Abstract
A Mycobacterium strain (RP1) was isolated from a contaminated activated sludge collected in a wastewater treatment unit of a chemical plant. It was capable of utilizing morpholine and other heterocyclic compounds, such as pyrrolidine and piperidine, as the sole source of carbon, nitrogen, and energy. The use of in situ 1H nuclear magnetic resonance (1H NMR) spectroscopy allowed the determination of two intermediates in the biodegradative pathway, 2-(2-aminoethoxy)acetate and glycolate. The inhibitory effects of metyrapone on the degradative abilities of strain RP1 indicated the involvement of a cytochrome P-450 in the biodegradation of morpholine. This observation was confirmed by spectrophotometric analysis and 1H NMR. Reduced cell extracts from morpholine-grown cultures, but not succinate-grown cultures, gave rise to a carbon monoxide difference spectrum with a peak near 450 nm, which indicated the presence of a soluble cytochrome P-450. 1H NMR allowed the direct analysis of the incubation medium containing metyrapone, a specific inhibitor of cytochrome P-450. The inhibition of morpholine degradation was dependent on the morpholine/metyrapone ratio. The heme-containing monooxygenase was also detected in pyrrolidine- and piperidine-grown cultures. The abilities of different compounds to support strain growth or the induction of a soluble cytochrome P-450 were assayed. The results suggest that this enzyme catalyzes the cleavage of the C—N bond of the morpholine ring.
Morpholine (C4H9NO) is a simple heterocyclic compound (Fig. 1) with great industrial importance. It is used as an anticorrosive agent in water boiling systems, as a chemical intermediate (in catalysts, solvents, antioxidants, pharmaceuticals, bactericides, and pesticides), in the textile industry, in photographic developers, in hair conditioners, in waxes, and in the preservation of book paper. Because of its solubility in water, significant amounts of this chemical compound could be released, via industrial effluents, in the environment. It would then move with soil moisture and running water and would not sorb sediment or organic matter (12a). Morpholine, like other secondary amines, is subject to N nitrosation. This reaction can be catalyzed by various bacteria from secondary amines and nitrites or nitrates at neutral pH (3) but can also take place, in vivo, in mice by the action of NO2 on morpholine (29). Nitroso compounds are of particular concern because they are mutagens and carcinogens. N-Nitrosomorpholine was reported to be mutagenic in microbial gene mutation assays with Salmonella typhimurium (32), and recent studies assessing the carcinogenic activities of nitroso compounds have shown that this compound could enhance, even at low doses, the development of early stages of hepatocarcinogenesis in rats (8).
FIG. 1.
Structures of the compounds used.
Removal of this pollutant from contaminated wastewater and the environment is possible by biological treatment, since Knapp et al. (12) have clearly established that morpholine is biodegradable. A Mycobacterium strain, MorG, could utilize this compound as the sole source of carbon, nitrogen, and energy via an inducible pathway. Some bacteria that are capable of aerobic degradation of morpholine have been isolated from activated sludge, soils, and water. Except for two bacteria belonging to the genus Arthrobacter (7), all morpholine-degrading bacteria are mycobacteria (2, 4).
Biochemical studies of morpholine catabolism have been limited to those carried out with the environmental Mycobacterium strain MorG by Knapp et al. (12) and Swain et al. (24). These authors proposed a pathway for morpholine degradation by this strain in which the later stages of catabolism gave two C2-unit products: glycolate and ethanolamine. However, the early reaction mechanisms were not elucidated.
In the companion paper (5) our results on the pathway of biodegradation of morpholine by another strain, Mycobacterium aurum MO1, are presented. For that study, in situ 1H nuclear magnetic resonance (1H NMR) was used. This method allows direct detection and quantification of the metabolites formed, by analyzing the incubation medium at different times. We showed that M. aurum completely degraded morpholine (10 mM) in 10 h. Two intermediary compounds, 2-(2-aminoethoxy)acetate and glycolate, were identified.
In this paper, we report the isolation and the characterization of a Mycobacterium strain that metabolizes morpholine as the sole source of carbon, nitrogen, and energy. Experiments performed with inhibitors and spectrophotometric analysis implicated a cytochrome P-450 in this degradation. 1H NMR methodology, as described in the companion paper (5), was used to quantitatively monitor the degradation of morpholine and to identify the intermediates. The results were used to propose a pathway for morpholine degradation by this strain.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Strain RP1 was isolated, by enrichment culture with morpholine as the sole source of carbon and energy, from an activated sludge collected in a chemical wastewater treatment plant. The strain was deposited in the Institut Pasteur collection (CIP105337). This bacterium was grown and maintained on peptone-beef (PB) medium (casein peptone, 3 g · liter−1; beef extract, 5 g · liter−1), Trypticase soy broth (bioMérieux, Marcy l’Etoile, France), or a mineral salts medium (11) which contained (per liter) 1 g of KH2PO4, 1 g of K2HPO4, 0.04 g of MgSO4 · 7H2O, 0.004 g of FeCl3 · 6H2O, and 1 g of (NH4)2SO4 (when the carbon source did not contain nitrogen or when the amine was only used as a source of carbon). The pH was adjusted to 7.0 with NaOH or HCl after the addition of the carbon source. On mineral salts medium the following carbon sources were used: thiomorpholine, tetrahydrofuran, and tetrahydropyran at 5 mM and morpholine, piperidine, pyrrolidine, and succinate at 10 mM. Solid medium was prepared from mineral salts and PB media by the addition of 1.5% (wt/vol) Noble agar (Difco, Detroit, Mich.) or agar, respectively.
The assays of growth inhibition were performed in test tubes at 30°C under agitation on a gyratory shaker (180 rpm). Bacterial growth was determined by monitoring the optical density at 600 nm (OD600).
Chemicals.
Morpholine, glycolic acid, and ethanolamine were purchased from Aldrich Chemical (Sigma Aldrich Sarl, St. Quentin Fallavier, France); piperidine, pyrrolidine, thiomorpholine, tetrahydrofuran, and tetrahydropyran were from Fluka (Sigma Aldrich Sarl); methimazole and metyrapone were obtained from Sigma; and tetradeuterated sodium trimethylsilylpropionate (TSPd4) was purchased from EurisoTop (St. Aubin, France).
Analytical methods.
The morpholine concentration was routinely estimated spectrophotometrically by the method of Stevens and Skov (23) as modified by Knapp et al. (12). In situ 1H NMR spectroscopy was used when a greater precision in morpholine concentration was required and to identify degradation intermediates. The protein concentration was determined by the Bradford method (1).
Spectrophotometric analysis of cytochrome P-450.
Mycobacterial cells were harvested by centrifugation (8,000 × g, 10 min) at 4°C, washed, and resuspended in 50 mM phosphate buffer (pH 7.4) containing 0.3 mM phenylmethylsulfonyl fluoride. Bacterial walls were broken by three passages through a French pressure cell (SLM-Aminco) at 18,000 lb/in2 and centrifuged at 30,000 × g at 4°C for 30 min. The supernatant was reduced by dithionite sodium and divided equally between two optically matched cuvettes, and the difference spectrum was recorded in the presence of CO (19). An extinction coefficient of 91 mM−1 · cm−1 was used to determine the content of cytochrome P-450 in crude extracts (14).
To determine the induction of a cytochrome P-450 by tetrahydrofuran, tetrahydropyran, or thiomorpholine, the cells were grown in PB medium to obtain sufficient biomass, washed, and resuspended in mineral salts medium supplemented with one of these compounds at 5 mM.
All photometric analyses were done with a Shimadzu UV 160 A spectrophotometer.
1H NMR spectroscopy. (i) Microorganism growth.
For these experiments, strain RP1 cultures were grown in 100 ml of Trypticase soy broth (bioMérieux) in 500-ml Erlenmeyer flasks incubated at 30°C at 200 rpm. They were harvested after 48 h of culture.
(ii) Incubation with xenobiotics.
Incubations of cells with morpholine (10 mM), ethanolamine (15 mM), and glycolate (10 mM) were carried out as described in the companion paper (5).
For the assay of inhibition with metyrapone, different concentrations (5 and 10 mM) were tested. This inhibitor was added to the flasks containing the cells (5 g of wet cells in 50 ml of buffer) and morpholine (10 mM). Samples were taken every hour for 12 h and then from time to time until 72 h.
(iii) Preparation of the samples for 1H NMR.
The preparation of the samples for NMR, the materials used, and the quantification of the metabolites were as described elsewhere (5).
RESULTS
Enrichment and isolation of bacteria.
An enrichment culture was established with activated sludge collected from a chemical wastewater treatment plant (18). A subenrichment culture inoculated with an aliquot of this culture was made in mineral salts medium containing 10 mM morpholine. The culture was incubated on a gyratory shaker at 180 rpm and 30°C. Samples were regularly recovered, centrifuged, and assayed for morpholine content. When this amine was totally degraded, appropriate dilutions of the cell suspension were spread onto solid mineral salts medium supplemented with morpholine. Isolated colonies were selected, and their ability to degrade morpholine was determined in liquid mineral salts medium containing 10 mM morpholine.
Identification of a morpholine-degrading bacterium.
Enrichment and isolation procedures yielded only one bacterial strain (designated RP1) from this mixed culture that was able to utilize morpholine as a growth substrate. This culture was estimated to be pure after microscopic observation and several subcultures onto solid mineral salts agar medium containing morpholine and onto PB agar medium. Strain RP1 formed red, convex, slightly mucoid colonies on solid medium, and no diffusible pigment was observed. The coloration was affected neither by the growth conditions (PB agar medium, solid mineral salts medium supplemented with different compounds, and temperature) nor by the presence of light. The cells were nonmotile and exhibited an elementary rod-coccus life cycle. RP1 was gram positive, partially acid fast, catalase positive, and oxidase negative. On the basis of analysis of mycolic acids, polar lipids, wall sugars, and total proteins (Institut Pasteur, Paris, France), strain RP1 was assigned to the genus Mycobacterium: the cell wall peptidoglycan was based on meso-diaminopimelic acid, the major cell wall sugars were glucose and arabinose, and the wall envelope contained mycolic acids, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol, and phosphatidylinositol mannosides. Comparison of mycolic acids and total proteins of this bacterium with those of reference strains indicated that RP1 was closely related to Mycobacterium chlorophenolicum. Experiments to determine the 16S ribosomal DNA sequence of this microorganism and to establish its phylogenetic relationship to other mycobacteria are in progress.
Degradation of morpholine.
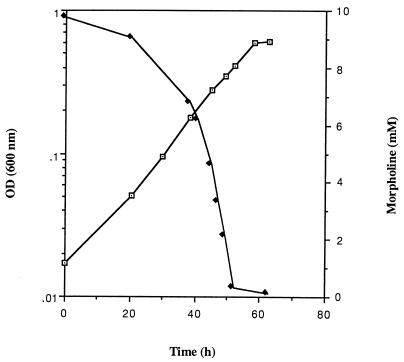
The ability of Mycobacterium sp. strain RP1 to degrade morpholine in pure culture was determined in liquid mineral salts medium (Fig. 2). When morpholine (10 mM) was used as the sole source of carbon, nitrogen, and energy, it was rapidly degraded. At the end of the logarithmic growth phase (50 h), no morpholine could be detected in the culture medium. During this time, the turbidity of the culture (OD600) increased from 0.018 to 0.62 and ammonia accumulated (data not shown). The medium had a uniformly turbid appearance, and no clumps or aggregates were visible. The natural clumping of the cells is a growth characteristic widely shared among members of the genus Mycobacterium, making it difficult to follow the growth and the degradation abilities of these bacteria (20). In contrast to M. aurum MO1, Mycobacterium sp. strain RP1 did not present this inconvenience. In addition, the doubling time in liquid medium supplemented with morpholine was about 9 h for strain RP1 versus about 12 h for M. aurum MO1, making it easier to study morpholine degradation with strain RP1.
FIG. 2.
Growth of Mycobacterium sp. strain RP1 on liquid mineral salts medium (⊡) and morpholine degradation (⧫).
In order to monitor the degradation of morpholine more precisely, we used the in situ 1H NMR spectroscopy methodology presented in the companion paper (5) for the study of the pathway of biodegradation of morpholine by M. aurum MO1. With this technique, the incubation medium is directly analyzed both qualitatively and quantitatively. It allows the identification of the intermediates formed during the degradation of morpholine.
Resting RP1 cells were incubated with 10 mM morpholine at 30°C with agitation (200 rpm) for 72 h. The conditions found to be best for M. aurum MO1 (100 g of wet cells in 1 liter of Knapp buffer) were used. Samples of the incubation medium were taken periodically and centrifuged, and the supernatants were analyzed by 1H NMR after adjustment of the pH to 10 (to avoid changes in chemicals shifts) and addition of a reference (TSPd4) for chemical shifts (0 ppm) and quantification.
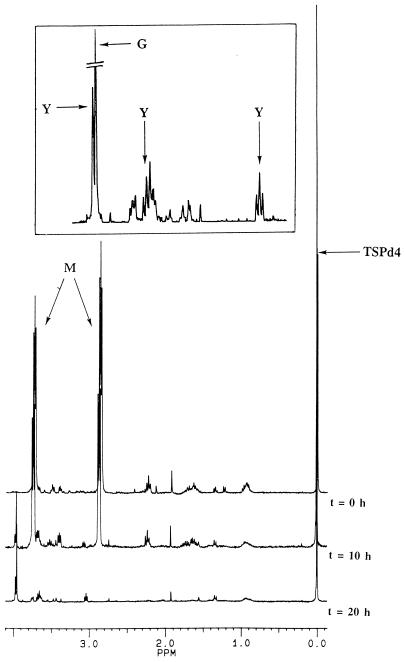
Figure 3 shows the kinetics of morpholine degradation as monitored by 1H NMR spectroscopy; only spectra recorded at 0, 10, and 20 h are plotted. The two pseudotriplets at 2.88 and 3.72 ppm correspond to the CH2 of morpholine. In the expanded region of the spectrum recorded at 20 h, different intermediates are observed: the three signals, named Y, at 3.96 (singlet) and 3.67 and 3.05 (pseudotriplets) have been identified as 2-(2-aminoethoxy)acetate after synthesis of this compound (5), and the singlet G at 3.95 ppm corresponds to glycolate, the second observed intermediate. The last singlet, at 0 ppm, corresponds to the CH3 of the TSPd4, our reference.
FIG. 3.
Kinetics of morpholine degradation by Mycobacterium sp. strain RP1. Resting cells (5 g of wet cells in 50 ml of Knapp buffer) were incubated with 10 mM morpholine at 30°C with agitation (200 rpm) for 72 h. Samples (1 ml) were collected every hour for 12 hours and from time to time until 72 h; after centrifugation, the supernatants of these samples were analyzed by 1H NMR spectroscopy at 300.13 MHz. TSPd4 was used as a reference for chemical shifts and quantification. The inset corresponds to an expanded scale, from 2.60 to 4.00 ppm, of the 20-h spectrum. M, morpholine; Y, 2-(2-aminoethoxy)acetate; G, glycolic acid.
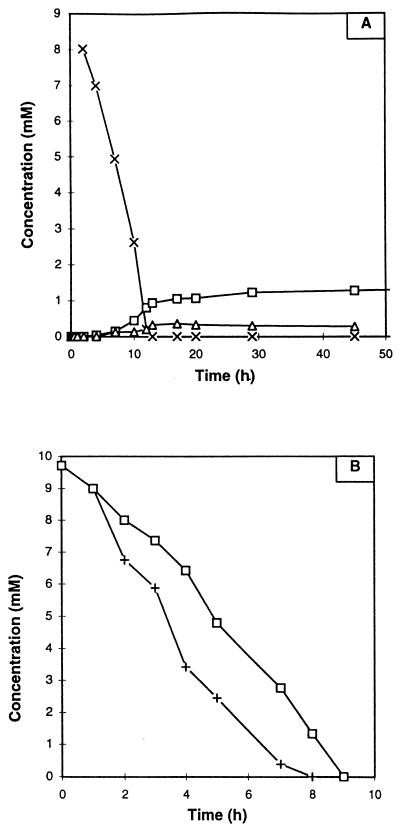
For quantification, the concentrations of morpholine and of the different intermediates were calculated by measuring the areas of the peaks and comparing them with that of the peak for TSPd4. The equation used for the calculation is described in the companion paper (5). Figure 4A shows the time courses for the concentrations of morpholine, 2-(2-aminoethoxy)acetate, and glycolate.
FIG. 4.
(A) Time courses for the concentrations of morpholine (×), glycolate (□), and 2-(2-aminoethoxy)acetate (▵) during the degradation of morpholine (10 mM) by Mycobacterium sp. strain RP1 cells at 100 g · liter−1. (B) Kinetics of degradation of ethanolamine (15 mM) (+) and glycolate (10 mM) (□) by Mycobacterium sp. strain RP1 (100 g · liter−1). The quantification was done by integrating the signals in 1H NMR spectra relative to the area of the reference TSPd4 signal.
Under these conditions, morpholine was degraded in 13 h at a rate of about 0.8 mM/h. The two intermediary compounds, 2-(2-aminoethoxy)acetate and glycolate, appeared after about 10 h of incubation. In contrast to the case for M. aurum MO1 (5), glycolate was not completely degraded, and its concentration in the supernatant increased.
Degradation of ethanolamine and glycolic acid.
The degradation of ethanolamine and glycolic acid, supposed intermediates of the biodegradation pathway according to Swain et al. (24), was also tested with this strain. The concentrations of ethanolamine and glycolic acid were, respectively, 15 and 10 mM. The kinetics of these two degradations are presented in Fig. 4B. Ethanolamine and glycolic acid were completely degraded within 10 h. No metabolite was detected in the medium.
Inhibition of morpholine degradation by selective inhibitors.
Degradation of a saturated heterocycle ring is likely to begin by the breakage of a bond between the heteroatom and an adjacent carbon atom. It was demonstrated that xenobiotic compounds bearing amine and ether functional groups could serve as substrates for flavin-containing monooxygenase or cytochrome P-450 (9, 21, 31). Previous work (12) showed that morpholine degradation was associated with oxygen consumption. According to these results and the chemical structure of morpholine, it was possible that the enzyme responsible for the ring cleavage was a monooxygenase.
In order to check the involvement of such enzymes in the first steps of morpholine degradation by Mycobacterium sp. strain RP1, the influence of selected inhibitors on the degradation ability of this strain was tested. Metyrapone (2-methyl-1,2-di-3-pyridyl-1-propanone) was chosen as a specific cytochrome P-450 inhibitor (25), and methimazole (2-mercapto-1-methylimidazole) was chosen as a competitive inhibitor of flavin-containing monooxygenase (26).
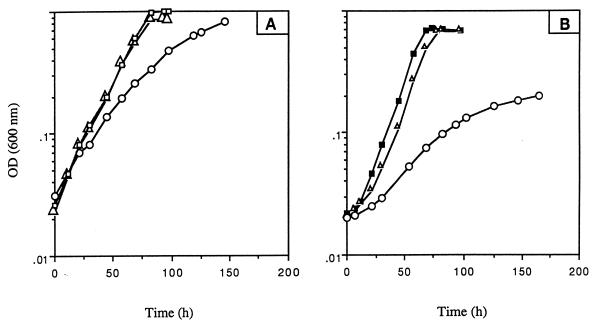
As shown in Fig. 5A, the growth of Mycobacterium sp. strain RP1 on liquid mineral salts medium supplemented with succinate was slightly affected by the presence of metyrapone and was not affected by the addition of an equivalent concentration of methimazole. The same experiments performed with morpholine as the sole source of carbon, nitrogen, and energy (Fig. 5B) indicated that without inhibitors in the medium or with methimazole, the stationary phase (OD600 of 0.7) was reached within 60 h. After the same time, the OD600 in the culture with metyrapone was only 0.06, thus indicating that this compound inhibited the growth of strain RP1 on morpholine. Metyrapone did not affect the viability of Mycobacterium sp. strain RP1, since the addition of this chemical to succinate-containing medium did not prevent cell growth. Consequently, the observed effects of metyrapone on the growth of the bacterium RP1 in morpholine-containing medium were due to its inhibitory properties. These results suggest that a cytochrome P-450 is involved in the oxidative catabolism of this amine. However, no flavin-containing monooxygenase seems to be implicated in morpholine degradation by strain RP1.
FIG. 5.
Influence of methimazole (▵) and metyrapone (○) on the growth of Mycobacterium sp. strain RP1 on succinate (A) and on morpholine (B). The inhibitors were added to the medium at a final concentration of 300 μg · ml−1. Control cultures without an inhibitor were grown on succinate (□) and morpholine (▪).
To obtain direct evidence of the inhibitory effect of metyrapone, experiments with different concentrations of this compound were carried out, and the results were analyzed by 1H NMR. To the flasks containing the cells (100 g · liter−1) were added metyrapone (5 and 10 mM) and morpholine (10 mM). The kinetics of morpholine degradation are reported in Fig. 6.
FIG. 6.
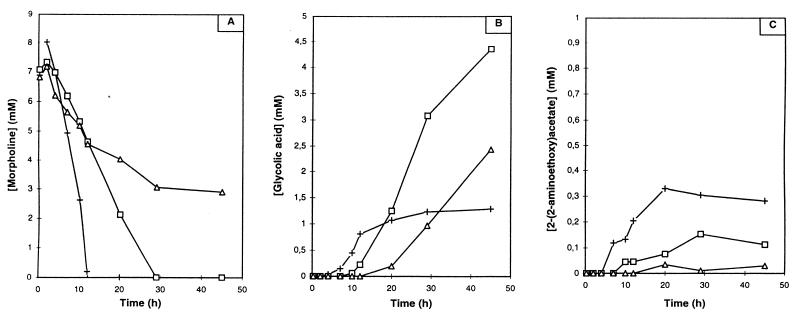
Incubation of Mycobacterium sp. strain RP1 cells (100 g · liter−1) with morpholine (10 mM) in the presence of 5 mM (□) or 10 mM (▵) metyrapone or in the absence of metyrapone (+). Time courses for the concentrations of morpholine (A), glycolic acid (B), and 2-(2-aminoethoxy)acetate (C) are shown.
The addition of metyrapone led to a concentration-dependent inhibition of the morpholine degradation reactions. When the concentration of metyrapone was increased, the following effects were observed: (i) the rates of morpholine degradation (Fig. 6A) and 2-(2-aminoethoxy)acetate formation (Fig. 6B and C) were decreased, (ii) the appearance of the intermediates was delayed (Fig. 6B and C), and (iii) the final concentration of 2-(2-aminoethoxy)acetate was decreased, while that of glycolate was increased.
These results confirm the presence of an activity due to a cytochrome P-450 in the morpholine degradation pathway. In addition, the effects of metyrapone on 2-(2-aminoethoxy)acetate formation indicate that the oxidation leading to the opening of the heterocycle takes place during the early stages of morpholine degradation. The accumulation of glycolate was unexpected, and it shows that morpholine is not completely mineralized. This interesting phenomenon is not yet explained.
Spectrophotometric evidence of the induction of a cytochrome P-450.
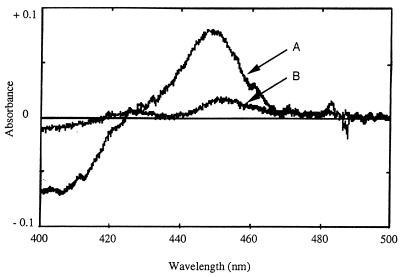
Cytochrome P-450s contain a ferriprotoporphyrin IX prosthetic group (heme). The heme is anchored in the active site by an axial iron-sulfur bond between the heme ferric iron and a cysteine sulfydryl group. Under reduced conditions, the ferrous iron can bind carbon monoxide. This binding gives rise to a distinctive absorption band at approximately 450 nm. Cell extracts from cultures grown on morpholine and on succinate were treated with sodium dithionite and CO. In the spectrum of the CO-treated reduced extract, but not in that of the nontreated reduced extract, of morpholine-grown bacteria, a peak at 449 nm was observed (Fig. 7, spectrum A). This result demonstrates the presence of a soluble cytochrome P-450 in this cell extract. The cytochrome P-450 content was about 90 pmol per mg of protein. Such a monooxygenase was not detected (but could be present at a low level) in the protein extracts of succinate-grown (Fig. 7, spectrum B) or acetate-grown (data not shown) bacteria. This indicated that the presence of a soluble cytochrome P-450 in Mycobacterium sp. strain RP1 was induced by growth on morpholine.
FIG. 7.
Carbon monoxide difference spectra of crude extracts of morpholine-grown (spectrum A) and succinate-grown (spectrum B) cells of Mycobacterium sp. strain RP1. The protein concentration was 10 mg · ml−1.
Growth of Mycobacterium sp. strain RP1 on other substrates and induction of a cytochrome P-450.
The abilities of different substrates to support the growth of Mycobacterium sp. strain RP1 and to induce a cytochrome P-450 were tested (Table 1). Mycobacterium sp. strain RP1 was not able to grow on thiomorpholine. Pyrrolidine and piperidine could support growth of RP1 and could be used as the sole source of carbon, nitrogen, and energy. These compounds, and also thiomorpholine, induced the production of a cytochrome P-450. No induction of the synthesis of a cytochrome P-450 occurred with tetrahydrofuran and tetrahydropyran. These two last molecules could support slow growth (OD600 = 0.15 in 12 days).
TABLE 1.
Growth of Mycobacterium sp. strain RP1 on different substrates and induction of a cytochrome P-450
| Substrate | Growtha | Cytochrome P-450 (pmol · mg of protein−1) |
|---|---|---|
| Acetate | + | NDb |
| Succinate | + | ND |
| Morpholine | + | 90 |
| Thiomorpholine | − | 80 |
| Pyrrolidine | + | 140 |
| Piperidine | + | 132 |
| Tetrahydrofuran | −/+ | ND |
| Tetrahydropyran | −/+ | ND |
+, growth; −/+, slow growth; −, no growth.
ND, not detected.
DISCUSSION
An actinomycete that grew on morpholine as the sole source of carbon, nitrogen, and energy was isolated from an activated sludge. This microorganism was identified as Mycobacterium sp. strain RP1 and found to be related to M. chlorophenolicum on the basis of physiological and biochemical characteristics. Mycobacterium sp. strain RP1 was also able to grow on two other cyclic amines, pyrrolidine and piperidine. Other morpholine-degrading bacteria, such as M. aurum MO1, can use these two compounds (13).
The use of 1H NMR showed the complete degradation of morpholine in 13 h at a rate of 0.8 mM/h and unambiguously identified two intermediates, 2-(2-aminoethoxy)acetate and glycolate. This suggests that Mycobacterium sp. strain RP1 cleaves the C—N bond of the morpholine ring. The morpholine biodegradation pathway observed with this strain seems to be very similar to that obtained with M. aurum MO1.
We have also shown that the enzymes required for the biodegradation of ethanolamine and glycolic acid are present in strain RP1. Both of these possible intermediates were degraded by this strain within 10 h. The pathway via ethanolamine has not been evidenced, as ethanolamine has not been detected during the biodegradation of morpholine. However, this intermediate might have built up below the limit for NMR detection (50 μM). Only glycolate and 2-(2-aminoethoxy)acetate were demonstrated to be intermediary compounds in this process.
Metyrapone and not methimazole inhibited the growth of Mycobacterium sp. strain RP1 on morpholine, strongly suggesting that a cytochrome P-450 is involved in the degradation of this compound. The same results were obtained for growth on pyrrolidine and piperidine. Purification of cytochrome P-450 is being considered in order to obtain conclusive evidence on this point. In parallel experiments, we have directly shown that morpholine is a substrate for cytochrome P-450: when metyrapone was added in morpholine-containing mineral salts medium, analysis by 1H NMR indicated that the degradation of morpholine was inhibited, the kinetics of formation of 2-(2-aminoethoxy)acetate were slowed, and no other compound (except glycolate) was detected. The appearance of the intermediary metabolites was delayed with increasing amounts of metyrapone. In addition, there was an unexpected accumulation of glycolate. Further knowledge of the biodegradation pathway of Mycobacterium sp. strain RP1 is needed to understand this phenomenon.
The presence of a soluble heme-containing monooxygenase was confirmed by the CO difference spectrum of cell extracts of Mycobacterium sp. strain RP1 grown on liquid mineral salts medium amended with morpholine. A cytochrome P-450 was also detected when this bacterium was grown on pyrrolidine, piperidine, and thiomorpholine but not tetrahydrofuran, tetrahydropyran, or succinate. Among the different compounds tested, only the cyclic amines induced the synthesis of a heme-containing monooxygenase, suggesting the cleavage of the C—N bond.
The involvement of a cytochrome P-450 in the degradation of morpholine represents another example of the intervention of these enzymes in xenobiotic metabolism (15), in which actinomycetes have a significant role (16, 17, 22). Although the presence of different inducible cytochrome P-450s in the same microorganism has been demonstrated, it seems probable that the synthesis of a single cytochrome P-450 could be induced by these cyclic amines: (i) morpholine, thiomorpholine, pyrrolidine, and piperidine are closely related compounds which have common properties; (ii) Knapp et al. (12) have noticed that morpholine-grown, but not acetate-grown, Mycobacterium sp. strain MorG was capable of immediately oxidizing pyrrolidine and piperidine; and (iii) all morpholine-negative mutants of MorG obtained by Swain et al. (24) failed to utilize pyrrolidine. To our knowledge, the implication of cytochrome P-450 in degradation mediated by mycobacteria has been demonstrated only for halogenated phenols. These monooxygenases were membrane associated (27, 28).
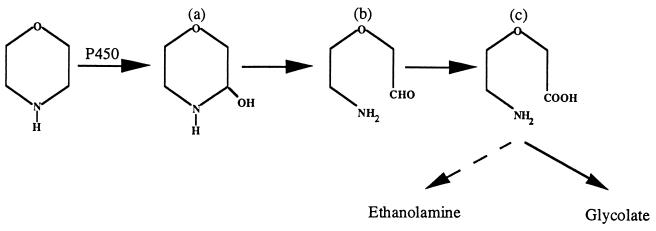
The most probable reaction catalyzed by this enzyme on these cyclic amines is C—N bond cleavage by a mechanism similar to an N dealkylation (Fig. 8). The monooxygenase catalyzes a hydroxylation on a C atom adjacent to the amine group which gives an unstable compound (compound a). This mechanism (rather than N oxygenation) is favored when α-protons are available (10). The compound so formed could be linearized to give 2-(2-aminoethoxy)acetaldehyde (compound b), which could undergo oxidation to form 2-(2-aminoethoxy)acetate (compound c).
FIG. 8.
Hypothetical pathway for morpholine degradation by Mycobacterium sp. strain RP1. a, 2-hydroxymorpholine; b, 2-(2-aminoethoxy)acetaldehyde; c, 2-(2-aminoethoxy)acetate.
In conclusion, the present results show that Mycobacterium sp. strain RP1 catalyzes the degradation of morpholine, pyrrolidine, and piperidine. This strain can use these compounds, which are the secondary amines most used in industry, as the sole source of carbon, nitrogen and energy. These degradations involve a soluble cytochrome P-450. The growth of strain RP1 on different substrates and the induction of a heme-containing monooxygenase suggest that this enzyme attacks morpholine at the C—N position. This reaction could be followed by ring cleavage to form 2-(2-aminoethoxy)acetate which is further degraded to glycolic acid, as shown by the detection of these intermediates in the 1H NMR spectra. This study, together with previous work (6, 11, 30), underlines the importance of mycobacteria in the degradation of xenobiotic compounds. Further investigations to identify metabolites of the early reactions in the catabolism of morpholine by Mycobacterium sp. strain RP1 are in progress.
ACKNOWLEDGMENTS
This work was supported by interdisciplinary programs of CNRS “PIRSEM” and “ECOTECH.” P. Poupin and B. Combourieu were recipients of a fellowship from the Ministère de la Recherche et de l’Enseignement Supérieur.
We acknowledge Anne-Lise Etienne, coordinator of the CNRS programs.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown V R, Knapp J S. The effect of withdrawal of morpholine from the influent and its reinstatement on the performance and microbial ecology of a model activated sludge plant treating a morpholine-containing influent. J Appl Microbiol. 1990;69:43–53. [Google Scholar]
- 3.Calmels S, Ohshima H, Bartsch H. Nitrosamine formation by denitrifying and non-denitrifying bacteria: implication of nitrite reductase and nitrate reductase in nitrosation catalysis. J Gen Microbiol. 1988;134:221–226. doi: 10.1099/00221287-134-1-221. [DOI] [PubMed] [Google Scholar]
- 4.Cech J S, Hartman P, Slosarek M, Chudoba J. Isolation and identification of a morpholine-degrading bacterium. Appl Environ Microbiol. 1988;54:619–621. doi: 10.1128/aem.54.2.619-621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combourieu B, Besse P, Sancelme M, Veschambre H, Delort A M, Poupin P, Truffaut N. Morpholine degradation pathway of Mycobacterium aurum MO1: direct evidence of intermediates by in situ 1H nuclear magnetic resonance. Appl Environ Microbiol. 1998;64:153–158. doi: 10.1128/aem.64.1.153-158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Ross D, Cerniglia C E. Degradation of pyrene by Mycobacterium flavescens. Appl Microbiol Biotechnol. 1996;46:307–312. doi: 10.1007/s002530050822. [DOI] [PubMed] [Google Scholar]
- 7.Dmitrenko G N, Gvozdyak P I, Udod V M. Selection of destructor microorganisms for heterocyclic xenobiotics. Khim Tekhnol Vody. 1987;9:442–445. [Google Scholar]
- 8.Enzmann H, Zerban H, Kopp-Schneider A, Loser E, Bannasch P. Effects of low doses of N-nitrosomorpholine on the development of early stages of hepatocarcinogenesis. Carcinogenesis. 1995;16:1513–1518. doi: 10.1093/carcin/16.7.1513. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich F P. Enzymatic oxidation of xenobiotic chemicals. Biochem Mol Biol. 1990;25:97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich F P, Macdonald T L. Mechanisms of cytochromes P450 catalysis. FASEB J. 1990;4:2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- 11.Knapp J S, Brown V R. Morpholine biodegradation. Int Biodeterior. 1988;25:299–306. [Google Scholar]
- 12.Knapp J S, Callely A G, Mainprize J. The microbial degradation of morpholine. J Appl Bacteriol. 1982;52:5–13. [Google Scholar]
- 12a.Lewis R J Sr, editor. Chemical review: morpholine. Danger Prop Ind Mater Rep. 1995;15:270–297. [Google Scholar]
- 13.Mazure N, Truffaut N. Degradation of morpholine by Mycobacterium aurum MO1. Can J Microbiol. 1994;40:751–765. doi: 10.1139/m94-120. [DOI] [PubMed] [Google Scholar]
- 14.Miles J S, Munro A W, Rospendowski B N, Smith W E, McKnight J, Thomson A J. Domains of the catalytically self-sufficient cytochrome P450 BM-3. Genetic construction, overexpression, purification and spectroscopic characterization. Biochem J. 1992;288:503–509. doi: 10.1042/bj2880503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro A W, Lindsay J G. Bacterial cytochromes P450. Mol Microbiol. 1996;20:1115–1125. doi: 10.1111/j.1365-2958.1996.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Keefe D P, Harder P A. Occurrence and biological function of cytochrome P450 monooxygenases in the actinomycetes. Mol Microbiol. 1991;5:2099–2105. doi: 10.1111/j.1365-2958.1991.tb02139.x. [DOI] [PubMed] [Google Scholar]
- 18.Penaud F. Ph.D. thesis. Compiègne, France: University of Compiègne; 1989. [Google Scholar]
- 19.Peterson J A, Lu J-Y. Bacterial cytochromes P450: isolation and identification. Methods Enzymol. 1991;206:612–620. doi: 10.1016/0076-6879(91)06131-l. [DOI] [PubMed] [Google Scholar]
- 20.Poupin P, Mazure N, Truffaut N. Morpholine degradation by strain Mycobacterium aurum MO1: improvement of cells growth and morpholine degradation rate by cell immobilization. In: Wijffels R H, Buitlaar R M, Blucke C, Tramper J, editors. Progress in biotechnology. 11. Immobilized cells: basis and applications. Amsterdam, The Netherlands: Elsevier Science, B.V.; 1996. pp. 770–776. [Google Scholar]
- 21.Sariaslani S F. Microbial cytochromes P450 and xenobiotic metabolism. Adv Appl Microbiol. 1991;36:133–178. doi: 10.1016/s0065-2164(08)70453-2. [DOI] [PubMed] [Google Scholar]
- 22.Sariaslani S F, Omer C A. Actinomycetes cytochromes P450 involved in oxidative metabolism: biochemistry and molecular biology. Crit Rev Plant Sci. 1992;11:1–16. [Google Scholar]
- 23.Stevens W H, Skov K. A rapid spectrophotometric method for determining parts per million of morpholine in boiler water. Analyst. 1965;90:182–183. [Google Scholar]
- 24.Swain A, Waterhouse K V, Venables W A, Callely A G, Lowe S E. Biochemical studies of morpholine catabolism by an environmental mycobacterium. Appl Microbiol Biotechnol. 1991;35:110–114. [Google Scholar]
- 25.Testa B, Jenner P. Inhibitors of cytochrome P450s and their mechanism of action. Drug Metab. 1981;12:1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- 26.Tomasi I, Artaud I, Bertheau Y, Mansuy D. Metabolism of polychlorinated phenols by Pseudomonas cepacia AC1100: determination of the first two steps and specific inhibitory effect of methimazole. J Bacteriol. 1995;177:307–311. doi: 10.1128/jb.177.2.307-311.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uotila J S, Salkinoja-Salonen M S, Apajalahti J H A. Degradation of pentachlorophenol by membrane bound enzymes from Rhodococcus chlorophenolicus PCP-1. Biodegradation. 1991;2:25–31. doi: 10.1007/BF00122422. [DOI] [PubMed] [Google Scholar]
- 28.Uotila J S, Kitunen V H, Saastamoinen T, Coote T, Häggblom M M, Salkinoja-Salonen M S. Characterization of aromatic dehalogenases of Mycobacterium fortuitum CG-2. J Bacteriol. 1992;174:5669–5675. doi: 10.1128/jb.174.17.5669-5675.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Stee E W, Slone R A, Simmons J E, Moorman M P, Brunnemann K D. Endogenous formation of N-nitrosomorpholine in mice from 15NO2 by inhalation and morpholine gavage. Carcinogenesis. 1995;16:89–92. doi: 10.1093/carcin/16.1.89. [DOI] [PubMed] [Google Scholar]
- 30.Wang R F, Cao W W, Cerniglia C E. Phylogenetic analysis of polycyclic aromatic hydrocarbon degrading mycobacteria by 16S rRNA sequencing. FEMS Microbiol Lett. 1995;130:75–80. doi: 10.1016/0378-1097(95)00186-9. [DOI] [PubMed] [Google Scholar]
- 31.White G F, Russel N J, Tidswell E C. Bacterial scission of ether bonds. Microbiol Rev. 1996;60:216–232. doi: 10.1128/mr.60.1.216-232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Morpholine: health and safety guide. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]