Abstract
Isoprene (2-methyl-1,3 butadiene) is a low-molecular-weight hydrocarbon emitted in large quantities to the atmosphere by vegetation and plays a large role in regulating atmospheric chemistry. Until now, the atmosphere has been considered the only significant sink for isoprene. However, in this study we performed both in situ and in vitro experiments with soil from a temperate forest near Ithaca, N.Y., that indicate that the soil provides a sink for atmospheric isoprene and that the consumption of isoprene is carried out by microorganisms. Consumption occurred rapidly in field chambers (672.60 ± 30.12 to 2,718.36 ± 86.40 pmol gdw−1 day−1) (gdw is grams [dry weight] of soil; values are means ± standard deviations). Subsequent laboratory experiments confirmed that isoprene loss was due to biological processes: consumption was stopped by autoclaving the soil; consumption rates increased with repeated exposure to isoprene; and consumption showed a temperature response consistent with biological activity (with an optimum temperature of 30°C). Isoprene consumption was diminished under low oxygen conditions (120 ± 7.44 versus 528.36 ± 7.68 pmol gdw−1 day−1 under ambient O2 concentrations) and showed a strong relationship with soil moisture. Isoprene-degrading microorganisms were isolated from the site, and abundance was calculated as 5.8 × 105 ± 3.2 × 105 cells gdw−1. Our results indicate that soil may provide a significant biological sink for atmospheric isoprene.
Isoprene (2-methyl-1,3 butadiene [C5H8]), is a low-molecular-weight alkene hydrocarbon emitted to the atmosphere by many plant species (13, 16, 18). Isoprene emission by vegetation is estimated at 5 × 1014 g year−1, an amount comparable to annual global methane (CH4) emissions (6, 17, 18, 22, 27). Atmospheric isoprene strongly influences atmospheric chemistry (14). In atmospheres enriched in nitrogen oxides (NOx; NOx = NO, NO2), reactions with nonmethane hydrocarbons (of which isoprene is the dominant biogenic species) leads to the production of ozone via secondary photochemical oxidation reactions (14, 15). In addition, isoprene oxidation in the atmosphere produces carbon monoxide (CO) (7, 16). Therefore, there is considerable interest in identifying all potential sources and sinks for the hydrocarbon.
One significant sink for many atmospheric trace gases (e.g., H2, CO, CH4, OCS, NO, and N2O) is soil. These gases serve as electron donors in oxidation reactions and/or as an energy source for microorganisms, which help moderate the chemical composition of the atmosphere (9). For example, Bender and Conrad showed that the oxidation of atmospheric methane in soil is a significant portion of the global CH4 budget, amounting to 5 to 20% of the total global CH4 sink (5). Similarly, Shorter et al. showed that soil microorganisms strongly influence atmospheric methyl bromide (CH3Br) concentrations by acting as a sink (33). Although ambient concentrations of CH3Br are ∼1,000 times less than isoprene concentrations, the amount of this compound consumed by soil is estimated at 4.2 × 1010 g year−1 (33). These results demonstrate the ability of microorganisms to consume atmospheric trace gases at extremely low concentrations. Because atmospheric isoprene concentrations, which typically range from 0.5 to 10 ppb (4, 11), are high relative to methyl bromide concentrations, this suggests the possibility of a potentially significant isoprene sink in soil.
The possibility of a biological sink for isoprene in soil has never been investigated. Van Ginkel et al. showed that pure cultures of a Nocardia sp. were able to degrade isoprene and to use the compound as their sole carbon and energy source, but the intricacies and potential significance of the process were not explored (35). Additionally, Hou et al. suggested that propene-grown Xanthobacter spp. and methanotrophs were able to oxidize isoprene but that it did not serve as the sole carbon and energy source (21). However, these studies offer very little insight into the importance of isoprene consumption in situ and provide little information on the physiological controls on the process. In this paper we describe the results of both in situ and in vitro investigations of isoprene consumption by soil microorganisms in a temperate forest soil. Our findings suggest that microorganisms consume isoprene even at trace-level concentrations and that soil microorganisms may provide a significant, previously undocumented biological sink for atmospheric isoprene.
MATERIALS AND METHODS
We measured isoprene consumption in soil from McGowan Woodlot, a 24-acre temperate mixed-hardwoods forest located near Ithaca, N.Y. (42°25′N, 76°28′W). The soil at McGowan Woodlot is classified as a mesic Glossoboric Hapludalf (Alfisol) with a pH of 5.8 to 6.0. Soil organic matter content ranges from 9.3% in the 0- to 3-cm depth interval (from horizon) to 6.5% in the 9- to 12-cm depth interval (from horizon). The soil has an average bulk density of 1.18 g cm−3 and a total porosity of 57%.
We measured isoprene flux in the field using semitransparant polyethelene static chambers. The circular chambers had a diameter of 18 cm and a total volume of 1.4 liters. Each chamber was fitted with a butyl-rubber septum for gas sampling. On each visit, we removed leaves from the soil surface, placed the five chambers randomly on the soil to a depth of ∼3 cm, and packed soil around the outside of each chamber to prevent leakage. On each visit, all chambers were placed within an area of 100 m2. An aliquot of an isoprene standard was injected into each chamber and was mixed thoroughly, and we then collected gas samples in 1-ml glass, gastight syringes every 15 min for 1 h. In most cases, the initial headspace concentration was ∼385 ppb. Measurements were made in the same general area on each visit, but we repositioned chambers to avoid previously disturbed locations. Standards were also prepared in the field and sampled at the beginning and end of each experiment. This was done to verify that the removal of isoprene in the chambers was not due to chemical reactions with oxidizing radicals in the atmosphere. We reasoned that if concentrations in the standards (which are presumably influenced only by chemical oxidations in the atmosphere of the standard vessels) did not decrease over the course of each experiment then we could conclude that chemical oxidation in the atmospheres of the chambers was negligible. In addition, on each visit we measured soil temperature to a depth of 5 cm with a thermistor and we measured soil moisture gravimetrically. Sampling occurred on five dates during the summer of 1996 (6 June, 12 June, 22 July, 12 September, and 1 October), and gas samples were analyzed within 2 h of collection.
Soil cores 15 to 18 cm in length were collected from the McGowan Woodlot site in polyvinyl chloride tubes (inside diameter, 10 cm; wall thickness, 4 mm) by removing the forest litter, cutting around the perimeter with a knife, and tapping the tube with a rubber mallet. After extraction, the top and bottom of each core was sealed with a rubber cap: the top cap had a butyl-rubber septum for gas sampling. A headspace of ∼785 cm3 was left after sealing the tubes. Repeat additions of isoprene were made at different times to the closed vessels to achieve final concentrations of 100 to 650 ppb, and cores were incubated in the dark at 25°C. We collected gas samples from the headspace every 15 min until the isoprene concentration was <5 ppb. Vessels were then opened to reaerate the headspaces, and the experiment was repeated. Experiments were initiated within 2 h of collection of the soil cores.
Bulk soil samples were obtained at 3-cm intervals to a depth of 12 cm from McGowan Woodlot. Bulk soil samples were brought to the laboratory, all stones and plant material were removed, and soils were sieved to aggregate sizes of <4 mm. Following collection, 30- to 35-g samples of each soil were placed in 1-liter mason jars to provide a soil layer of ∼0.5 cm. Soil water content was adjusted to 40% (dry weight), vessels were sealed, and initial isoprene headspace concentrations were adjusted to 508 ppb. Jar lids had a butyl-rubber septum for gas sampling and were sampled for 12 h to determine the time course of isoprene consumption in soil. Unless otherwise indicated, soil incubations were performed in the dark at 25°C. To verify biological rather than physical consumption of isoprene, a set of soil samples were autoclaved three times for 1 h at 120°C and 0.10 MPa (15 lb/in−2), and sterile, distilled water was added to restore the desired soil moisture level. Blanks (no soil) were also included in each assay to verify that atmospheric oxidation within the jars was not responsible for declines in isoprene headspace concentrations and to confirm that the experimental setup was not prone to leakage. In all cases, experiments were performed within 72 h of soil collection.
Isoprene consumption as a function of soil temperature.
The effect of temperature on isoprene consumption was determined by preincubating fresh soil from McGowan Woodlot at treatment temperatures for 24 h (40% soil moisture [SM]) to ensure that the samples were at the desired temperature at the time of the experiment. The vessels were then injected with an aliquot of isoprene standard, and different samples were incubated for 12 h at 5, 15, 25, 30, 35, 40, and 50°C in temperature incubators or in controlled temperature water baths. Headspace isoprene concentrations were sampled every 3 h to determine isoprene consumption.
Isoprene consumption as a function of SM.
The effect of SM content on isoprene consumption was determined by using freshly collected soil samples from McGowan Woodlot (40% SM [fresh], sieved to aggregate sizes of <4 mm). SM contents of 5, 10, 25, 40, 60, 75, and 100% were obtained by air drying excessively moist soils or by adding a mist of sterile, deionized water with a spray bottle to dry samples. Samples were acclimated for 24 h to the altered SM content, vessels were sealed and incubated at 25°C with an isoprene headspace concentration of 508 ppb, and headspaces were samples at 3-h intervals for 12 h to determine the time course of isoprene consumption.
Effect of oxygen concentration on isoprene consumption in soil.
To determine whether the consumption of isoprene in soil is an aerobic process (i.e., the microorganisms responsible for isoprene consumption require oxygen to mineralize isoprene), incubations were performed in vessels which had been purged of headspace oxygen (O2). Fresh soil from McGowan Woodlot was placed in vessels, which were sealed and from which oxygen was removed with a vacuum pump equipped with a needle to penetrate the septa on the jars. Pressure inside the vessels was brought to −0.3 MPa for 2 min, and vessel headspaces were then filled to standard pressure with high purity N2 (99.99%). The process was repeated three times before the headspace isoprene concentration was amended by using the premixed isoprene standard. Soils were incubated for 12 h, and headspace isoprene concentration was sampled every 3 h to determine isoprene consumption.
Gas analysis.
Isoprene concentration was measured on a gas chromatograph (model 3400; Varian Chromatography Systems, Walnut Creek, Calif.) equipped with a photoionization detector and a 50-m fused silica column (Megabore SPB-1, 5-μm coating; Supelco, Inc., Supelco Park, Pa.). The carrier gas was highly purified helium (99.99%). Gas samples were injected on-column by splitless injection. Column temperature was 55°C, and the retention time of the isoprene was 4.5 min with a carrier gas linear velocity of 18.5 cm s−1. Isoprene concentration was determined by peak area measured with a peak integrator (Shimadzu, model CR 501). The gas chromatograph had a detection limit of 5 ± 1 ppb and was calibrated daily by using premixed standards.
Isolation of isoprene-degrading microorganisms.
Soil samples were collected from the top 1 cm of the soil profile at McGowan Woodlot. Following collection, serial dilutions of samples were performed according to the procedure described by Madsen (25), and dilutions were streaked onto agar plates prepared with Stanier’s Basal Salt Medium (34). Plates were then divided into two groups and transferred to airtight, stainless steel containers. One container was equipped with a glass test tube containing 0.5 ml of 99.9% pure liquid isoprene, which volatilized to provide a carbon substrate in an oxic headspace. Control plates that were inoculated but lacked isoprene added as a growth substrate were incubated in a separate vessel. Plates were incubated at 25°C in the dark and checked every 3 days until large, prolific colonies were obvious on plates incubated with volatilized isoprene in the headspace compared to the growth on control plates (25). Isolated colonies were quantified according to Madsen (25).
RESULTS
Isoprene flux in the field occurred rapidly to concentrations below our detection limit within 1 h. Reaction rate constants (k) of isoprene in the chambers ranged from −0.012 ± 0.006 min−1 to −0.048 ± 0.002 min−1 with consumption rates ranging from 672.60 ± 30.12 to 2,718.36 ± 86.40 pmol gdw−1 day−1 (gdw is g [dry weight] of soil; values are means ± standard deviations) (Table 1). The diffusive loss rate of an inert tracer (CH3F) was measured at −0.0016 min−1, indicating that diffusion alone was not responsible for isoprene loss in the chambers. Flux rates increased gradually through the summer, with the highest values in late summer (12 September) and values decreasing on the final sampling date (1 October) following leaf senescence (Table 1).
TABLE 1.
In situ mean (± SD) uptake rate constants (k) and isoprene flux rates from static field chambers (n = 5) in McGowan Woodlot during the summer of 1996
| Date (mo/day/yr) | k (min−1) | Isoprene flux rate (pmol gdw−1 day−1) | Soil temp (°C) | SM (% dry weight) |
|---|---|---|---|---|
| 6/6/96 | −0.012 ± 0.006 | 672.60 ± 30.12 | 14.98 | 53.67 |
| 6/12/96 | −0.039 ± 0.002 | 2,192.76 ± 106.20 | 18.32 | 39.49 |
| 7/22/96 | −0.027 ± 0.004 | 1,516.32 ± 247.80 | 16.43 | 47.95 |
| 9/12/96 | −0.048 ± 0.002 | 2,718.36 ± 86.40 | 17.76 | 27.86 |
| 10/1/96 | −0.037 ± 0.005 | 2,072.76 ± 184.44 | 13.50 | 44.80 |
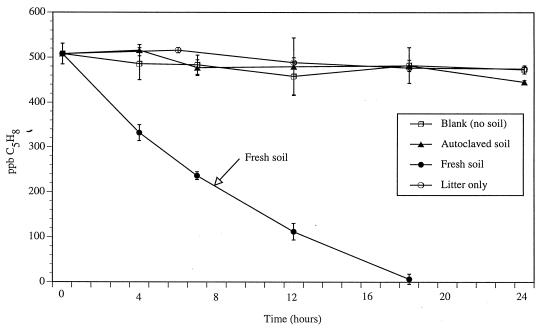
Laboratory studies indicated that isoprene consumption was biologically mediated: after a 24-h incubation, sterile soil consumed <5% of the isoprene added to the headspace, while >95% of the isoprene added to blanks (no soil) was detectable 24 h later (Fig. 1). In addition, assays performed with leaves only (no soil) showed that consumption of isoprene in the litter layer was negligible (Fig. 1). The results with the experimental controls contrast with results observed with nonsterile soil samples, which showed a linear decrease in isoprene headspace concentration (r2 = 0.96) until concentrations decreased below the detection limit 18 h after the experiment began (Fig. 1).
FIG. 1.
Time courses of isoprene consumption in 30-g samples of fresh soil, autoclaved (sterile) soil, leaf litter, and blank (no soil) incubations. Error bars represent ±1 standard deviation from the means of triplicate samples.
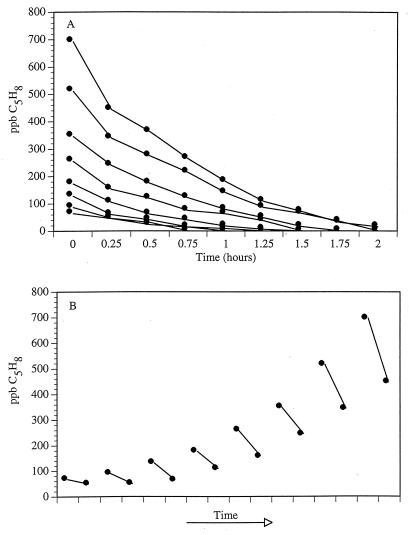
Isoprene concentrations in the core segment headspaces were always depleted within 2 h (Fig. 2A). Soil cores consumed isoprene over a sevenfold concentration range with no lag in response regardless of initial isoprene concentration. At all concentrations of isoprene tested the logarithm of the concentration decreased linearly as a function of time (i.e., isoprene consumption in cores was a first-order reaction) (31, 36). The two cores also showed an increasing propensity for isoprene consumption as each series of experiments progressed. In the first experiment, isoprene concentrations decreased from 86.53 to 45.75 ppb and from 63.98 to 45.26 ppb in 0.25 h in cores 1 and 2, respectively. However, after being exposed to isoprene of various concentrations, isoprene concentrations decreased from 692.24 to 442.82 ppb and from 511.8 to 338.65 ppb in 0.25 h in cores 1 and 2, respectively, several hours later (Fig. 2B). The initial depletion rate curves for the two cores show that after exposure to isoprene, the subsequent treatment with a higher isoprene concentration resulted in a more rapid removal of isoprene than in the previous experiment.
FIG. 2.
(A) Time courses of isoprene consumption in whole-soil cores exposed to different initial isoprene headspace concentrations. (B) Enrichment of soil microorganisms with increasing isoprene concentrations resulting in an acceleration of the rate of isoprene consumption.
We found that consumption rates decreased monotonically with increasing soil depth (Table 2). Consumption of isoprene was most rapid at the surface (0- to 3-cm) soil layer (585.48 ± 6.94 pmol gdw−1 day−1), while the other samples from lower profiles showed lower rates of consumption. A Tukey’s test of multiple comparisons showed that consumption was statistically significantly higher in the 0- to 3-cm sample than in the 6- to 9-cm sample (t20 = 2.76; P = 0.01) or the 9- to 12-cm sample (t20 = 2.74; P = 0.01). However, the slopes of the regression lines for the 0- to 3-cm and 3- to 6-cm samples were not statistically different (t20 = 1.09; P = 0.3).
TABLE 2.
Mean isoprene consumption rates from four depth intervals incubated in the laboratory at 25°C
| Depth interval (cm below surface) | Consumption rate (pmol gdw−1 day−1)a | SM (% dry weight) | LOI (%)b | pH |
|---|---|---|---|---|
| 0–3 | 585.48 ± 6.94 | 35.15 | 6.54 | 5.63 |
| 3–6 | 468.41 ± 20.88 | 37.83 | 7.04 | 5.60 |
| 6–9 | 394.05 ± 5.71 | 44.59 | 8.43 | 6.30 |
| 9–12 | 378.81 ± 11.80 | 49.87 | 8.98 | 6.33 |
Values are means ± standard deviations for four replicate experiments at each depth interval.
LOI, loss on ignition, determined by dry combustion at 625°C for 6 h, to estimate soil organic matter content.
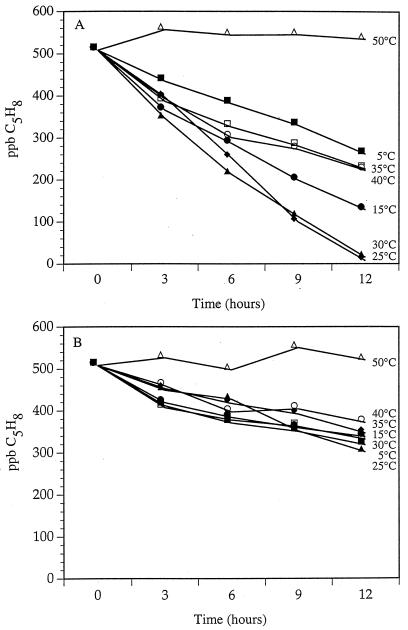
Time courses of isoprene consumption (0- to 3-cm depth interval) at a wide range of temperatures are presented in Fig. 3A. For all treatments (except blanks), the decrease in isoprene in the headspace was linear; blanks showed no measurable isoprene loss. Multiple comparisons revealed that consumption was significantly different at each temperature, with the exception of the rates at 25 and 30°C, for which regression analysis revealed no difference (t16 = 1.00; P = 0.34). Linear regression showed significant, negative slopes at all temperatures except for 50°C, at which temperature the consumption rate was not significantly different from zero (F = 0.34; P = 0.575). For the 0- to 3-cm depth interval, rates of isoprene consumption were not significantly different at 25 and 30°C (t16 = 1.00; P = 0.34) but were significantly higher than rates at all other temperatures at α = 0.05.
FIG. 3.
Time courses of isoprene consumption in duplicate 30-g soil samples from 0- to 3-cm (A) and 15- to 18-cm (B) layers of a temperate forest soil incubated at different temperatures.
Contrary to the results obtained with soil from the 0- to 3-cm horizon, temperature variability had no significant effect on the consumption of isoprene in the 15- to 18-cm layer (Fig. 3B). Rates of isoprene consumption were not statistically different at any temperature except for 50°C, at which temperature the slope of the line was not statistically different from zero (F = 0.55; P = 0.51). Isoprene consumption rates for the 15- to 18-cm samples were significantly lower at all temperatures than the lowest rate observed for the 0- to 3-cm samples (i.e., at 4°C).
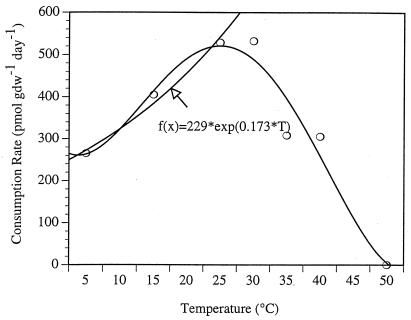
Consumption rates at each temperature were plotted to depict the optimum temperature and to estimate the Q10 for isoprene consumption (Fig. 4). Our empirical data showed an optimum temperature of 30°C for isoprene consumption. Within a temperature range of 5 to 25°C, isoprene consumption rates increased exponentially (r2 = 0.98) and gave an average Q10 of 1.42 (Q10 = [KT + 10]/KT, where KT + 10 and KT denote rate constants at temperatures T + 10 and T, respectively). The Arrhenius relationship was also applied to the data between 5 and 25°C, and the activation energy (Ea) of the reaction was calculated at 20.0 kJ mol−1. At temperatures of >30°C, consumption rates declined, and they became negligible at 50°C, as depicted in Fig. 4.
FIG. 4.
Isoprene consumption rate versus temperature. An exponential (n = 4) function is fit to the data from 5°C to 30° C shown in Fig. 3A. The curve fit to all data points merely illustrates the increase in isoprene consumption rates between 5°C and 30°C, followed by a rapid decrease at higher temperatures.
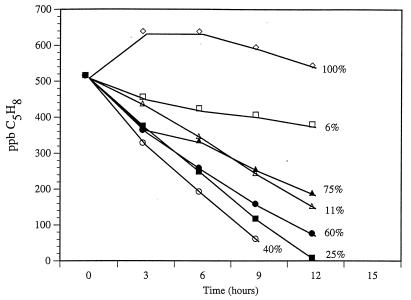
Time courses for isoprene consumption at different SMs are presented in Fig. 5. Isoprene consumption was highest at SM contents of 25 and 40% (40% was the SM of the freshly collected soil). All treatments below 100% SM showed linear decreases in headspace isoprene concentration over 12 h, indicating that isoprene consumption was moisture dependent. At 100% SM, the slope of the line was not significantly different from zero (F = 0.01; P = 0.92). Multiple comparisons revealed that consumption at 10 and 75% SM were not significantly different (t16 = 1.58; P = 0.14), and rates at 25 and 40% SM were not significantly different (t16 = 0.08; P > 0.5). The rate was lowest at 5% SM (Fig. 5).
FIG. 5.
Time courses of isoprene consumption in duplicate 30-g surface (0- to 3-cm) soil samples from a temperate forest incubated in the laboratory with different soil moisture contents.
Isoprene consumption was diminished by ∼80% in jars purged of headspace oxygen. Rates of consumption in jars with anoxic headspaces were 120.00 ± 7.44 pmol gdw−1 day−1 versus a rate of 528.36 ± 7.68 pmol gdw−1 day−1 in jars incubated with air containing ambient atmospheric oxygen. Under ambient O2 conditions, consumption of isoprene was linear (r2 = 0.96), but under anoxic headspace conditions, an exponential curve fit the data best (r2 = 0.94).
We were able to isolate and enumerate isoprene-degrading microorganisms from fresh soil samples from McGowan Woodlot: large (diameter, 1 to 2 mm) colonies formed 2 to 3 weeks after incubations began. Two distinct colony types were observed on the test plates with isoprene as the sole carbon substrate, and these types were absent from control plates not exposed to isoprene vapor. The abundance of isoprene-degrading microorganisms in surface soil at the temperature forest site was calculated as 5.8 × 105 ± 3.2 × 105 cells gdw−1 (based on colony counts from four replicate plates at a dilution of 10−2).
DISCUSSION
Experiments with static chambers in the field suggest that soil can consume atmospheric isoprene at high rates. On all dates sampled, isoprene was rapidly and irreversibly removed from the chamber headspaces to lower than ambient concentrations (i.e., <10 ppb) within 1 h. Standards prepared and sampled in the field showed no detectable change in isoprene consumption over the course of each experiment, indicating that isoprene loss was not due to chemical oxidation with OH in the chambers. An inert tracer (CH3F) was also added to the chambers to determine the diffusive loss rate into the soil. Uptake rates for isoprene were all more than 10 times faster than the diffusive loss rates of the tracer, providing direct evidence that the soil at McGowan Woodlot actively consumes isoprene from the chamber headspaces and that diffusion alone is not responsible for the decrease.
Results of experiments with the intact soil cores from McGowan Woodlot also suggest that isoprene uptake is microbially mediated. Initially, with low concentrations of isoprene, consumption rates were relatively low. However, as isoprene concentrations were increased in successive experiments, rates of isoprene consumption also increased. This gradual increase in the consumption rates in the two cores in the sequence of experiments may reflect growth of microorganisms which followed exposure to isoprene, and the acceleration of consumption rates after repeated exposure to isoprene suggests that isoprene consumption is a growth-linked physiological process. This is consistent with the phenomenon of enrichment observed in the microbial degradation of herbicides (1). Enrichment is an increase in the number and/or the activity of the microorganisms metabolizing a particular compound following the addition of that compound to the soil (1). Isoprene additions in the first experiment and each successive experiment may have enriched the microbial community consuming isoprene, and the excess substrate may have allowed the isoprene-degrading population to grow in number. This growth would result in higher consumption rates in each successive experiment and would suggest that the decline in isoprene in the intact cores was due to microbial consumption (3).
Isoprene consumption was stopped by autoclaving the soil, further indicating that the uptake of isoprene by soil is a biological process. This also demonstrates that physical sorption to soil particles, container surfaces, or sampling components are negligible processes (33) and that the uptake of isoprene is not the result of a chemical transformation in the soil. Results of incubations with room air and isoprene in the headspace revealed no loss of isoprene, suggesting that chemical reactions with oxidative species in the atmosphere of the jars was not responsible for isoprene removal. In spite of the fact that isoprene has a reported atmospheric lifetime of 1.9 h in the troposphere in full sunlight (15), our experimental setup minimized isoprene loss due to reaction with oxidizing radicals over the course of the experiment.
Results of experiments with soil from different depth intervals revealed that isoprene consumption is most rapid at the surface (0- to 3-cm) layer and declines through the soil profile. At a depth of 12 cm, the isoprene consumption rate is 35% lower than the rate at 0 to 3 cm. There are several possible reasons for these patterns as a function of depth. For example, consumption showed positive correlations with soil organic matter (r = 0.913) and SM at each depth (r = 0.95) and a negative correlation with soil pH (r = −0.83), all of which varied through the soil profile. Next, since isoprene is produced in chloroplasts and emitted through the stomata of plants during photosynthesis, the primary source of isoprene in forests is the plant canopy (32). Thus, the highest concentrations of isoprene would be most accessible to microorganisms living at the soil surface. Finally, soil samples often reveal that microorganisms are most abundant at the surface and that numbers decline with depth (30). However, the consumption of isoprene at lower depths suggests that isoprene may diffuse into the soil beyond the surface layer and be consumed by microorganisms living well below the soil surface. Notably, the soil at McGowan Woodlot is well aerated (57% total pore space), and bulk soil analysis revealed the presence of earthworms throughout the soil profile; thus, it is likely that isoprene may diffuse well into the soil profile (30).
Isoprene consumption was linear at incubation temperatures of 5 to 50°C, indicating that isoprene consumption in soil is temperature dependent. Between 5 and 25°C, the average Q10 of 1.42 was similar to Q10 values obtained in studies of other microbial processes, such as soil respiration and CH4 consumption by soil (24, 28, 37), and similar to the Q10 values reported for many common microbial enzymes (2). The optimum temperature for isoprene consumption (30°C) was similar to those reported for many common genera of soil bacteria such as Arthrobacter, Pseudomonas, Streptomyces, and Bacillus (20). It is extremely unlikely that a nonbiological process would exhibit a maximum rate between 5 and 30°C (26). This indicates that the agents responsible for isoprene decline are microbial.
The lack of a temperature effect on isoprene consumption in subsoil (15 to 18 cm) is noteworthy because (i) many biological soil processes are strongly influenced by soil temperature (24, 30, 33, 37) and (ii) consumption of isoprene in the 0- to 3-cm soil horizon was highly temperature dependent. The reason for this result is not immediately clear, and any attempt at providing one would be speculative. However, there are examples of other biological processes that are relatively insensitive to temperature. For instance, methane oxidation in soil has been shown to be much less temperature sensitive than many other biological processes, owing to the depth distribution of methane consumption and the transport mechanism (12, 23). Nonetheless, the reason for the discrepancy between the effects of temperature on isoprene consumption in the 0- to 3-cm soil profile and the 15- to 18-cm soil profile that we observed is uncertain.
Laboratory incubations suggest a close link between SM and isoprene consumption in soil. Adjusting soil water content to between 25 and 60% did not significantly affect the rate of isoprene consumption. However, increasing SM to 75% decreased consumption to 64% of the maximum rate, and increasing SM to 100% resulted in no consumption of isoprene in laboratory incubations. It is possible that diffusion of isoprene into soil is diminished when the soil is saturated, thus limiting isoprene consumption. In studies involving CH4 consumption in soil, Whalen et al. found that a change from the gas phase to aqueous molecular diffusion resulted in a 104-fold less rapid rate of methane transport to cells (37). On the other hand, soil drought diminishes microbial activity by leading to cell desiccation of the soil microbial community and hence a decrease in microbial activity (30).
Incubations of soil under low oxygen concentrations suggest that the consumption of isoprene by soil is an aerobic process. Isoprene consumption rates decreased by 80% under low O2 conditions, and the rate of isoprene consumption declined over the course of the experiment. This decline, coinciding with a decrease in oxygen, is evidence that the organisms degrading isoprene are aerobic. The diminished rate of consumption observed under low O2 conditions implies that some microsites in the soil aggregates may have retained enough oxygen to allow aerobic metabolism to occur or that perhaps other anaerobic reactions may have contributed to the biological degradation of isoprene under low O2 concentrations (29). Harder and Probian found that microorganisms were able to oxidize many organic hydrocarbons anaerobically, including the acyclic monoterpenes and the mono- and bicyclic monoterpenes (19). Isoprene consumption under both high O2 and low O2 conditions may suggest that at least two different organisms may be capable of isoprene consumption in soil, although a more rigorous test of anaerobic metabolism would be required to verify this. However, since the rate of isoprene consumption began to decline over the course of the experiment, this suggests that microsites contained available O2 at the beginning of the experiment, but as this microsite oxygen became scarce, microorganisms could no longer consume isoprene.
Isoprene-degrading microorganisms were abundant in soil sampled from McGowan Woodlot, and preliminary evidence suggests they belong to the genus Arthrobacter, a heterogeneous group of soil bacteria with considerable nutritional versatility (8a). Few previous studies of the distribution and abundance of isoprene-biodegrading microorganisms have been undertaken (35). However, it has been shown in studies of other atmospheric trace gases that degradation by microbial cultures in vitro corroborates in situ field studies of processes which are globally significant (10, 29). The facts that (i) isoprene is a naturally abundant, highly reduced organic compound, (ii) microorganisms have been isolated from soil samples which can utilize the compound as their sole carbon and energy source, and (iii) isoprene consumption occurs in soil from a number of different ecosystems worldwide (8) all suggest that consumption of atmospheric isoprene by soil microorganisms may be a significant component of the global isoprene budget.
ACKNOWLEDGMENTS
This research was supported in part by an Andrew W. Mellon Student Research Grant from Cornell University and by a Kieckhefer Fellowship from Cornell University.
This work also benefited from the insight provided by Michael Keller and Dan Miller, from Eugene Madsen and Tim Fahey, and from two anonymous reviewers who provided valuable comments.
REFERENCES
- 1.Ashton F M. Persistence and biodegradation of herbicides. In: Matsumura F M, Krishna Murti C R, editors. Biodegradation of pesticides. New York, N.Y: Plenum Press; 1982. [Google Scholar]
- 2.Atlas R M, Bartha R. Microbial ecology. Redwood City, Calif: The Benjamin/Cummings Publishing Company; 1993. [Google Scholar]
- 3.Audus L J. Herbicide behavior in the soil. In: Audus L J, editor. The physiology and biochemistry of herbicides. New York, N.Y: Academic Press; 1964. [Google Scholar]
- 4.Baldocci D, Guenther A, Harley P, Klinger L, Zimmerman P, Lamb B, Westberg H. The fluxes and air chemistry of isoprene above a deciduous hardwood forest. Philos Trans R Soc London. 1995;350:279–296. [Google Scholar]
- 5.Bender M, Conrad R. Kinetics of methane oxidation in oxic soils. Chemosphere. 1993;26:687–696. [Google Scholar]
- 6.Brasseur G P, Chatfield R B. The fate of biogenic trace gases in the atmosphere. In: Sharkey T D, Holland E A, Mooney H A, editors. Trace gas emissions by plants. New York, N.Y: Academic Press; 1991. [Google Scholar]
- 7.Cicerone R J. Fires, atmospheric chemistry and the ozone layer. Science. 1994;263:1243–1244. doi: 10.1126/science.263.5151.1243. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland C C, Yavitt J B. Consumption of atmospheric isoprene in soil. Geophys Res Lett. 1997;24:2379–2382. [Google Scholar]
- 8a.Cleveland C C. Consumption of atmospheric isoprene in soil. M.S. Thesis. Ithaca, N.Y: Cornell University; 1997. [Google Scholar]
- 9.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad R, Meyer O, Seiler W. Role of carboxydobacteria in consumption of atmospheric carbon monoxide in soil. Appl Environ Microbiol. 1981;42:211–215. doi: 10.1128/aem.42.2.211-215.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoso L, Romero R, Rondón A, Fernandez E, Oyola P, Sanhueza E. Natural and anthropogenic C2 to C6 hydrocarbons in the central-eastern Venezuelan atmosphere during the rainy season. J Atmos Chem. 1996;25:201–214. [Google Scholar]
- 12.Dunfield P, Knowles R, Dumont R, Moore T R. Methane production and consumption in temperate and subarctic peat soils. Soil Biol Biochem. 1993;25:321–326. [Google Scholar]
- 13.Evans R C, Tingey D T, Gumpertz M L, Burns W F. Estimates of isoprene and monoterpene emission rates in plants. Bot Gaz. 1982;143:304–310. [Google Scholar]
- 14.Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther A, Hewitt C N, Lamb B, Liu S, Trainer M, Westberg H, Zimmerman P. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem Cycles. 1992;6:389–430. [Google Scholar]
- 15.Finlayson-Pitts B J, Pitts J N., Jr . Atmospheric chemistry. New York, N.Y: John Wiley and Sons; 1986. [Google Scholar]
- 16.Greenberg J P, Zimmerman P R, Chatfield R B. Hydrocarbons and carbon monoxide in African savannah air. Geophys Res Lett. 1985;12:113–116. [Google Scholar]
- 17.Guenther A, Hewitt C N, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay W A, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100:8873–8892. [Google Scholar]
- 18.Guenther A B, Zimmerman P, Wildermuth M. Natural volatile organic emission rate estimates for U.S. woodland landscapes. Atmos Environ. 1993;28:1197–1210. [Google Scholar]
- 19.Harder J, Probian C. Microbial degradation of monoterpenes in the absence of molecular oxygen. Appl Environ Microbiol. 1995;61:3804–3808. doi: 10.1128/aem.61.11.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt J G, Krieg N R, Sneath P H A, Staley J T, Williams S T, editors. Bergey’s manual of determinative bacteriology. Baltimore, Md: Williams and Wilkins; 1994. [Google Scholar]
- 21.Hou C T, Patel R N, Laskin N, Barnabe N, Barist I. Epoxidation and hydroxylation of C4- and C5-branched-chain alkenes and alkanes by methanotrophs. Ind Microbiol. 1981;23:477–482. [Google Scholar]
- 22.Houghton J T, Callander B A, Varney S K, editors. Climate change 1992: the supplementary report to the IPCC scientific assessment. Cambridge, United Kingdom: Cambridge University Press; 1992. [Google Scholar]
- 23.King G M, Adamsen A P S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl Environ Microbiol. 1992;58:2758–2763. doi: 10.1128/aem.58.9.2758-2763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd J, Taylor J A. On the temperature dependence of soil respiration. Funct Ecol. 1994;8:315–323. [Google Scholar]
- 25.Madsen E L. Methods for determining biodegradability. In: Hurst C, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C: American Society of Microbiology; 1997. pp. 709–720. [Google Scholar]
- 26.Madsen E L, Morgan M D, Good R E. Simultaneous photoreduction and microbial oxidation of iron in a stream in the New Jersey Pinelands. Limnol Oceanogr. 1986;31:832–838. [Google Scholar]
- 27.Monson R K, Guenther A B, Fall R. Physiological reality in relation to ecosystem- and global-level estimates of isoprene emission. In: Sharkey T D, Holland E A, Mooney H A, editors. Trace gas emissions by plants. New York, N.Y: Academic Press; 1991. [Google Scholar]
- 28.Nesbit S P, Breitenbeck G A. A laboratory study of factors influencing methane uptake in soils. Agric Ecosys Environ. 1992;41:39–54. [Google Scholar]
- 29.Oremland R S, Miller L G, Culbertson C W, Connell T L, Jahnke L. Degradation of methyl bromide by methanotrophic bacteria in cell suspensions and soils. Appl Environ Microbiol. 1994;60:3640–3646. doi: 10.1128/aem.60.10.3640-3646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul E A, Clark F E. Soil microbiology and biochemistry. New York, N.Y: Academic Press; 1989. [Google Scholar]
- 31.Reasoner D J, Geldreich E E. New medium for enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharkey T D. Isoprene synthesis by plants and animals. Endeavour. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- 33.Shorter J H, Kolb C E, Crill P M, Kerwin R A, Talbot R W, Hines M E, Harriss R C. Rapid degradation of atmospheric methyl bromide in soils. Nature. 1995;377:717–719. [Google Scholar]
- 34.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic Pseudomonas, a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 35.Van Ginkel C G, De Jong E, Tilanus J W R, Bont J A M. Microbial oxidation of isoprene, a biogenic foliage volatile and of 1,3-butadiene, an anthropogenic gas. FEMS Microbiol Ecol. 1987;45:275–279. [Google Scholar]
- 36.Vemulapalli G K. Physical chemistry. Englewood Cliffs, N.J: Prentice Hall, Inc.; 1993. [Google Scholar]
- 37.Whalen S C, Reeburgh W S, Sandbeck K A. Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol. 1990;56:3405–3411. doi: 10.1128/aem.56.11.3405-3411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]