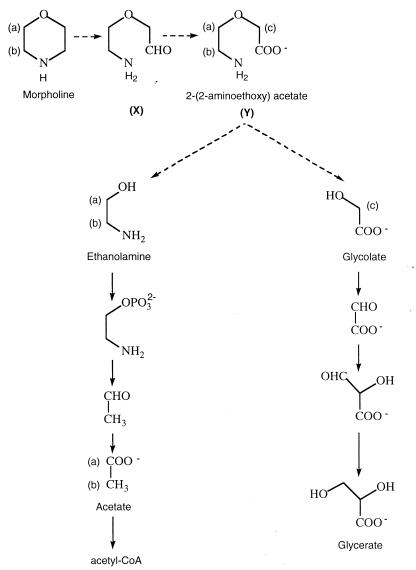
More recently, a few studies were carried out in order to understand the morpholine biodegradation process and its regulation. Swain et al. (28) proposed a hypothetical pathway for the biodegradation of morpholine by Mycobacterium chelonae (Fig. 1) that could proceed via 2-(2-aminoethoxy)acetate and glycolate and/or ethanolamine. Mazure and Truffaut (19) described the degradation of morpholine by M. aurum MO1. They proposed that M. aurum grown on morpholine could degrade intermediary compounds via the ethanolamine and glycolate routes. Depending on the morpholine concentration in the medium, one pathway could be used while the other was inhibited.
FIG. 1.
Hypothetical morpholine biodegradation pathway of M. chelonae proposed by Swain et al. (28). CoA, coenzyme A.
However, to date, no tool for the direct detection of intermediates, or even of morpholine, has been available. Only indirect strategies were developed, such as chemical oxygen demand, optical density, or NH3 measurements, growth on intermediates, or in vitro enzyme assays. Consequently, only hypothetical pathways were proposed, and limited interpretations of various experiments were made.
In this work we describe the degradation of morpholine by M. aurum MO1 by using in situ 1H nuclear magnetic resonance (1H-NMR) spectroscopy. This technique has been previously applied in medical fields for analyzing biological fluids (20, 23) and also for studying microbial physiology with extracts or culture medium (8, 30, 31). More recently, Gaines et al. (7) described the first use of 1H-NMR spectroscopy to monitor the catabolism of mixtures of aromatic compounds by Acinetobacter calcoaceticus grown in fully deuterated medium. This method is very interesting and allowed the identification and quantification of metabolites which accumulated during growth by rendering invisible the fully deuterated microbial cultures. However, it cannot be used in all circumstances, because it requires specialized growth medium and large quantities of D2O. In this work, we studied the degradative metabolism of M. aurum MO1 by directly analyzing the incubation medium in H2O. Our method, using normal water, can be more generally used and does not perturb the system being studied (the deuterated compounds can affect the enzymatic reactions). 1H-NMR spectroscopy is both qualitative and quantitative, so it allowed us to establish unambiguously some steps of morpholine degradation by this strain.
MATERIALS AND METHODS
Chemicals.
Morpholine, sodium acetate, 2-(2-aminoethoxy)ethanol, and glycolic acid were purchased from Aldrich Chemical (Sigma Aldrich Sarl, St. Quentin Fallavier, France), ethanolamine was purchased from Prolabo (Vault en Velin, France), and tetradeuterated sodium trimethylsilylpropionate (TSPd4) was purchased from EurisoTop (St. Aubin, France).
Growth conditions.
M. aurum MO1 cultures were grown in 100 ml of Trypticase soy broth (bioMérieux, Marcy-L’Etoile, France) in 500-ml Erlenmeyer flasks incubated at 30°C with agitation at 200 rpm. They were harvested after 48 h of culture.
Incubation with xenobiotics.
Cells were harvested by centrifugation at 9,000 × g for 15 min at 5°C. The supernatant was eliminated, and the pellet was washed twice with Knapp buffer (containing, per liter of distilled water, KH2PO4 [1 g], K2HPO4 [1 g], FeCl3 [4 mg], and MgSO4 · 7H2O [40 mg], pH 6.6) and finally resuspended in this buffer (5 g of wet cells in 50 ml of buffer). The cells were incubated with 10 mM morpholine, 10 mM ethanolamine, or 13 mM glycolic acid as the only source of energy in a 500-ml Erlenmeyer flask at 30°C with agitation (200 rpm). Incubation of cells under the same conditions in the absence of substrate constituted a negative control, as did incubation of the substrate in the buffer without cells. Samples (1 ml) were taken every hour for 12 h and from time to time until 72 h. They were centrifuged at 12,000 × g for 5 min. The supernatants were isolated and immediately frozen until NMR analysis.
1H-NMR spectroscopy. (i) Preparation of NMR samples.
The supernatant (540 μl) was supplemented with 60 μl of a 8 mM solution of TSPd4 in D2O and adjusted to pH 10 with 4 N NaOH. pH adjustment avoided changes in chemical shifts. D2O was used for locking and shimming. TSPd4 constituted a reference for chemical shifts (0 ppm) and quantification.
(ii) 1H-NMR spectra.
1H NMR was performed at 300.13 MHz on a 300 MSL Bruker spectrometer at 21°C with 5-mm-diameter tubes containing 500 μl of sample; water was suppressed by saturation with a classical NOE Bruker program. About 150 scans were collected (90° pulse, 3.7 μs; relaxation delay, 6 s; acquisition time, 1.024 s; 8,000 data points; saturation time, 2 s). No filter was applied before Fourier transformation, but a baseline correction was performed on spectra before integration with Bruker software. Under these conditions, the limit of quantification was in the range of 0.05 mM.
(iii) Quantification of metabolites.
The concentration of metabolites was calculated as follows: [m] = (9A0 × [TSPd4])/(b × Aref), where [m] is the concentration of metabolite m, A0 is the area of metabolite m resonance in the 1H-NMR spectrum, [TSPd4] is the concentration of the reference, Aref is the area of reference resonance in the 1H-NMR spectrum, b is the number of protons of metabolite m in the signal integrated, and 9 is the number of protons resonating at 0 ppm.
Synthesis of 2-(2-aminoethoxy)acetate. (i) Protection of the amino function.
The reaction was conducted as described previously (25). To a solution of 500 mg (4.8 mmol) of 2-(2-aminoethoxy)ethanol in 10 ml of ethyl acetate, stirred at room temperature, was added 1.25 g (1.2 equivalents, 5.8 mmol) of di-tert-butyldicarbonate. The mixture was stirred overnight at room temperature. The solvent was then evaporated under vacuum, and the residue was purified by column chromatography (the eluent was pentane-ether [50:50, vol/vol]). The yield was 92%, with 1H NMR (400.13 MHz, CDCl3) δ ppm 1.40 (s, 9H), 3.15 (s, 1H), 3.25 (t, 2H, J = 5 Hz), 3.46 to 3.56 (m, 4H), 3.75 (t, 2H, J = 4 Hz), 5.30 (s, 1H); 13C NMR (100.62 MHz, CDCl3) δ ppm 27.9 (C-7), 39.8 (C-4), 60.7 (C-1), 69.7 (C-3), 71.8 (C-2), 78.4 (C-6), 155.9 (C-5).
(ii) Oxidation of the N-protected alcohol.
The oxidation of the N-protected alcohol was performed as previously described (2). A flask was charged with 4.9 ml of carbon tetrachloride, 4.9 ml of acetonitrile, 7.3 ml of water, 0.5 g (2.45 mmol) of the N-protected alcohol, and 2.14 g (4.1 equivalents, 10 mmol) of sodium periodate (NaIO4). The mixture was stirred vigorously for 15 min. To this biphasic solution, 12.2 mg (0.06 mmol) of RuCl3 · 3H2O was added, and the mixture was stirred vigorously for 6 h at room temperature. Then, 24.4 ml of dichloromethane was added, and the phases were separated. The upper aqueous layer was extracted three times with dichloromethane. The combined organic extracts were dried over anhydrous MgSO4 and concentrated. The residue was purified by column chromatography (the eluent was ethyl acetate with a few drops of acetic acid until the elution of the aldehyde and then methanol). The yield was 28%, with 1H NMR (400.13 MHz, CDCl3) δ ppm 1.45 (s, 9H), 3.35 (t, 2H, J = 5 Hz), 3.65 (t, 2H, J = 5 Hz), 4.10 (s, 2H), 5.30 (s, 1H), 8.20 (s, 1H).
(iii) Deprotection of the amino group.
The amino group was deprotected as described previously (26). A suspension of 40 mg (0.18 mmol) of the N-protected acid in 10 ml of a 3 N HCl solution in ethyl acetate was stirred vigorously at room temperature for 30 min. The solution was removed in vacuo, and the oil was triturated with ether (3 ml). The ether layer was removed, and the aqueous phase was evaporated. After the sample was dried overnight, the pure chlorhydrate of 2-(2-aminoethoxy)acetate was obtained with a yield of 85%, with 1H NMR (400.13 MHz, D2O) δ ppm 3.97 (s, 1H), 3.69 (t, 2H, J = 3.6 Hz), 3.07 (t, 2H, J = 3.6 Hz).
RESULTS
Degradation of morpholine by M. aurum MO1.
Resting M. aurum MO1 cells (5 g of wet cells in 50 ml of Knapp buffer) were incubated with 10 mM morpholine at 30°C with agitation (200 rpm) for 72 h.
In order to find the best conditions for complete morpholine degradation, the first experiments were carried out with different [morpholine]/[bacterium] ratios. The amount of bacteria was varied from 100 to 10 g of wet cells in 1 liter of Knapp buffer, while the morpholine concentration was kept constant (10 mM [0.87 g · liter−1]). In all cases, the same intermediates were detected in 1H-NMR spectra. However, when the cell concentration was low (10 g · liter−1), the degradation of morpholine stopped before completion. This could result from the inhibitory effect of ammonia resulting from the degradation of morpholine, as Mazure and Truffaut (19) showed that ammonia was toxic to this strain. The best results were obtained with a cell concentration of 100 g · liter−1. The following experiments were carried out with this concentration.
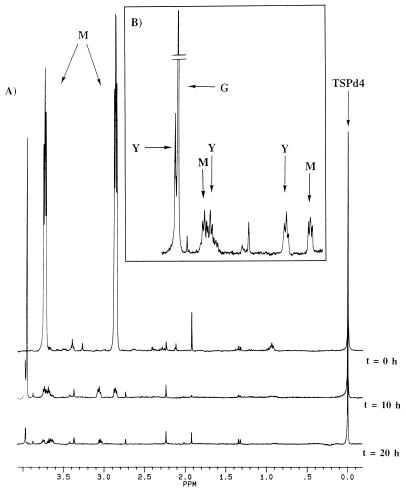
Samples (1 ml) were taken every hour, and the supernatants (500 μl) were analyzed by 1H-NMR spectroscopy after adjustment of the pH to 10 to avoid changes in chemical shifts. The spectra obtained were compared with those of the negative controls (incubation of cells under the same conditions in the absence of substrate and incubation of the substrate in the buffer without cells). Spectra collected at 0, 10, and 20 h are presented in Fig. 2A.
FIG. 2.
(A) Kinetics of morpholine degradation by M. aurum MO1. Resting cells (5 g of wet cells in 50 ml of Knapp buffer [KH2PO4, 1 g · liter−1; K2HPO4, 1 g · liter−1; FeCl3, 4 mg · liter−1; MgSO4 · 7H2O, 40 mg · liter−1; pH 6.6]) were incubated with 10 mM morpholine at 30°C with agitation (200 rpm) for 72 h. Samples (1 ml) were collected every hour for 12 h and from time to time until 72 h; after centrifugation, the supernatants of these samples were analyzed by 1H-NMR spectroscopy at 300.13 MHz. TSPd4 was used as a reference for chemical shifts and quantification. (B). Expanded scale, from 2.60 to 3.98 ppm, of the 10-h spectrum. M, morpholine; G, glycolate; Y, 2-(2-aminoethoxy)acetate.
In the spectrum obtained at time zero, three main signals are visible: a singlet at 0 ppm that belongs to the methyl groups of TSPd4 and two pseudotriplets at 2.88 and 3.72 ppm that correspond to CH2(b) and CH2(a) of morpholine (Fig. 1). The singlet corresponding to the NH of morpholine was not detected in water because of the quadrupolar moment of 14N. At 10 h (Fig. 2B), the signals of morpholine were decreasing while a singlet at 3.95 ppm was increasing. This signal was assigned to glycolate as evidenced by addition of the commercial compound to the sample. Three new signals were also present, resonating at 3.96 ppm (singlet), 3.67 ppm (pseudotriplet), and 3.05 ppm (pseudotriplet). These signals are compatible with those of 2-(2-aminoethoxy)acetate CH2(c), CH2(a), and CH2(b), respectively (Fig. 1). At 20 h, morpholine was exhausted and all the intermediate compounds were decreasing and had almost disappeared.
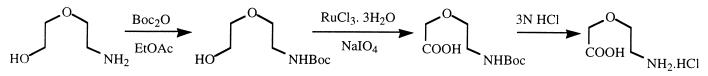
In order to confirm the assignment for the intermediate, 2-(2-aminoethoxy)acetate was synthesized. Vieles and Séguin (32) first described the synthesis of this compound, but the yield obtained was too low to be used. Therefore, we developed a new synthesis strategy in three steps (Fig. 3). The first step corresponded to the protection of the amino function with di-tert-butyldicarbonate in order to avoid the reaction of this function during the oxidation. This reaction was almost quantitative (yield, 92%). For the next step, which was oxidation of the alcohol into acid, the choice of the oxidant was more difficult, since it should not be too acidic or too strong in order not to remove the N protection. RuCl3 · 3H2O was chosen (2). A mixture of the aldehyde and the acid was obtained. The most difficult point was the purification of this acid; column chromatography on silica gel with a polar eluent was performed. The last step was the deprotection of the amino group. The N-protected acid was placed in a 3 N HCl solution in ethyl acetate, and the chlorhydrate of 2-(2-aminoethoxy)acetic acid was obtained with a good yield of 85%. After adjustment of the pH to 10, 2-(2-aminoethoxy)acetate was added to the sample collected at 12 h. The resonances were perfectly overlapping (data not shown).
FIG. 3.
Synthesis of the chlorhydrate of 2-(2-aminoethoxy)acetate. Boc2O, di-tert-butyldicarbonate; EtOAc, ethyl acetate.
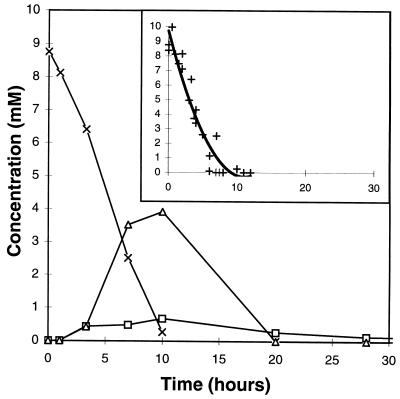
Quantitative analysis of the kinetics of degradation of morpholine was performed by integrating the signals of the different metabolites in 1H-NMR spectra; the measured areas were compared to the integral of the TSPd4 signal. The different concentrations of metabolites were calculated from these integrals as described in Materials and Methods. Under these conditions, the limit of quantification for an individual metabolite was estimated to be 0.05 mM. Figure 4 shows one example of the time courses for the concentrations of morpholine, glycolate, and 2-(2-aminoethoxy)acetate. The kinetics were quite reproducible, as shown in the inset, where data from five independent experiments are gathered.
FIG. 4.
Time courses for the concentrations of morpholine (×), glycolate (▵), and 2-(2-aminoethoxy)acetate (□) during the degradation of morpholine (10 mM) by M. aurum MO1 cells at 100 g · liter−1. The quantification was made by integrating the signals in 1H-NMR spectra relative to the area for the reference TSPd4 (see Fig. 2). (Inset) Data from five independant experiments concerning the degradation of morpholine; a polynomial calculation was used to plot the final curve.
Morpholine was almost exhausted after 10 h of incubation. Its rate of degradation was about 0.85 mM/h. Glycolate and 2-(2-aminoethoxy)acetate concentrations increased with time until 10 to 12 h and then decreased. No more glycolate was detected after 20 h or even longer. Consequently, glycolate is likely to be degraded in the cells.
Degradation of ethanolamine and glycolic acid by M. aurum MO1.
Mazure and Truffaut (19) showed that M. aurum MO1 was able to grow on ethanolamine in the absence of morpholine. In contrast, under the same conditions, no growth was observed on glycolic acid. This prompted us to monitor the kinetics of degradation of these two compounds, which are thought to be intermediates in the morpholine biodegradation pathway (Fig. 1). The most efficient condition for morpholine degradation, i.e., 100 g of bacteria · liter−1, was used, with 10 mM ethanolamine or 13 mM glycolate.
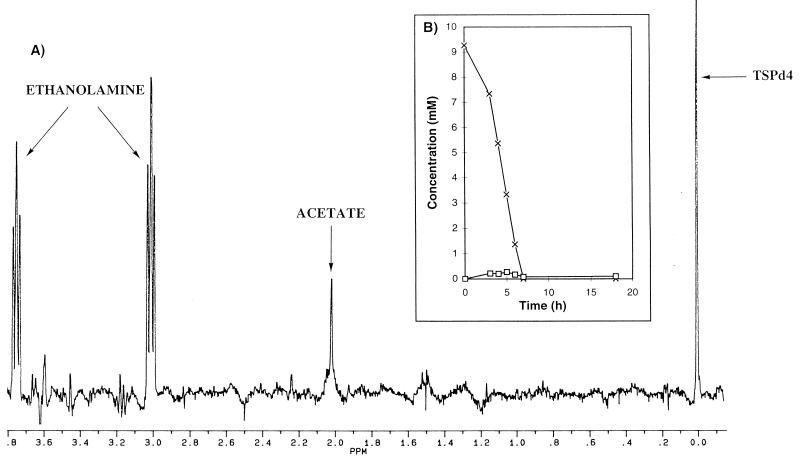
An example of a 1H-NMR spectrum collected after 5 h of incubation with ethanolamine is shown in Fig. 5A. The triplets resonating at 3.02 and 3.75 ppm correspond, respectively, to CH2(b) and CH2(a) of ethanolamine (Fig. 1). A resonance at 2.01 ppm (singlet) was assigned to acetate after addition of the commercial compound. The resonance at 3.83 ppm was also present in the control sample and thus is not a metabolite from morpholine degradation. The time courses for the metabolite concentrations are presented in Fig. 5B. Ethanolamine was degraded in 8 h at a rate of about 1.25 mM/h. During this degradation, the acetate level remained very low (about 0.3 mM).
FIG. 5.
Kinetics of degradation of ethanolamine (10 mM) by M. aurum MO1 (100 g · liter−1). The conditions were as described for Fig. 2. (A) 1H-NMR spectrum collected after 5 h of incubation. (B) Time courses for the concentrations of ethanolamine (×) and acetate (□).
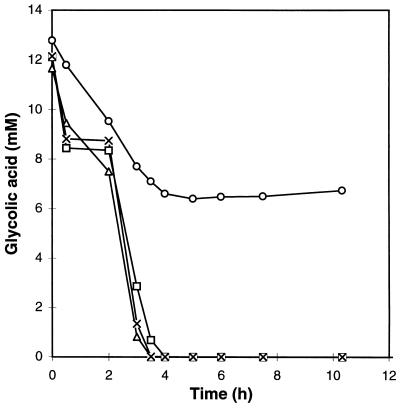
As shown before, glycolate has been identified as one of the intermediates in the morpholine degradation pathway. Study of its degradation kinetics is therefore particularly interesting (Fig. 6). When glycolic acid was added directly to the flask, under the usual conditions, the degradation was rapidly stopped (in about 4 h), whereas the 1H-NMR spectrum analysis of the morpholine degradation showed an increase and then a total disappearance of glycolic acid in 20 h. The pH was checked and was found to be very acidic (pH 3). Cells can no longer degrade glycolic acid at such a pH. The pH of the culture medium was then adjusted to 7 with various bases (KOH, NH4OH, and morpholine) before the addition of the bacteria. Under these conditions, the degradation of glycolate was complete in 4 h, no matter which base was used. The rate of degradation was very high, 3.25 mM/h.
FIG. 6.
Kinetics of degradation of glycolic acid (13 mM) by M. aurum MO1 (100 g · liter−1) without pH adjustment (○) and with adjustment of the pH to 7 by KOH (×), NH4OH (▵), and morpholine (□).
DISCUSSION
In this paper, 1H-NMR spectroscopy, performed directly on the incubation medium, was shown to be a useful tool to study the degradation of morpholine by M. aurum MO1. This approach was useful to directly quantify the degradation of morpholine. The NMR spectra collected at different incubation times showed that this strain is able to degrade morpholine at a rate of 0.85 mM/h under our conditions. It is worth noting that biodegradation began immediately; no latency period was observed. This observation shows that either no induction existed or the induction time was very short (less than 1 h).
The 1H-NMR technique also allowed us to identify unambiguously, for the first time, glycolate and 2-(2-aminoethoxy)acetate as intermediates of the biodegradation pathway. This suggests that M. aurum MO1 cleaved the C-N bond of the morpholine ring. These intermediates were suggested by Swain et al. (28) in the case of M. chelonae MorG but were never directly evidenced. In our experiments we did not find evidence of intermediates of the ethanolamine branch (acetaldehyde or acetate) during incubation with morpholine. However, we have shown that M. aurum cells could degrade ethanolamine through the classical pathway via acetate. This confirms the results of Mazure and Truffaut (19) concerning growth on this substrate without induction of morpholine. It is interesting that the rate of degradation of ethanolamine measured under our conditions (1.25 mM/h) was threefold higher than that of morpholine (0.85 mM/h).
Glycolate has been identified as the major intermediate of the morpholine degradation pathway. Although M. aurum MO1 was not able to grow on glycolic acid (19), we have shown that glycolic acid was degraded very rapidly by this strain (about 3.25 mM/hour). This degradation is pH dependent and can occur only if the pH is not too acidic. The nature of the base used for pH adjustment is not important. Mazure and Truffaut (19) reported that no growth was detected in the absence of morpholine with glycolic acid at pH 6.5 but that the presence of 0.1 g of morpholine · liter−1 induced degradation of this acid. Our results are different, showing a complete degradation of glycolate in the absence of morpholine (assays with KOH or NH4OH). Therefore, the enzymes required for this degradation are present in the microorganism, and no induction by morpholine is necessary.
In conclusion, this work is a pioneer in the direct quantification and identification of the morpholine degradation pathway of M. aurum MO1. The use of in situ 1H-NMR spectroscopy allows direct determination of the intermediates formed. Work is now in progress to identify other metabolites (particularly by analyzing the intracellular medium) and to understand the regulation of this pathway.
The methodology described here, using 1H-NMR spectroscopy, is general and easy to apply and could be used to investigate many biodegradative processes. This technique was used in the following companion paper (24) to study the morpholine biodegradation pathway with another strain. In this second article, we show evidence for the involvement of a cytochrome P-450 in morpholine degradation.
ACKNOWLEDGMENTS
This work was supported by interdisciplinary programs of CNRS “PIRSEM” and “ECOTECH.”
We acknowledge Anne-Lise Etienne, coordinator of the CNRS programs. We greatfully acknowledge Christelle Roland and Tran Thi Minh Ngoc for their help. We thank J. S. Cech for the gift of M. aurum MO1.
REFERENCES
- 1.Brown V R, Knapp J S, Heritage J. Instability of the morpholine degradative phenotype in mycobacteria isolated from activated sludge. J Appl Bacteriol. 1990;69:54–62. [Google Scholar]
- 2.Carlsen P H J, Katsuki T, Martin V S, Sharpless K B. A greatly improved procedure for rhutenium tetraoxide catalyzed oxidations of organic compounds. J Org Chem. 1981;46:3936–3938. [Google Scholar]
- 3.Cech J S, Hartman P, Slosarek M, Chudoba J. Isolation and identification of a morpholine-degrading bacterium. Appl Environ Microbiol. 1988;54:619–621. doi: 10.1128/aem.54.2.619-621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean-Ross D, Cerniglia C E. Degradation of pyrene by Mycobacterium flavescens. Appl Microbiol Biotechnol. 1996;46:307–312. doi: 10.1007/s002530050822. [DOI] [PubMed] [Google Scholar]
- 5.Dmitrenko G N, Udod V M, Gvozdyak P I. Destruction of morpholine by fixed bacteria. Khim Teknol Vody. 1985;7:97–99. [Google Scholar]
- 6.Emtiazi G, Knapp J S. The biodegradation of piperazine and structurally-related linear and cyclic amines. Biodegradation. 1994;5:83–92. [Google Scholar]
- 7.Gaines G L, III, Smith L, Neidle E L. Novel nuclear magnetic resonance spectroscopy methods demonstrate preferential carbon source utilization by Acinetobacter calcoaceticus. J Bacteriol. 1996;178:6833–6841. doi: 10.1128/jb.178.23.6833-6841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudet G, Forano E, Dauphin G, Delort A-M. Futile cycling of glycogene in Fibrobacter succinogenes as shown by in situ1H NMR and 13C NMR investigation. Eur J Biochem. 1992;207:155–162. doi: 10.1111/j.1432-1033.1992.tb17032.x. [DOI] [PubMed] [Google Scholar]
- 9.German Chemical Society. Advisory Committee on Existing Chemicals of Environmental Relevance, Morpholine BUA report 56, December 1990. S. Stuttgart, Germany: Hirzel; 1993. [Google Scholar]
- 10.Guerin W F, Jones G E. Mineralization of phenanthrene by a Mycobacterium sp. Appl Environ Microbiol. 1988;54:937–944. doi: 10.1128/aem.54.4.937-944.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Häggblom M M, Nohynek L J, Salkinoja-Salonen M S. Degradation and O methylation of chlorinated phenolic compounds by Rhodococcus and Mycobacterium strains. Appl Environ Microbiol. 1988;54:3043–3052. doi: 10.1128/aem.54.12.3043-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmans S, de Bont J A M. Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Appl Environ Microbiol. 1992;58:1220–1226. doi: 10.1128/aem.58.4.1220-1226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitkamp M A, Cerniglia C E. Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosms containing sediment and water from a pristine ecosystem. Appl Environ Microbiol. 1989;55:1968–1973. doi: 10.1128/aem.55.8.1968-1973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleespies M R M, Kroppenstedt F A, Rainey L E, Webb L E, Stackebrandt E. Mycobacterium hodleri sp. nov., a new member of the fast-growing mycobacteria capable of degrading polycyclic aromatic hydrocarbons. Int J Syst Bacteriol. 1996;46:683–687. doi: 10.1099/00207713-46-3-683. [DOI] [PubMed] [Google Scholar]
- 15.Knapp J S, Callely A G, Mainprize J. The microbial degradation of morpholine. J Appl Bacteriol. 1982;52:5–13. [Google Scholar]
- 16.Knapp J S, Brown V R. Morpholine biodegradation. Int Biodeterior. 1988;24:299–306. [Google Scholar]
- 17.Knapp J S, Whytell A J. The biodegradation of morpholine in river water and activated sludge. Environ Pollut. 1990;68:67–79. doi: 10.1016/0269-7491(90)90013-3. [DOI] [PubMed] [Google Scholar]
- 18.Kretzer A, Andreesen J R. A new pathway for isonicotinate degradation by Mycobacterium sp. INA1. J Gen Microbiol. 1991;137:1073–1080. doi: 10.1099/00221287-139-11-2763. [DOI] [PubMed] [Google Scholar]
- 19.Mazure N, Truffaut N. Degradation of morpholine by Mycobacterium aurum MO1. Can J Microbiol. 1994;40:761–765. doi: 10.1139/m94-120. [DOI] [PubMed] [Google Scholar]
- 20.McGowan P E, Reglinoki J, Wilson R, Walker J J, Wisdom S, McKillop J H. Quantitative 1H NMR analysis of amniotic fluid. J Pharm Biomed Analysis. 1988;11:629–632. doi: 10.1016/0731-7085(93)80167-y. [DOI] [PubMed] [Google Scholar]
- 21.Mirwish S S, Salmasi S, Cohen S M, Patil K, Mahboubi E. Liver and forestomach tumors, and other forestomach lesions in rats treated with morpholine and sodium nitrite with and without sodium ascorbate. JNCI. 1983;71:81–85. [PubMed] [Google Scholar]
- 22.Mjos K. Kirk-Othmer encyclopaedia of chemical technology. 3rd ed. Vol. 2. New York, N.Y: Wiley Interscience; 1978. Cyclic amines; pp. 295–308. [Google Scholar]
- 23.Naughton D P, Haywood R, Blake D R, Edmonds S, Hawkes G E, Grootveld M. A comparative evaluation of the metabolic profiles of normal and inflammatory knee-joint synovial fluids by high resolution proton NMR spectroscopy. FEBS Lett. 1993;332:221–225. doi: 10.1016/0014-5793(93)80636-9. [DOI] [PubMed] [Google Scholar]
- 24.Poupin P, Truffaut N, Combourieu B, Besse P, Sancelme M, Veschambre H, Delort A M. Degradation of morpholine by an environmental Mycobacterium strain involves a cytochrome P-450. Appl Environ Microbiol. 1998;64:159–165. doi: 10.1128/aem.64.1.159-165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saïto S, Nakajima H, Inaba M, Moriwake T. One-pot transformation of azido-group to N-(t-butoxycarbonyl)amino group. Tetrahedron Lett. 1989;30:837–838. [Google Scholar]
- 26.Stahl G L, Walter R, Smith C W. General procedure for the synthesis of mono-N-acylated 1,6-diaminohexanes. J Org Chem. 1978;43:2285–2287. [Google Scholar]
- 27.Suzuki S, Mitsuoka T. N-nitrosamine formation by intestinal bacteria. IARC Sci Publ. 1984;57:275–282. [PubMed] [Google Scholar]
- 28.Swain A, Waterhouse K V, Venables W A, Callely A G, Lowe S E. Biochemical studies of morpholine catabolism by an environmental mycobacterium. Appl Microbiol Biotechnol. 1991;35:110–114. [Google Scholar]
- 29.Tiehm A, Fritzsche C. Utilisation of solubilized and crystalline mixtures of polycyclic aromatic hydrocarbons by a Mycobacterium sp. Appl Microbiol Biotechnol. 1995;42:964–968. [Google Scholar]
- 30.Tran-Dinh S, Wietzerbin J, Courtois A, Hervé M. A novel approach for investigating reaction mechanisms in cells. Mechanism of deoxy-trehalose synthesis in Saccharomyces cerevisiae by 1H NMR spectroscopy. Eur J Biochem. 1995;228:727–731. doi: 10.1111/j.1432-1033.1995.tb20316.x. [DOI] [PubMed] [Google Scholar]
- 31.Veiga-da-Cunha M, Firme A, San Romao V, Santos H. Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl Environ Microbiol. 1992;58:2271–2279. doi: 10.1128/aem.58.7.2271-2279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieles, P., and J. Séguin. 1953. Synthèses de morpholones-3 et d’oxazolidone-2. Bull. Soc. Chim. Fr. 287–289.
- 33.Wackett L P, Brusseau G A, Householder S R, Hanson R S. Survey of microbial oxygenases: trichloroethylene degradation by propane-oxidizing bacteria. Appl Environ Microbiol. 1989;55:2960–2964. doi: 10.1128/aem.55.11.2960-2964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]