Abstract
O-Methyltransferase I, which catalyzes conversions both of demethylsterigmatocystin (DMST) to sterigmatocystin (ST) and of dihydrodemethylsterigmatocystin (DHDMST) to dihydrosterigmatocystin (DHST) during aflatoxin biosynthesis, was purified to apparent homogeneity from the cytosol fraction of the mycelia of Aspergillus parasiticus NIAH-26 through the following chromatography series: phenyl-Sepharose, DEAE-Sepharose, phenyl-Sepharose, Sephacryl S-300, and Matrex gel Green A. The apparent molecular mass was estimated at 150 kDa based on Sephacryl S-300 gel filtration chromatography, and the denaturing molecular mass was 43 kDa based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The pI of the enzyme was 4.4, and the optimal pH for activity was broad, from 6.5 to 9.0. In competition experiments using the purified enzyme, the formation of ST from DMST was suppressed when DHDMST was added to the reaction mixture and DHST was newly formed. These results indicate that DMST and DHDMST commonly serve as substrates for the enzyme. The Km of the enzyme for DMST was 0.94 μM, and that for DHDMST was 2.5 μM. Interestingly, MT-I kinetics deviated substantially from standard Michaelis-Menten kinetics, demonstrating substrate inhibition at a higher substrate concentration.
Aflatoxins constitute a family of acutely toxic, teratogenic, potent carcinogenic, and mutagenic metabolites produced by certain strains of common molds Aspergillus flavus and A. parasiticus (13). Recently, some strains of A. nomius (18) and A. tamarii (15) were also reported to produce aflatoxins. Sterigmatocystin (ST) is also a toxic and carcinogenic intermediate in the aflatoxin biosynthetic pathway but is not as potent as aflatoxins, and ST is produced by strains belonging to 20 species Aspergillus, including A. nidulans (11). Food contamination by aflatoxins and ST can seriously and adversely affect the health of animals and humans.
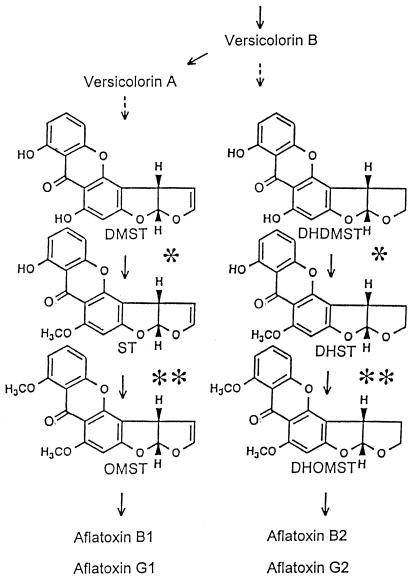
The biosynthetic pathway of aflatoxins is mostly known, and Fig. 1 shows the latter part leading to formation of aflatoxins (2, 4, 5, 12, 23, 27, 29, 30, 32). Aflatoxins B1 (AFB1), G1 (AFG1), B2 (AFB2), and G2 (AFG2) are major, naturally occurring substances. AFB1 and AFG1 contain dihydrobisfuran rings, and AFB2 and AFG2 contain tetrahydrobisfuran rings in their moiety. In the pathways, AFB1 and AFG1 are produced from demethylsterigmatocystin (DMST), and AFB2 and AFG2 are produced from dihydrodemethylsterigmatocystin (DHDMST) (32). These different bisfuran rings are produced at the branching step between versicolorin B and versicolorin A (30), and common enzymes are suspected of being involved in independent pathways leading to the formation of AFB1/AFG1 and AFB2/AFG2. DMST and DHDMST have two hydroxyl groups in their molecules; the C-6-OH groups among them are first methylated by O-methyltransferase I (MT-I) to produce ST and dihydrosterigmatocystin (DHST), and the remaining C-7-OH groups are then methylated by O-methyltransferase II (MT-II) to make O-methylsterigmatocystin (OMST) and dihydro-O-methylsterigmatocystin (DHOMST). This methylation sequence is strictly determined by either enzyme substrate specificity (32). These methyltransferases differ in molecular masses and sensitivity to N-ethylmaleimide (NEM); i.e., MT-II activity is resistant to this reagent, and MT-I is inactivated by it (32).
FIG. 1.
Metabolic scheme for aflatoxin (AFB1, AFB2, AFG1, and AFG2) biosynthesis showing structures of critical intermediates. Reactions catalyzed by MT-I are indicated by single asterisks, and those catalyzed by MT-II are indicated by double asterisks. Solid arrow, confirmed reaction; dashed arrow, unconfirmed reaction.
Recently, several enzymes involved in aflatoxin biosynthesis have been purified to homogeneity. O-Methyltransferase (6) and 40-kDa methyltransferase (17), which correspond to MT-II, have been purified, and the same enzyme was shown to be involved in both ST-to-OMST and DHST-to-DHOMST reactions (4, 29, 32). Norsolorinic acid reductase (3, 9), cyclase (21, 27), versiconal hemiacetal acetate reductase (22), and esterase (19) have been also purified. Our objective herein was to purify and characterize the MT-I enzyme. In this study, we devised a coupling assay using NEM-treated cytosol fraction to specifically and conveniently detect MT-I activity, where we changed the resultant ST and DHST produced by MT-I to OMST and DHOMST.
MATERIALS AND METHODS
Microorganisms and growth conditions.
We purified MT-I activity from A. parasiticus NIAH-26, a UV-irradiated mutant of A. parasiticus SYS-4 (NRRL-2999) (33). In YES medium (2% yeast extract, 20% sucrose), this strain expressed all enzymes in the aflatoxin biosynthetic pathway from norsolorinic acid to aflatoxins, although it produced no aflatoxins, anthraquinone, or xanthone precursors (29–32, 34).
A. parasiticus NIAH-26 was grown in a malt-extract agar medium at 28°C for 1 week, and then the conidiospores were collected as described before (31).
Standard metabolite samples.
High concentrations of metabolites were made as a stock solution in dimethylformamide. After dilution with a large volume of methanol, the concentration of the metabolites in methanol was determined from UV absorption spectra by using molar absorption coefficients (11, 32) as follows (in M−1cm−1): DMST (335 nm), 19,100; DHDMST (335 nm), 19,400; ST (329 nm), 13,100; DHST (325 nm), 16,300; OMST (310 nm), 16,500; and DHOMST (311 nm), 17,300. A standard kit (Makor Chemicals Ltd., Jerusalem, Israel) was used for the analysis of AFB1, AFB2, AFG1, and AFG2.
Enzyme assay.
The NEM-treated cytosol fraction was used as a crude MT-II enzyme fraction containing no MT-I enzyme. It was prepared by incubating the cytosol (3.7 mg of protein per ml) of A. parasiticus NIAH-26 with 18 mM NEM at 37°C for 60 min in a mixture containing 0.18 M potassium phosphate buffer (pH 7.5) and 10% glycerol. The reaction was terminated by adding one-seventh volume of 2 M 2-mercaptoethanol. To remove unreacted excess NEM from proteins, the treated cytosol was purified through a previously equilibrated Sephadex G-25 M column (Column PD-10; Pharmacia LKB, Uppsala, Sweden) and then eluted with a solution containing 100 mM Tris-HCl (pH 7.5) and 10% (vol/vol) glycerol. This NEM-treated cytosol had no MT-I activity and was stored at −80°C until used. MT-II activity of the resultant cytosol was stable for at least 6 months under these conditions.
We detected enzymatically active fractions through a coupled assay method where we changed the reaction products of MT-I, i.e., ST and DHST, to OMST and DHOMST, respectively, because OMST and DHOMST brilliantly fluoresce under UV light. A 2- to 5-μl aliquot was added to the reaction mixture (60 mM potassium phosphate buffer [pH 7.5], 10% glycerol, 60 μM DMST or DHDMST, 0.3 mM S-adenosylmethionine, 0.46 mg of NEM-treated cytosol/ml [total volume, 20 μl]). The final concentration of dimethylformamide did not exceed 2% of the reaction volume to avoid inhibition of enzyme activity. After incubation at 37°C for 5 to 30 min, the reaction was terminated by adding 35 μl of water-saturated chloroform and mixing with a Vortex mixer. After centrifugation at 10,000 × g for 1 min, 20 μl of the lower chloroform layer was spotted onto a silica gel (SIL) thin-layer chromatography (TLC) plate, and the plate was developed with a solution of chloroform–ethyl acetate–90% formic acid (6:3:1 [vol/vol/vol]). After developing, plates were inspected under long-wavelength (365-nm) UV light to detect the yellow fluorescence of OMST or DHOMST. Fluorescence photographs were taken as described elsewhere (32).
To examine MT-I activity quantitatively, we used SIL high-performance chromatography (HPLC). Two microliters of each enzyme fraction was added to 50 μl of reaction mixture as described above and incubated for 10 min. The reaction was terminated by the addition of 80 μl of water-saturated chloroform and mixing with a Vortex mixer. After centrifugation, 20 μl of the lower chloroform layer was directly injected into a Shimadzu HPLC apparatus (model CL-6A; Shimadzu Co., Kyoto, Japan) equipped with a SIL column (0.46 by 15 cm; Shim-pack CLC-SIL; Shimadzu) and a guard column (0.4 by 1 cm; Shim-pack G-SIL). Absorbance at 240 nm was monitored for detection of OMST and DHOMST by using a solution of isopropyl alcohol–n-hexane (1:9 [vol/vol]) at a flow rate of 1 ml/min. The retention times of standard samples were as follows: DMST and DHDMST, less than 2 min; ST, 3.58 min; DHST, 3.97 min; OMST, 11.82 min; and DHOMST, 13.67 min.
To determine the kinetic values of the purified enzyme, ST or DHST production was measured by using an octadecyl silane (ODS) column. Purified enzyme (0.9 μg/ml) was incubated with different concentrations of either DMST or DHDMST without adding NEM-treated cytosol at 37°C for 30 min in a final volume of 50 μl. The reaction was terminated by adding 60 μl of water-saturated chloroform and mixing, and then 25 μl of the lower chloroform extract was injected into an HPLC apparatus equipped with a ODS column (0.46 by 15 cm; STR ODS; Shimadzu) and a guard column (0.4 by 1 cm). The elution solution of acetonitrile-water (6:4 [vol/vol]) was monitored at a flow rate of 1 ml/min and absorbance at 240 nm. The retention times of the standard samples were as follows: DMST, 14.70 min; DHDMST, 13.53 min; ST, 5.83 min; and DHST, 5.42 min.
Purification.
The cytosol fraction was prepared from the mycelia of A. parasiticus NIAH-26 by successive centrifugation as described previously (22). All subsequent purification steps were performed at 0 to 4°C.
Cytosol (225 ml) was brought to 0.8 M with (NH4)2SO4, and loaded onto a phenyl-Sepharose CL-4B column (2.5 by 8.8 cm; Pharmacia LKB) previously equilibrated with buffer A (20 mM Tris-HCl [pH 7.5], 10% [vol/vol] glycerol, 10 mM MgCl2, 0.4 mM EDTA, 1 mM 2-mercaptoethanol) supplemented with 0.8 M (NH4)2SO4. The column was washed with the equilibration solution, and proteins bound to the column were eluted with a linear gradient of 0.8 to 0 M (NH4)2SO4 in buffer A (180 ml) and then with buffer A (80 ml). Active fractions were collected and then loaded onto a DEAE-Sepharose CL-6B column (1.2 by 13 cm; Pharmacia LKB) previously equilibrated with buffer A. The column was washed with buffer A, and activity was eluted with a linear gradient of 0 to 0.5 M KCl in buffer A (80 ml). Active fractions were collected and pooled. The pooled solution was brought to 0.8 M (NH4)2SO4 by adding one-third volume of 3.2 M (NH4)2SO4 in buffer A. Phenyl-Sepharose chromatography (0.7 by 18.5 cm) was repeated with successive solutions: 5 ml of buffer A supplemented with 0.4 M (NH4)2SO4, 40 ml of linear gradient 0.4 to 0 M (NH4)2SO4 in buffer A, and then 35 ml of buffer A. The active fractions were pooled and concentrated by ultrafiltration in a Centriprep-10 concentrator (Amicon, Div. W. R. Grace & Co., Danvers, Mass.). The concentrated fraction was loaded onto a Sephacryl S-300 column (1.6 by 68 cm; Pharmacia LKB) previously equilibrated with buffer A. The active fractions of MT-I were pooled and loaded onto a Matrex gel Green A column (0.5 by 7.5 cm; Amicon) previously equilibrated with a buffer A. The protein unbound to the column was pooled as a pure MT-I enzyme.
Characterization of MT-I.
To examine competition between DMST and DHDMST for MT-I, purified enzyme (0.9 μg/ml) was incubated with different concentrations (1 to 300 μM) of DHDMST in the presence of 20 μM DMST without NEM-treated cytosol in a final volume of 50 μl at 37°C for 30 min. The resulting ST and DHST were measured by HPLC as described above.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (20), and proteins were stained with Coomassie brilliant blue.
The molecular mass of the native enzyme was determined in comparison with the following molecular mass standards (Bio-Rad Laboratories or Pharmacia LKB): thyroglobulin (670 kDa), ferritin (450 kDa), catalase (240 kDa), gamma globulin (158 kDa), albumin (68 kDa), ovalbumin (44 kDa), and chymotrypsinogen (25 kDa).
The pI of MT-1 activity was determined by using a Rotofore cell (Bio-Rad). Ten milliliters of cytosol (41 mg of protein) was concentrated by ammonium sulfate fractionation (25 to 80% saturation) and desalted with column PD-10 (Pharmacia LKB). Ampholyte solution (pH range, 3.5 to 10) was added to the protein solution, and the sample was diluted to 50 ml. Rotofore treatment was performed, and then each fraction was examined for MT-I activity by routine TLC analysis using NEM-treated cytosol coupling as described above.
Protein concentration was determined by Bradford dye binding (7) using a protein assay solution (Bio-Rad) and bovine serum albumin as the standard. To assess pH dependence of enzyme activity, buffer systems used were 0.1 M sodium acetate for pH 3.5 to 6.0, 0.1 M sodium phosphate for pH 5.5 to 8.5, and 0.1 M glycine-NaOH for pH 8.0 to 10.5. The reaction was carried out at 37°C for 15 min. The conversion of DMST to ST by purified MT-I was measured by HPLC as described above.
RESULTS
Enzyme purification.
MT-I purification consisted of five steps using four types of resins. Typical results from MT-I purification are summarized in Table 1, column chromatography steps are shown in Fig. 2, and SDS-PAGE of the protein at different stages of purification is shown in Fig. 3.
TABLE 1.
Purification of MT-I
| Step | Vol (ml) | Total protein (mg) | Total enzyme activity (pmol/min)a | Sp act (pmol/μg/min)a | Purification
|
|
|---|---|---|---|---|---|---|
| Fold | % | |||||
| Cytosol | 225 | 738 | 390 | 0.53 | 1 | 100 |
| 431b | 0.58b | 1b | 100b | |||
| 1st phenyl-Sepharose chromatography | 52 | 24.5 | 71.1 | 2.9 | 5.5 | 18.2 |
| DEAE-Sepharose chromatography | 9 | 7.4 | 29.4 | 2.1 | 4.0 | 7.5 |
| 2nd phenyl-Sepharose chromatography | 12.5 | 0.9 | 32.0 | 18.6 | 35.2 | 8.2 |
| Sephacryl S-300 gel filtration | 6 | 0.3 | 15.4 | 27.6 | 52.3 | 4.0 |
| Matrex gel Green A | 6 | 0.09 | 5.7 | 60.8 | 115 | 1.5 |
| 4.0b | 43.1b | 73.9b | 0.9b | |||
Production of OMST from DMST through ST formation was measured by coupling as described in Materials and Methods.
Where given, the second line represents production of DHOMST from DHDMST through DHST formation.
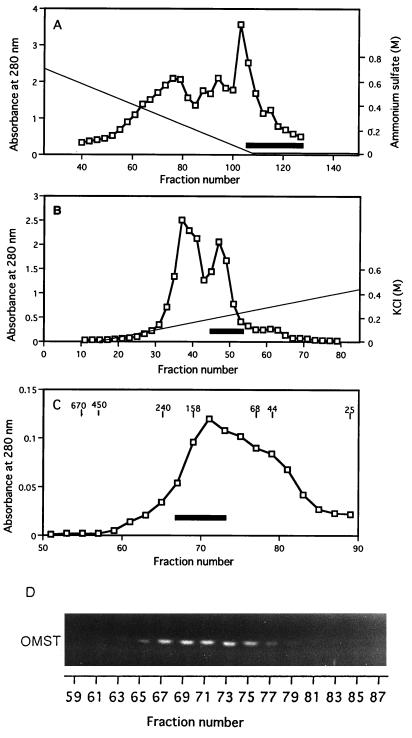
FIG. 2.
MT-I chromatographies. (A) First phenyl-Sepharose fractionation. Cytosol fraction added with ammonium sulfate was loaded onto a phenyl-Sepharose column, and the column effluent after a linear gradient of (NH4)2SO4 (solid line) followed by washing with the solution without (NH4)2SO4 was assayed for protein (absorbance at 280 nm; open squares) and MT-1 activity (open squares) by measuring the production of OMST from DMST. The volume of each fraction was 2 ml, and the solid bar indicates active fractions collected. (B) DEAE-Sepharose ion-exchange chromatography. Proteins were eluted by successive linear gradient washes with KCl. The volume of each fraction was 1 ml, and the solid bar indicates active fractions collected. (C) Sephacryl S-300 gel filtration chromatography. After rechromatography of the pooled fractions with phenyl-Sepharose, active fractions were collected, concentrated, and applied to a Sephacryl S-300 column. The solid line indicates active fractions collected. Molecular standards are indicated in kilodaltons, and the volume of each fraction was 1 ml. (D) Fluorescence photographs showing the production of OMST from DMST by each fraction after Sephacryl S-300 gel filtration. The NEM-treated cytosol fraction was added into each reaction mixture containing an aliquot of each fraction.
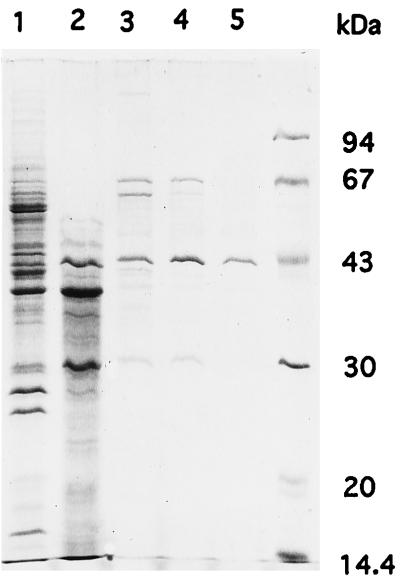
FIG. 3.
SDS-PAGE of MT-I. Pooled fractions following elution of MT-I were analyzed by SDS-PAGE on a 13% polyacrylamide gel, and polypeptide bands were stained with Coomassie brilliant blue R-250. Lane 1, pooled fraction after first phenyl-Sepharose fractionation; lane 2, pooled fraction after DEAE-Sepharose fractionation; lane 3, MT-I after second phenyl-Sepharose fractionation; lane 4, MT-I after Sephacryl S-300 pool; lane 5, flowthrough fraction (pure MT-I) from Matrex gel Green A. Molecular mass standards (Pharmacia LKB) were bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa).
A single peak of activity was recovered on the first phenyl-Sepharose column (Fig. 2A), and activity eluted close to 0 M (NH4)2SO4 during the gradient of (NH4)2SO4 concentration. Since MT-I activity was efficiently separated from pigments and other proteins, a band corresponding to the MT-I protein could be detected by SDS-PAGE analysis even after this first step (Fig. 3, lane 1). The pooled fraction was applied to DEAE-Sepharose, where high-molecular-weight proteins were effectively separated from MT-1 (Fig. 3, lane 2). The second phenyl-Sepharose step was very effective in reducing the number of contaminating proteins (Fig. 3, lane 3). The active fraction following concentration was loaded onto a Sephacryl S-300 column (Fig. 2C). A single activity peak showing brilliant yellow OMST fluorescence eluted at approximately 150 kDa (Fig. 2D). The absence of spots on parts corresponding to side fractions at this stage indicates that NEM-treated cytosol did not contain any MT-I activity. At this step, MT-I became a major band on SDS-PAGE (Fig. 3, lane 4). Enzyme fractions pooled after gel filtration chromatography were applied to a Matrex gel Green A column. Enzyme activity was recovered in flowthrough fractions, and we finally obtained 90 μg of purified MT-I.
Enzyme characterization.
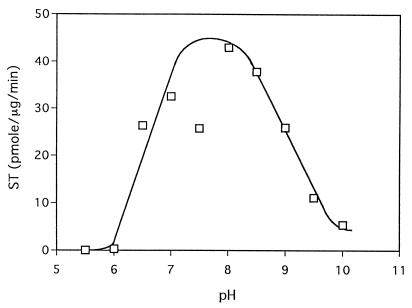
Purified MT-1 has a molecular mass of 43 kDa, as determined by SDS-PAGE (Fig. 3, lane 5). The molecular mass of the native enzyme was estimated to be 150 kDa (Fig. 2C). Preparatory isoelectric focusing between pH 3.5 and pH 10.0 showed MT-I activity pI to be 4.4. The MT-I pH profile showed broad activity between pH 6.5 and 9.0. No activity was detected below pH 5.5 (Fig. 4).
FIG. 4.
Effect of pH on enzyme activity. Production of ST from DMST was measured by using pure enzyme and different pH solutions.
The specific activity of the pure enzyme was 60.8 pmol of OMST/μg of protein/min when DMST was used as a substrate, and it was 43.1 pmol of DHOMST/μg/min when DHDMST was used. Recovery of activity was 1.5% for OMST production and 0.9% for DHOMST production.
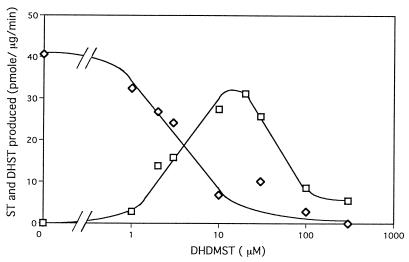
We conducted competition experiments between DMST and DHDMST for the purified enzyme to determine whether both DMST and DHDMST serve as substrates for the MT-I enzyme and then measured the amount of ST or DHST produced (Fig. 5). Only ST was produced from DMST in the absence of DHDMST, and ST production gradually decreased with increasing DHDMST concentration, whereas DHST production increased. These results demonstrate that DMST and DHDMST compete for the same substrate site on the same enzyme. Interestingly, DHDMST exceeding 100 μM inhibited enzyme activity.
FIG. 5.
Competition between DMST and DHDMST for MT-I enzyme. Pure MT-I enzyme was incubated in reaction mixtures containing 20 μM DMST and different DHDMST concentrations, and then reaction products ST (◊) and DHST (□) were measured as described in Materials and Methods.
Double-reciprocal plots of MT-I activity show that in the range of low substrate concentration, Km and Vmax of MT-I for DMST were estimated to be 0.94 μM and 78.1 pmol/μg of protein/min, while those for DHDMST were estimated to be 2.5 μM and 106.7 pmol/μg of protein/min. On the other hand, this enzyme was inhibited by higher concentrations of either DMST or DHDMST, and the apparent Kis of DMST and DHDMST were about 108 and 171 μM.
DISCUSSION
Enzyme purification requires simple and sensitive detection for the reaction product. However, reaction products of MT-I, ST and DHST, appear as dull or faint red or dark orange fluorescence on TLC plates under UV light. Spraying the TLC plate with an AlCl3 solution followed by heat treatment increases fluorescence intensity (25), but the sensitivity is still limited. Although HPLC analysis confirmed the presence of ST and DHST, measurement of activity for many fractions after every chromatography is very time-consuming compared to TLC analysis. Using radioactive tracers, e.g., S-adenosyl-[methyl-3H]methionine, increases the assay’s sensitivity (32), but even then, MT-I activity could not be completely separated from MT-II activity in the early stages of purification.
In contrast, OMST and DHOMST following ST and DHST in aflatoxin biosynthetic pathway (Fig. 1) show brilliant yellow fluorescence under UV light and can be easily detected on a TLC plate. Therefore, in this study, we used a coupled assay where we enzymatically changed the ST and DHST produced by MT-I to OMST and DHOMST, respectively, by routinely adding an NEM-treated cytosol fraction to the reaction mixture. MT-II activity could not be measured with this assay because DMST and DHDMST added to the reaction mixture were substrates for MT-I but not for MT-II. Unless ST or DHST was produced from either substrate by MT-I, neither OMST nor DHOMST formed even in the presence of MT-II. As shown in Fig. 2D, we could detect MT-I activity specifically by using TLC chromatography. Moreover, detection of OMST or DHOMST by the coupling assay confirmed that the protein purified was the MT-I enzyme catalyzing formation of ST or DHST. In this study, we observed that the peak fraction of MT-I activity differed from that of MT-II in either DEAE-Sepharose or isoelectric focusing chromatography, and MT-I activity was completely separated from MT-II in the isoelectric focusing (data not shown). These results further confirmed our previous finding that MT-I and MT-II are different enzymes (32).
Specific activity of MT-I generally increased as purification progressed (Table 1). However, it specifically decreased in the DEAE-Sepharose step compared to the first phenyl-Sepharose step. Total activity was markedly low in the DEAE step and then recovered to some extent in the second phenyl-Sepharose step. This result may indicate that an MT-I inhibitor or a DMST competitor may be concentrated together with this enzyme in the DEAE step, and this factor separated in the subsequent step.
Our previous study suggested that the same MT-I was involved in both DMST→ST and DHDMST→DHST conversions, since the two activities formed the same peak pattern through gel filtration of cytosol (32). As shown in Table 1, the similarity of enzyme activity between OMST and DHOMST production may indicate that the same enzyme is involved in both reactions. Moreover, the results from competition experiments between DMST and DHDMST for the purified enzyme demonstrate that DMST and DHDMST compete for the substrate site on the same enzyme (Fig. 5).
The molecular mass of the native enzyme under low-salt conditions, i.e., in solution A, was estimated to be 150 kDa (Fig. 2C). In contrast, we previously reported that MT-I in the cytosol fraction was about 210 kDa, using the HPLC gel filtration column. On the other hand, in different experiments, we also estimated the mass of the MT-I enzyme activity as about 90 kDa when the cytosol fraction was applied to an Ultrogel AcA 34 column in a buffer solution supplemented with 0.5 M KCl (data not shown). The difference in molecular mass of the native enzyme may reflect differences in gel filtration conditions. MT-I may aggregate to form a homopolymer in the low-salt solution, and the functional unit may be a homodimer under physical conditions. The detailed structure of the enzyme remains to be elucidated.
Aflatoxin production depends on the type of carbon source contained in the culture medium (1). We previously reported that most enzyme activities in this pathway, including MT-I, were dependent on the carbon source (29–32, 34). Recent molecular genetic analyses have shown that many of the genes involved in aflatoxin biosynthesis are clustered on one chromosome (8, 24, 28, 35), and it is generally accepted that transcriptional expression of the enzyme genes is commonly regulated by the aflR gene product, which is a transcriptional activator (10, 14, 24, 26). In the present study, however, we found that MT-I activity was regulated by the concentration of its substrate. Kinetic analysis of the enzyme as well as the results in Fig. 5 indicate that this enzyme has at least two substrate binding sites: a high-affinity catalytic substrate site and a low-affinity inhibitory site. This is the first study to show that aflatoxin production may be regulated at the enzyme level. Many intermediates in the aflatoxin pathway have been isolated from A. versicolor, and substrate inhibition may contribute to the presence of many intermediates of aflatoxins in the cell of this mold. The role of this inhibition in aflatoxin biosynthesis, however, requires further study.
Recently, Kelkar et al. reported that the A. nidulans stcP gene encodes a methyltransferase responsible for conversion of DMST to ST (16), and this A. nidulans gene may be a homolog of the MT-I gene in A. parasiticus. Cloning and characterization of the MT-I gene are in progress.
ACKNOWLEDGMENTS
We thank K. Kawai, Chukyo Women’s University, for advice on metabolite solubilization.
This work was supported in part by a grant-in-aid (Bio-Media Program) from the Ministry of Agriculture, Forestry and Fisheries (BMP 97-V-1-○3-4-4).
REFERENCES
- 1.Abdollahi A, Buchanan R L. Regulation of aflatoxin biosynthesis: induction of aflatoxin production by various carbohydrates. J Food Sci. 1981;46:633–635. [Google Scholar]
- 2.Bennett J W, Christensen S B. New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol. 1983;29:53–92. doi: 10.1016/s0065-2164(08)70354-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar D, Cleveland T E. Purification and characterization of a reductase from Aspergillus parasiticus SRRC 2043 involved in aflatoxin biosynthesis. FASEB J. 1990;4:2727. [Google Scholar]
- 4.Bhatnagar D, Cleveland T E, Kingston D G I. Enzymological evidence for separate pathways for aflatoxin B1 and B2 biosynthesis. Biochemistry. 1991;30:4343–4350. doi: 10.1021/bi00231a033. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar D, McCormick S P, Lee L S, Hill R A. Identification of O-methylsterigmatocystin as an aflatoxin B1 and G1 precursor in Aspergillus parasiticus. Appl Environ Microbiol. 1987;53:1028–1033. doi: 10.1128/aem.53.5.1028-1033.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar D, Ullah A H J, Cleveland T E. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep Biochem. 1988;18:321–349. doi: 10.1080/00327488808062532. [DOI] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang P K, Skory C S, Linz J E. Cloning of a gene associated with aflatoxin biosynthesis in Aspergillus parasiticus. Curr Genet. 1992;21:231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- 9.Chuturgoon A A, Dutton M F. The affinity purification and characterization of a dehydrogenase from Aspergillus parasiticus involved in aflatoxin B1 biosynthesis. Prep Biochem. 1991;21:125–140. doi: 10.1080/10826069108018008. [DOI] [PubMed] [Google Scholar]
- 10.Cleveland T E, Bhatnagar D. Evidence for de novo synthesis of an aflatoxin pathway methyltransferase near the cessation of active growth and the onset of aflatoxin biosynthesis in Aspergillus parasiticus mycelia. Can J Microbiol. 1990;36:1–5. doi: 10.1139/m90-001. [DOI] [PubMed] [Google Scholar]
- 11.Cole R J, Cox R H. Handbook of toxic fungal metabolites. New York, N.Y: Academic Press, Inc.; 1981. pp. 67–93. [Google Scholar]
- 12.Dutton M F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988;52:274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvorackova I. Aflatoxins and human health. Boca Raton, Fla: CRC Press; 1990. [Google Scholar]
- 14.Feng G H, Chu F S, Leonard T J. Molecular cloning of genes related to aflatoxin biosynthesis by differential screening. Appl Environ Microbiol. 1992;58:455–460. doi: 10.1128/aem.58.2.455-460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto T, Wicklow D T, Ito Y. Aflatoxin and cycropiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl Environ Microbiol. 1996;62:4036–4038. doi: 10.1128/aem.62.11.4036-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelkar H S, Keller N P, Adams T H. Aspergillus nidulans stcP encodes an O-methyltransferase that is required for sterigmatocystin biosynthesis. Appl Environ Microbiol. 1996;62:4296–4298. doi: 10.1128/aem.62.11.4296-4298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller N P, Dischinger H C, Jr, Bhatnagar D, Cleveland T E, Ullah A H J. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl Environ Microbiol. 1992;59:479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtzman C P, Horn B W, Hesseltine C W. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Leeuwenhoek. 1987;53:147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- 19.Kusumoto K, Hsieh D P H. Purification and characterization of the esterase involved in aflatoxin biosynthesis in Aspergillus parasiticus. Can J Microbiol. 1996;42:804–810. doi: 10.1139/m96-101. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lin B-K, Anderson J A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch Biochem Biophys. 1992;293:67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima K-I, Ando Y, Hamasaki T, Yabe K. Purification and characterization of two versiconal hemiacetal acetate reductases involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1994;60:2561–2567. doi: 10.1128/aem.60.7.2561-2567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire G M, Brobst S W, Graybill T L, Pal K, Townsend C A. Partitioning of tetrahydro- and dihydrobisfuran formation in aflatoxin biosynthesis defined by cell-free and direct incorporation experiments. J Am Chem Soc. 1989;111:8308–8309. [Google Scholar]
- 24.Payne G A, Nystrom G J, Bhatnagar D, Cleveland T E, Woloshuk C P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl Environ Microbiol. 1993;59:156–162. doi: 10.1128/aem.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott P M. AOAC official method 973.38, sterigmatocystin in barley and wheat, chapter 49. In: Cunniff P, editor. Official methods of analysis of AOAC International. Baltimore, Md: AOAC International; 1995. pp. 44A–44B. [Google Scholar]
- 26.Skory C D, Chang P-K, Linz J E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend C A, McGuire S M, Brobst S W, Graybill T L, Pal K, Barry C E., III . Examination of tetrahydro- and dihydrobisfuran formation in aflatoxin biosynthesis: from whole cells to purified enzymes. In: Petroski R J, McCormick S P, editors. Secondary-metabolite biosynthesis and metabolism. New York, N.Y: Plenum Press, Inc.; 1991. pp. 141–154. [Google Scholar]
- 28.Trail F, Mahanti N, Linz J. Molecular biology of aflatoxin biosynthesis. Microbiology. 1995;141:755–765. doi: 10.1099/13500872-141-4-755. [DOI] [PubMed] [Google Scholar]
- 29.Yabe K, Ando Y, Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl Environ Microbiol. 1988;54:2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabe K, Ando Y, Hamasaki T. Desaturase activity in the branching step between aflatoxins B1 and G1 and aflatoxins B2 and G2. Agric Biol Chem. 1991;55:1907–1911. [Google Scholar]
- 31.Yabe K, Ando Y, Hamasaki T. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J Gen Microbiol. 1991;137:2469–2475. doi: 10.1099/00221287-137-10-2469. [DOI] [PubMed] [Google Scholar]
- 32.Yabe K, Ando Y, Hashimoto J, Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989;55:2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yabe K, Nakamura H, Ando Y, Terakado N, Nakajima H, Hamasaki T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl Environ Microbiol. 1988;54:2096–2100. doi: 10.1128/aem.54.8.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yabe K, Nakamura N, Nakajima H, Ando Y, Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 1991;57:1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Chang P-K, Cary J W, Wright M, Bhatnagar D, Cleveland T E, Payne G A, Linz J E. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl Environ Microbiol. 1995;61:2365–2371. doi: 10.1128/aem.61.6.2365-2371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]