Abstract
Visual responses in the cortex are strongly influenced by shifts in spatial attention. This modulation of visual processing includes changes in firing rate, decreased response variability, and decreased interneuronal correlations; all of which are thought to underlie enhanced perception near the center of attention at the cost of perception at other locations. Information from the retina is relayed to primary visual cortex via neurons in the lateral geniculate nucleus (LGN) of the thalamus. Although early studies describe an enhancement of LGN activity with spatial attention, more recent work has cast doubt on this view. Given its strategic position as the gateway to the cortex, an understanding of the effects of attention on visual processing in the LGN is important. We therefore performed experiments to reexamine the influence of spatial attention on spiking activity in macaque LGN (one male, one female) and applied a broad set of analyses and functional metrics to assess possible effects. Our results reveal a statistically significant effect of spatial attention in the LGN: firing rates were slightly higher and more reliable when monkeys directed attention toward the receptive fields of recorded neurons compared with when attention was directed to different retinotopic locations. However, effects were much smaller than previously reported (∼1 vs ∼4%) and further analyses suggest that effects are weak, inconsistent, and restricted to a small subset of parvocellular and magnocellular neurons. Thus, while spatial attention does exert an influence in the LGN, its effects are weak and may have limited impact on downstream processing.
Keywords: cortex, thalamus
Significance Statement
The lateral geniculate nucleus (LGN) is a critical relay in the visual system, shaping the flow of sensory information from the eye to the brain. Although higher-order brain regions show strong modulation by attention, it remains unclear whether the LGN is similarly affected. By directly recording LGN activity in monkeys performing a covert attention task, we found that spatial attention produces only weak and inconsistent modulation of visual responses. These findings suggest that the LGN largely operates independently of spatial attention, highlighting a potential boundary between early sensory processing and cognitive control.
Introduction
Spatial attention refers to the allocation of perceptual resources to enhance the processing of visual information at a specific spatial location, improving stimulus detectability and behavioral responsivity (Posner, 1980). While a full understanding of spatial attention is still emerging, the neural correlates of volitional spatial attention are generally believed to involve top–down processes (Maunsell, 2015; Boshra and Kastner, 2022; Martinez-Trujillo, 2022). These processes modulate fundamental aspects of visual responses in the cerebral cortex, including changes in the firing rate, decreased response variability, and decreased neuronal correlations (Luck et al., 1997; McAdams and Reid, 2005; Cohen and Maunsell, 2009; Mitchell et al., 2009). Consistent with a top–down framework, the influence of spatial attention on visual response properties is greatest toward the top of the visual hierarchy (e.g., IT cortex) and weakest in the primary visual cortex (V1), the initial stage of visual processing in the cortex (Luck et al., 1997; Kastner and Ungerleider, 2000; McAdams and Reid, 2005; Lauritzen et al., 2010; Ni and Maunsell, 2019; Meyyappan et al., 2025).
Along with the cortex, subcortical areas also play a role in spatial attention (Saalmann and Kastner, 2011; Zénon and Krauzlis, 2012; Zhou et al., 2016; Sherman and Usrey, 2021). Two of these areas, the superior colliculus in the midbrain and the pulvinar in the thalamus, receive strong, driving input from the cortex (Lynch et al., 1985; Sherman and Guillery, 2002; Cerkevich et al., 2014; Baldwin et al., 2017), and, thus, a route exists for cortical mechanisms to influence their response properties and communication with target structures. In contrast, neurons in the lateral geniculate nucleus (LGN) lack driving cortical input, instead receiving their driving input exclusively from the retina which is then relayed to the cortex (reviewed in Usrey and Alitto, 2015). Despite this, LGN activity has been reported to be influenced by spatial attention (O’Connor et al., 2002; McAlonan et al., 2008), potentially mediated by modulatory feedback pathways from the cortex and/or inhibitory circuits involving the thalamic reticular nucleus (McAlonan et al., 2008). The impact of attention on LGN activity remains unclear, though, as more recent work has not found effects of attention on visual processing in the LGN (Jiang et al., 2015; Shah et al., 2022).
Given the differences in past results and the importance of understanding the dynamics of visual signals delivered to the cortex, we reexamined the influence of spatial attention on the spiking activity of LGN neurons in the macaque monkey and applied a broad set of analyses and functional metrics to assess possible effects. Moreover, recognizing that effects may be small, we collected data from a large sample of LGN neurons to strengthen the statistical analyses. Our results reveal small but significant effects of attention on the firing rate and reliability of LGN responses to visual stimuli. However, a deeper analysis of the data reveals that these effects are weak and inconsistent. Specifically, we found that shifting spatial attention toward LGN receptive fields (RFs) was the equivalent of increasing the stimulus contrast by 1.25 or 1.0% for parvocellular and magnocellular neurons, respectively. Likewise, an ROC analysis indicates that an ideal observer would only be able to distinguish attend-toward trials from attend-away trials with 52% accuracy. Additionally, we did not observe a change in burst activity with shifts in spatial attention. Given that changes in burst firing are considered a reliable indicator of changes in thalamic network state (Sherman, 2001; McCormick et al., 2015), this is further evidence of the weak influence of spatial attention on LGN responses. Finally, by applying a linear classifier to pseudopopulations constructed from our recorded sample of LGN neurons, we found that attentional state could indeed be decoded at the population level. However, consistent with our single-cell analysis, this signal was largely driven by a small subset of neurons with stronger attentional modulation, rather than emerging from a distributed population code. Collectively, these results indicate that the modulation of LGN responses by spatial attention is statistically significant but functionally ineffectual.
Materials and Methods
Two adult rhesus monkeys (Macaca mulatta; one male and one female) were used for electrophysiological recordings in this study. All experimental procedures conformed to the National Institutes of Health and the United States Department of Agriculture guidelines and were approved by the Institutional Animal Care and Use Committee at University of California, Davis. Under full surgical anesthesia, the animals received a cranial implant consisting of a head post for head stabilization and a recording cylinder that allowed access to the LGN.
Electrophysiological recordings
Extracellular recordings from the LGN were made with platinum-glass electrodes (1–2 MΩ; Alpha Omega Engineering) using a microdrive (40 mm electrode travel; Thomas Motorized Electrode Manipulator, Thomas RECORDING) mounted on the recording chamber. Online continuous voltage signals were amplified (A-M Systems), filtered (0.1–5 kHz), and recorded using a Micro1401 data acquisition system (28 kHz) and Spike2 software (Cambridge Electronic Design). To extract action potentials, extracellular signals were high-pass filtered (stopband edge frequency, 500 Hz), thresholded, and clustered within the Spike2 software.
Visual stimulation and receptive field mapping
Visual stimuli were generated with a ViSaGe (Cambridge Research Systems) and presented on a gamma-corrected CRT monitor (Sony or Mitsubishi) positioned ∼80 cm in front of the animal. The display had a resolution of 1,024 × 768, a refresh rate of 100–140 Hz, and a mean luminance of ∼38 cd/m2. LGN RFs were manually mapped using small (<0.5°) user-controlled, computer-generated visual stimuli (custom software with Spike2 interface). The visual stimuli used for assessing spatial attention are described below.
Behavioral training and performance
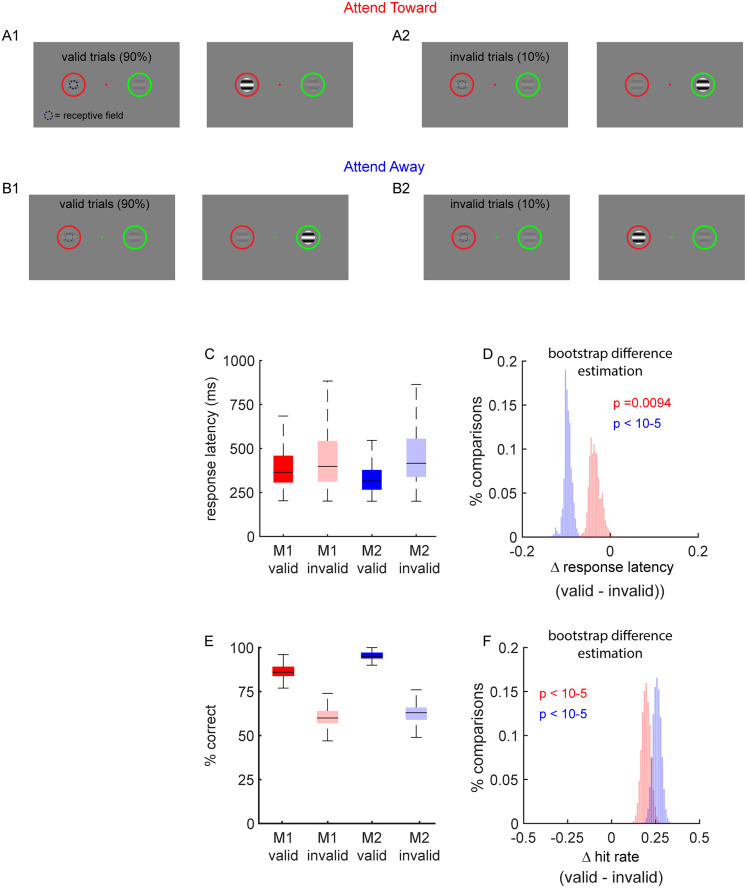
Data were collected while the animals performed a spatial attention task (Fig. 1A,B), while eye position and pupil size were monitored using an Eye-Trac6 infrared eye tracker (Applied Science Laboratories) with a custom Spike2 interface. For this task, the animals initiated a trial by fixating a central fixation dot similar to the fixation dot used for passive fixation (hand-mapping trials). The fixation dot was ∼0.2° diameter, and the fixation window was 0.8–1.2° diameter. After a short delay (0.5–1.0 s), two sine wave gratings (temporal frequency, 5 Hz; spatial frequency, 1.0 cycles/°) appeared at equidistant locations from the fovea, typically at an eccentricity of 4–8°. During recording sessions, one stimulus was positioned inside the recorded LGN RF (dashed black circle; note that the dashed circle was not present in the actual stimulus display presented to the animals). Each sine wave grating was surrounded by a colored circle (one green, one red), and the color of the circles at each location was constant throughout the task. The color of the fixation dot (either red or green) indicated to the animal which grating was 90% likely to change contrast (valid trials) after a delay with an exponential hazard function of 0.6–1.8 s. On 10% of the trials (invalid trials), the uncued grating increased in contrast (same hazard function). Because one stimulus was always inside the RF and the other outside, trials were further categorized based on the spatial relationship between the cued location and the RF. “Attend-toward” trials (A) occurred when the cued stimulus—i.e., the one most likely to change contrast—was positioned inside the RF. “Attend-away” trials (B) occurred when the cued stimulus was positioned outside the RF. This classification was used in all subsequent analyses to assess how spatial attention modulated LGN responses. The base contrast of the grating was set to be near the C50 (contrast to evoke a half-maximum response) of the recorded LGN neuron, as estimated through hand mapping. After the contrast change, the animal was given 1 s to respond by making a saccade to the location of the change, and the animal was rewarded with a small juice reward. Psychophysical performance (accuracy and reaction time) for valid and invalid trials were compared to ensure that the animals were applying spatial attention as anticipated. Based on these comparisons, the contrast change was titrated so that the animal performed 20–30% better on cued trials relative to uncued trials.
Figure 1.
Spatial attention task and psychophysical measure of spatial attention. Two macaque monkeys (Monkey 1, M1, and Monkey 2, M2) were trained to perform a covert spatial attention task. A, B, On each trial, animals maintained fixation on a central green dot while two peripheral sine wave gratings appeared, each surrounded by a colored circle. One grating was positioned over the RF of a recorded LGN neuron (dashed black circle) and the other at an equidistant location. The color of the fixation point, in conjunction with the colors of the peripheral circles, indicated the probability that each grating would undergo a contrast increase after a brief delay. In 90% of trials (A1, B1), termed “valid trials,” the contrast change occurred at the location where the circle color matched the fixation cue. In 10% of trials (A2, B2), termed invalid trials, the contrast change occurred at the nonmatching location. Since one stimulus was positioned inside the RF and one was positioned outside the RF, trials were further categorized based on the spatial relationship between the cued location and the RF. “Attend-toward” trials (A) occurred when the cued stimulus was positioned inside the RF, while “attend-away” trials (B) occurred when the cued stimulus was positioned away from the RF. The error bars in C and B indicate the 25th and 75th percentiles. Animals were rewarded for making a saccade to the grating that increased in contrast. Both animals respond faster (C, D) and more accurately (E, F) on valid trials compared with invalid trials. Statistical significance was assessed using BDE. Panels D and F show the distribution of median differences (valid–invalid) computed over 10,000 bootstrap resamples for M1 (red) and M2 (blue). These distributions provide graphical estimates of the effect size, variability, and statistical confidence (see Methods and Materials for details).
Analysis
Classification of neurons. Based on the hand-mapping procedures described above, we classified LGN neurons on two axes: cell type and response polarity (Alitto et al., 2011). Cell types were defined as magnocellular or parvocellular based on the C50 (C50 >35% were classified as parvocellular; C50 <35% were classified as magnocellular). Response polarity (on-center cells vs off-center cells) was based on responses to increases and decreases in luminance.
Firing rate and spatial attention. LGN neurons have linear responses to the presentation of drifting sine wave gratings that are modulated by the phase of visual stimulus. To extract this linear response, we calculated the average cycle histogram (cycle duration, 200 ms; bin size, 7.7 ms) for each trial and extracted the linear response as the F1 from a Fourier transform with a Hanning window (custom MATLAB script). Based on the F1 and F0 (mean firing rate), the attention index was defined as follows:
Here, the FR is the firing rate (either the F0 or F1) during attend-toward and attend-away trials.
Equivalent contrast change. Shifts in spatial attention are often analogized as being similar to changes in stimulus contrast (Carrasco et al., 2004; Reynolds and Chelazzi, 2004). Given that shifts in spatial attention cause changes in the firing rates of visual neurons, we wanted to determine the changes in contrast that would have produced equivalent changes in the firing rate. In the current study, the baseline stimulus contrast was individually specified for each LGN neuron, based on computer-aided hand mapping of the RF, to evoke 50% of the cell's maximum response (C50). Since complete contrast response functions were not available for the current dataset, we created standardized parvocellular and magnocellular contrast response functions from a separate sample of LGN neurons (Fig. 3a; magnocellular, n = 33; parvocellular, n = 87; 5–8 stimulus repeats per contrast) and applied the attentional changes in firing onto this value to determine an effective/equivalent contrast change. For each recorded neuron, we estimated the attention-driven change in the firing rate as follows:
Using the standardized magnocellular and parvocellular contrast response functions, we then estimated the contrast that would have evoked an equivalent response difference relative to the C50. For example, if the attend-toward firing rate was 1.2× greater than the attend-away firing rate, we would identify the contrast that would have evoked a response that was 1.2× the firing rate evoked at C50. Based on this calculation, we then defined attention equivalent contrast change (AECC) as follows:
ROC analysis of attentional modulation. To further quantify the influence of spatial attention on visual responses in the LGN, we performed an ROC analysis to determine how well an ideal observer would be able to determine the focus of the animal's attention based solely on the trial-by-trial distribution of F1s across attend-toward and attend-away trials. Using the MATLAB function perfcurve (bootstrap n = 1,000), we determined if (1) attend-toward trials were distinguishable from spontaneous activity, (2) attend-away trials were distinguishable from spontaneous activity, and (3) attend-toward trials were distinguishable from attend-away trials. For this analysis, spontaneous activity was based on the intertrial interval.
Figure 3.
The firing rate during spatial attention. A, Distribution of firing rates (F1) for attend-toward versus attend-away trials across all cells (M1 = red; M2 = blue; n = 283). B, Significance of these effects was determined via a BDE (see Materials and Methods). The red and blue data in panel B show the distribution of median difference values (toward–away) over 10,000 resamples with replacement for M1 and M2, respectively. These distributions provide a direct graphical illustration of statistical significance and confidence intervals (p values in text). C, D, Same as A and B for parvocellular (red) and magnocellular (blue) data. E, F, Same as A and B for on-center (red) and off-center (black).
Influence of attention on thalamic bursts. Spiking activity in the thalamus is often segregated into tonic and burst firing (Sherman, 2001). In tonic firing, thalamic neurons respond to excitatory stimuli in a linear, constant fashion where the mean firing rate is proportional to the strength of the stimulus. When thalamic neurons are sufficiently hyperpolarized to deinactivate T-type Ca++ channels, they begin to exhibit bursting activity (McCormick and Huguenard, 1992). As has been done previously (Reinagel et al., 1999; Alitto et al., 2019; Sanchez et al., 2023), we tracked burst and tonic firing based on the following criteria: (1) an ISI of >100 ms that preceded the first spike in a sequence and (2) one or more subsequent spikes that followed with ISIs of <4 ms. Past studies applying these criteria to intracellular recordings show that events defined as bursts co-occur with T-channel plateau potentials (Lu et al., 1992). Based on these criteria, we estimated the modulation of thalamic burst firing in the LGN by spatial attention.
Modulation of response reliability by spatial attention. To quantify the influence of spatial attention on response reliability in the LGN, we calculated the Fano factor for both attend-toward and attend-away trials (custom MATLAB function). The Fano factor is a common measure of response reliability and is defined as follows:
Support vector machine. To quantify the influence of spatial attention at the population level, we trained linear support vector machine (SVM) classifiers using MATLAB's fitcsvm function on pseudopopulations of sequentially recorded LGN neurons (Hastie et al., 2009). Pseudopopulations were constructed using two strategies, with and without cell identity, both incorporating bootstrap sampling over 10,000 iterations.
In the with cell identity condition, we constructed pseudopopulation trials by randomly sampling (with replacement) 20 attend-toward and 20 attend-away trials from each neuron. This resulted in feature vectors with 198 features (one per neuron) and 40 trials per bootstrap iteration. In the without-cell-identity condition, we generated feature vectors of the same size (198 features × 40 trials), but the firing rates were drawn randomly from the entire pool of trials, independent of neuron identity—effectively destroying the mapping between neurons and features.
In both approaches, the SVM was trained on the pseudopopulation and tested on a separate, nonoverlapping validation set constructed using the same sampling procedure. Classifier performance was quantified using MATLAB's predict and perfcurve functions, with performance defined as the area under the curve (AUC; receiver operator characteristic) on the validation data. To establish a chance-level baseline, we implemented a shuffle control in which trial labels (attend-toward vs attend-away) were randomized prior to validation. Shuffled-label decoding was performed using the same bootstrap framework. Statistical comparison between real and shuffled models was based on the proportion of bootstrap iterations in which the real-model AUC exceeded the shuffled AUC.
To assess the distribution of attentional modulation across the LGN population, we implemented two additional SVM-based analyses: greedy ablation and reverse greedy ablation. In the greedy ablation analysis, we began with the full pseudopopulation and iteratively removed the most informative neuron—defined as the neuron with the largest absolute SVM weight (beta coefficient). In the reverse greedy ablation analysis, we removed the least informative neuron at each step. In both cases, the classifier was retrained and evaluated after each ablation step using the same procedure described above.
Statistical tests. When testing for statistical differences between two populations, we performed bootstrap analyses to estimate median values, median difference values, 95% confidence intervals, and statistical significance. For this process, data were resampled with replacement 10,000 times while maintaining the original sample size in each resampling iteration. Within the text, statistics will be presented as follows:
for example:
Where applicable, a median bootstrap difference estimation (BDE) is also provided. Throughout the Results, the distributions from bootstrap analyses are illustrated within the figures (Fig. 1D–F). These histograms show the distribution of median values (or median difference values) generated from the 10,000 resamples with replacement. These distributions provide graphical estimates of the effect size, variability, and statistical confidence. For within-neuron statistical tests, trials were shuffled across attention conditions (e.g., attend toward vs attend away) and resampled with replacement 10,000 times per neuron. Statistical significance for each neuron was determined as the proportion of resampled values exceeding the observed effect size as follows:
For sample-level statistical tests involving index values (e.g., attention index, burst index), significance was defined as the proportion of resampled values having the different sign as the observed value. Specifically, for an observed attention index >0, significance was calculated as follows:
For an observed attention index <0, significance was calculated as follows:
Results
To study the influence of attention on visual processing in the LGN, we trained two macaque monkeys (M1 and M2) to perform a spatial attention task (Fig. 1A,B; see Materials and Methods). Briefly, the animals were trained to fixate on a colored dot while two drifting sinusoidal gratings, each surrounded by a thin colored circle, were shown in the periphery (mean eccentricity = 7.7 ± 3.3°, SD). The contrast of the drifting gratings was held constant for a randomized interval (0.6–2.0 s), at which point one of the gratings increased in contrast and the animal was rewarded for making a saccade to that location. The probability of each grating changing contrast was linked to the color of the fixation point. If the color of the fixation point matched the color of the surrounding circle, then that grating changed contrast on 90% of the trials (valid trials). On the remaining 10% of trials, the nonmatching grating changed contrast (invalid trials). Importantly, the color of the circles did not change from trial to trial; instead, the color of the fixation point changed. As expected, both monkeys were faster and more accurate on valid trials relative to invalid trials, indicating the animals were using covert spatial attention to perform the task (Fig. 1C–F; response latency, validM1 = 354 ms [347–365]; invalidM1 = 390 ms [361–405]; BDEM1 = −34 ms [−54 to −5]; pM1 = 0.0094; validM2 = 301 ms [298–303]; invalidM2 = 396 ms [378–417]; BDEM2 = −96 ms [−117 – −77]; pM2 < 10−5; hit rate, validM1 = 0.949 [0.932–0.964]; invalidM1 = 0.749 [0.701–0.796]; BDEM1 = 0.20 [0.152–0.249]; pM1 < 10−5; validM2 = 0.982 [0.979–0.985]; invalidM2 = 0.719 [0.672–0.767]; BDEM2 = 0.263 [0.217–0.311]; p < 10−5).
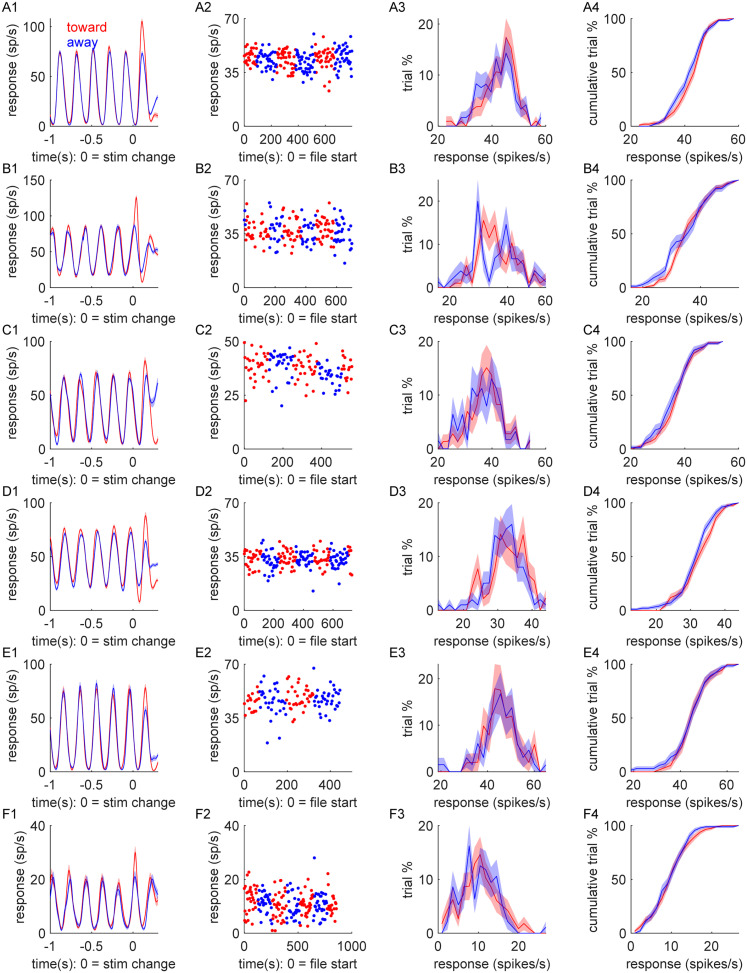
To quantify the influence of spatial attention on the spiking activity of LGN neurons, we recorded the responses of 283 isolated single units (Fig. 2; 103 magnocellular neurons, 180 parvocellular neurons; 151 off-cells, 132 on-cells) across both monkeys while they performed the spatial attention task. For each neuron, one of the drifting gratings was centered over the neuron's RF, and the other grating was shown at an equidistant location from the fixation point. Gratings were ∼2–3 times the estimated size of the recorded neuron's classical RF (typically 2° in diameter). Across cells and illustrated with the examples shown in Figure 2, LGN responses to the drifting sine wave grating largely matched the drift frequency of the grating (5 Hz). Thus, as is standard, we primarily focused on this component (first harmonic, F1) of the firing rate as quantified through Fourier analysis (see Materials and Methods).
Figure 2.
Single-unit examples of LGN responses recorded while the animal performed the spatial attention task. Each row (A–F) shows data from one neuron. Column 1. Peristimulus time histograms (PSTHs) showing firing rates for valid attend-toward (red) and attend-away (blue) trials, aligned to the time of contrast change (0 s). Column 2. The mean firing rate across all trials as a function of time relative to the start of recording. Column 3. Distribution of firing rates (F1) across all trials. Column 4. Cumulative distribution of firing rates across all trials. In all panels, the shaded areas indicate standard error of the mean.
Influence of spatial attention on the neuronal firing rate
Across our sample of LGN neurons, there was a small, but significant, increase in the F1 of the firing rate when the monkey directed attention toward the LGN cell's RF (toward trials) relative to when it directed attention away from the RF (away trials; Fig. 3). This effect was seen in M1 [Fig. 3A,B; (red data) toward = 26.1 [23.9–28.4] spikes/s; away = 25.2 [22.9–27.5] spikes/s; n = 161; BDE = 0.93 [0.56–1.32] spike/s; p < 10−5] and M2 [Fig. 3A,B; (blue data) toward = 25.8 [23.1–28.4] spikes/s; away = 24.9 [22.255–27.476]; n = 122; BDE = 0.48 [0.10–0.92] spikes/s; p = 0.0059]. This effect was also seen when data were divided based on the cell type [Fig. 3C,D; (parvocellular, red data) toward = 24.5 [22.8–26.2] spikes/s; away = 23.9 [22.2–25.7] spikes/s; BDE = 0.54 [0.24–0.84] spike/s; p = 0.0001; (magnocellular, blue data) toward = 29.1 [26.1–32.1] spikes/s, away = 28.0 [25.0–31.0], BDE = 1.1 [0.57–1.67] spikes/s, p < 10−5] and luminance polarity [Fig. 3E,F; (off-center, black data) toward = 26.9 [24.7–29.2] spikes/s; away = 26.2 [24.0–28.5]; BDE = 0.72 [0.31–1.17] spikes/s; p < 10−5; (on-center, red data) toward = 25.2 [ 23.0–27.4] spikes/s; away = 24.5 [22.4–26.7] spikes/s; BDE = 0.76 [0.43–1.1] spikes/s; p < 10−5]. The differences between parvocellular versus magnocellular (BDE = −0.16 [−0.73–0.33] spike/s; p = 0.31) and on-center versus off-center (BDE = 0.12 [−0.36–0.60] spikes/s; p = 0.65) were not statistically significant. Similar differences were seen for the mean response (F0; data not shown), for M1 (toward = 27.4 [24.9–30.0] spikes/sec; away = 26.5 [24.0–29.0] spikes/sec; BDE = 0.92 [0.54–1.32] spikes/s; p < 10−5) and M2 (toward = 30.6 [27.7–33.4] spikes/s; away = 30.0 [27.2–32.9] spikes/s; BDE = 0.54 [0.06–1.08] spikes/s; p < 0.0114). Across monkeys and cell types, the influence of attention on the firing rate was significant, but small, increasing the firing rate by ∼1 spike/s.
Given that the firing rate varies from cell to cell, a bounded index is often useful for making comparisons between conditions across a sample of cells. We therefore used an attention index based on each cell's F1 response, where
With the attention index, positive values indicate the firing rate increased with attention directed toward the neuron's RF, and negative values indicate the firing rate decreased with attention. For both monkeys, median attention index values were significantly greater than zero (Fig. 4A,D,E; M1, 0.014 [0.011–0.022]; p < 10−5; M2, 0.01 [0.001–0.012]; p = 0.0009). When data were combined across monkeys, an increased attention index was evident for parvocellular neurons (Fig. 4F,G; 0.011 [0.003–0.015]; p = 0.0004; n = 180), magnocellular neurons (Fig. 4F,G; 0.012 [0.009–0.020]; p < 10−5; n = 103), on-cells (Fig. 4H,I; 0.012 [0.007–0.018]; p < 10−5; n = 131), and off-cells (Fig. 4H,I; 0.012 [0.004–0.015]; p < 10−5; n = 152). The differences between parvocellular and magnocellular neurons (parvocellular–magnocellular, −0.002 [−0.012–0.004]; p = 0.22) as well as between on-center and off-center neurons (on-center–off-center, 0.001 [−0.006–0.009]; p = 0.41) were not statistically significant. On a cell-by-cell basis, 16.6% of cells (47/283) had a statistically significant attention index at a significance level of 0.05 (Fig. 4B). This distribution of statistically significant cells was marginally biased (Fig. 4C; p = 0.0479) toward significantly positive attention index values (11.1% of cells) compared with significantly negative attention values (5.3% of cells). For the remainder of the paper, no statistically significant differences were seen between on-center and off-center data. Thus, on-center versus off-center differences will not be discussed.
Figure 4.
Attention index. A, Distribution of attention index values across cell types (error bars = 25th and 75th percentiles). B, Overall distribution of attention index values. Significant values (p < 0.05) are indicated in black. C, Significance of the distribution of attention index values (positive > negative) was determined by a bootstrap null distribution analysis (red dashed line = observed difference). D, Distribution of attention index values for M1 (red) and M2 (blue) (0 = black dashed line). E, Significance of these distributions was determined via a bootstrap mean estimation. F, G, Same as D and E for parvocellular (red) and magnocellular (blue) data. H, I, Same as A and B for on-center (red) and off-center (black) data.
Given that the influence of attention on firing rates appeared to be relatively small, we wished to put this change into perspective and estimate its functional relevance. Since spatial attention is often viewed as effectively increasing the contrast of the visual stimuli at that attended location, we wanted to determine the change in stimulus contrast that would evoke the same change in the firing rate as shifting spatial attention toward the LGN RF (Fig. 5). We therefore calculated the AECC (see Materials and Methods). In the current study, the baseline contrast was set to be at the neuron's C50 (the contrast that evoked a half-maximum response), but since each neuron's C50 was estimated online with hand-mapping procedures, we used data from a separate population of neurons to generate standardized contrast response functions for magnocellular (n = 33) and parvocellular (n = 87) neurons (Fig. 5A). Using these standardized functions, we calculated the contrast, relative to the C50, that evoked the same change in the firing rate as shifting spatial attention. For example, if the change in the firing rate caused by shifting attention was equivalent to the change in the firing rate caused by increasing the contrast from 30 to 40%, then the AECC would be 0.33. For the parvocellular neurons in this study (Fig. 5B–D), the 0.52 [0.097–0.785] spikes/s increase in firing when spatial attention was shifted toward the cell's RF is equivalent to a relative increase in contrast of only 0.025 [0.017–0.032] that is a change equal to 2.5% of the C50. For a typical parvocellular neuron, this would be the equivalent of changing the contrast from 50.0 to 51.25%. For magnocellular neurons (Fig. 5B–D), the 0.681 [0.294–1.112] spikes/s increase in the firing rate with attention is equivalent to a relative increase in contrast of 0.058 [0.041–0.076]. For a typical C50 seen in magnocellular neurons, this would be the equivalent of changing the contrast from 18.0 to 19.0. Across cells, equivalent contrast change values were significantly greater for magnocellular neurons than for parvocellular neurons (magno–parvo, 0.033 [0.014–0.052]; p = 0.0002); however, for both cell types, the equivalent contrast change values were very small and likely would be below threshold for change detection.
Figure 5.
Equivalent contrast change. A, Population contrast response functions for parvocellular (red) and magnocellular (blue) data (shaded area = standard error of the mean). B, C, Distribution of equivalent contrast values across cell types (error bars = 25th and 75th percentiles). D, Significance of these distributions was determined via a bootstrap mean estimation.
A separate metric that can be applied to assess the influence of spatial attention on visual responses utilizes receiver operating characteristic (ROC) analysis (Fig. 6). With ROC analysis, one can ask how well an ideal observer would be able to determine, on a cell-by-cell basis, where the monkey was focusing its attention based solely on the response distributions (see Materials and Methods). A small difference in mean response would be discernable if there was also little variability (e.g., all trials with attention toward the cell's RF had the same small difference in the firing rate compared with all-away trials). At the opposite extreme, the same small difference in mean response would not be discernable if associated with much greater variability. In the first case with low variance, the ideal observer would successfully distinguish attend-toward and attend-away trials a high percentage of the time. In the second case, the ideal observer would do much worse. Applying this approach, results indicate an ideal observer would have no difficulty discriminating either toward or away trial contrast changes from the baseline contrast (Fig. 6A,B,D–I; toward = 0.912 [0.898–0.925]; p < 10−5; away = 0.902 [0.888–0.916]; p < 10−5). On a cell-by-cell basis, 97.2% of cells had a statistically significant toward trial versus baseline AUC and 96.5% of cells had a statistically significant away trial versus baseline AUC (p < 0.05). However, and more importantly, the same methodology revealed that toward and away trials could only be discriminated with an accuracy of 0.522 (Fig. 6C; 0.522 [0.513–0.532]). On a cell-by-cell basis, only 13.8% of cells had statistically significant toward versus away AUC (p < 0.05). However, it is worth nothing that significant AUCs were more common (p = 0.01) for cells with greater activity while attending toward the LGN RF (n = 28; 9.9% of cells) compared with cells with greater activity while attending away from the LGN RF (n = 11; 3.9% of cells). Overall, these results indicate the influence of spatial attention in the LGN is weak and not a reliable indicator of where the animal was attending on a trial-to-trial basis.
Figure 6.
ROC analysis of spatial attention. A, AUC for attend-toward trials versus baseline, across cell types. B, AUC for attend-away trials versus baseline, across cell types. C, AUC for attend-toward trials versus attend-away trials, across cell types. D, Distribution of AUC values for M1 (red = toward vs baseline; blue = away vs baseline; black = toward vs away). E, Same as D for M2. F, Same as D for parvocellular data. G, Same as D for magnocellular data.
Spatial attention and thalamic bursts
Across thalamic nuclei, relay cells produce spikes that can be categorized as burst spikes or tonic spikes (Sherman, 2001). Although burst spikes are less common in alert animals compared with anesthetized animals (Glenn and Steriade, 1982; Ramcharan et al., 2000; Alitto et al., 2011), spatial attention could serve to modulate the relative occurrence of burst and tonic spikes. Consistent with previous studies, results from this study show that burst spikes were infrequent in the alert animal (Fig. 7A). This was the case for trials when animals were directed to attend toward the recorded cells' RFs (proportion burst spikes, all cells = 0.017 [0.015–0.018]; M1 = 0.018 [0.016–0.021]; M2 = 0.014 [0.011–0.017]; parvocellular cells = 0.018 [0.015–0.020]; magnocellular cells = 0.016 [0.013–0.019]) and away from the recorded cells' RFs (proportion burst spikes: all cells = 0.016 [0.015–0.018]; M1 = 0.018 [0.016–0.021]; M2 = 0.015 [0.012–0.017], parvocellular cells = 0.017 [0.015–0.020]; magnocellular cells = 0.016 [0.013–0.019]). Furthermore, shifts in spatial attention did not significantly modulate the probability of an LGN neuron firing in the burst mode as assessed using a burst index as follows:
(Fig. 7B–D; burst index, M1 = 0.027 [−0.034–0.0088]; p = 0.27; M2 = −0.004 [−0.074–0.061]; p = 0.44; parvocellular cells = −0.017 [−0.072–0.029]; p = 0.35; magnocellular cells = −0.009 [−0.088–0.009]; p = 0.32). On a cell-by-cell basis, 11.7% of cells (33/283) had significant burst index values, evenly split between more bursts during attend-toward trials (5.6%) and more bursts during attend-away trials (6.0%) at a significance level of 0.05. There was, however, a significant negative correlation between the firing rate of recorded cells and burst percentage for both toward trials (Fig. 7E,F; r = −0.15 [−0.247 – −0.055]; p = 0.001) and away trials (r = −0.17 [−0.259 – −0.070]; p = 0.0005). This inverse relationship might be explained by the time–voltage relationship that governs the production of bursts which, in turn, may reflect variable levels of alertness across/within recording sessions. Importantly, there was no correlation between the burst rate and the magnitude of spatial attention effects on the firing rate of LGN cells (r = 0.03 [−0.150–0.091]; p = 0.31).
Figure 7.
Thalamic bursts during spatial attention. A, Distribution of thalamic burst percentage for attend-toward trials versus attend-away trials. B, Sample distributions for burst index values across cell types. C, D, Significance of these effects illustrated via a BDE. E, Correlation between burst percentage and firing rate across all cell types (red = attend-toward trials; blue = attend-away trials; dashed line = linear fit). F, Significance of these correlations illustrated via a bootstrap mean estimation.
Influence of spatial attention on response reliability
Past studies have shown that spatial attention increases response reliability in the visual cortex (Mitchell et al., 2007); however, this effect has not been previously demonstrated in the LGN. To examine the influence of spatial attention on LGN response reliability, we calculated the Fano factor (Fano factor = spike count variance / spike count mean) when animals were directed to attend toward and away from the recorded neurons' RFs (Fig. 8). Consistent with results from studies not examining attention (Kara et al., 2000; Alitto et al., 2011), Fano factors across our sample of LGN neurons were, on average, below 1.0, indicating the responses of LGN neurons are less variable than expected from a Poisson process. This was the case for trials when animals were cued to attend toward the recorded cells' RFs (Fig. 8A; Fano factor, M1 = 0.928 [0.908–0.958]; M2 = 0.924 [0.897–0.951]; parvocellular cells = 0.932 [0.900–0.955]; magnocellular cells = 0.922 [0.908–0.968]) and away from the recorded cells' RFs (Fano factor, M1 = 0.92 [0.91–0.94]; M2 = 0.95 [0.90–0.98]; parvocellular cells = 0.932 [0.900–0.955]; magnocellular cells = 0.922 [0.908–0.968]). Although Fano factors were extremely similar for attend-toward and attend-away trials, there was a significant decrease in the Fano factor (as assessed using a Fano factor index; see Materials and Methods) when attention was directed toward the RFs of recorded neurons (Fig. 8B–D; FF index, M1 = −0.005 [−0.010–0.000]; p = 0.0306; M2 = −0.008 [−0.013 – −0.003]; p = 0.0003; parvocellular cells = −0.004 [−0.008–0.000]; p = 0.036; magnocellular cells = −0.010 [−0.016 – −0.004]; p = 0.0005). On a cell-by-cell basis, 21.9% of cells had statistically significant Fano factor index values (62/283), at a significant level of 0.05, with a significant bias (p = 0.0002) toward more cells with significantly negative FF index values (18%, more reliable during attend-toward trials) compared with cells with significantly positive FF index values (4.6%, more reliable during attend-away trials). Thus, similar to the effects of attention on the firing rate, there was a small, but significant, influence of attention on the response reliability of LGN neurons.
Figure 8.
Response reliability during spatial attention. A, Distribution of Fano factor values for attend-toward trials versus attend-away trials. B, Sample distributions for Fano factor index values across cell types. C, D, Significance of these effects as illustrated via a BDE.
Linear classification of LGN population activity
Although the influence of spatial attention on visual responses in the LGN is statistically significant, we have provided several lines of evidence that suggest the influence is unreliable, weak, and unlikely to have a strong impact on downstream visual processing. However, our core analyses treat each LGN recording independently and do not consider whether a more reliable signal emerges at the population level. Ideally, this question would be addressed using large numbers of simultaneously recorded LGN neurons. Unfortunately, our dataset consists of many sequentially recorded single units, which limits our ability to study true population dynamics.
Nevertheless, by constructing pseudopopulations from our data, we can ask whether the attentional signal becomes more robust when LGN neurons are considered collectively. Specifically, we asked whether an ideal observer (e.g., downstream neurons in V1) could decode the attentional state of a given trial based on LGN population activity. To address this, we used a linear classifier (SVM) to test whether trials could be reliably separated into attend-toward and attend-away categories based on pseudopopulation activity patterns. Each pseudopopulation was constructed by randomly sampling (with replacement) 20 attend-toward trials and 20 attend-away trials from each neuron, resulting in 198 features (neurons) and 40 samples (trials). The SVM was trained on this dataset and tested on a separate, nonoverlapping validation set constructed in the same way.
Across 10,000 bootstrap iterations, the classifier achieved a mean classification accuracy of ∼75% (Fig. 9A; AUC = 0.754 ± 0.001), which was significantly better different than the label-shuffled control (AUC = 0.499 ± 0.001; p = 0.0164). The linear classifier performed worse when only parvocellular data (Fig. 9B; AUC = 0.634 ± 0.001; shuffle AUC = 0.500 ± 0.001; p = 0.1520) or magnocellular data (Fig. 9C; AUC = 0.659 ± 0.001; shuffle AUC = 0.500 ± 0.001; p = 0.1064) were used. This indicates that attentional modulation was not confined to either the parvocellular or magnocellular pathways, consistent with our single-cell analyses described above. Although both subpopulations performed worse than the full population, this was likely due to the reduced number of neurons in these models; a size-matched control population (containing both parvocellular and magnocellular neurons) also performed worse than the full model (data not shown; AUC = 0.642 ± 0.001). Thus, the pseudopopulation analysis suggests that some attentional information is indeed present at the population level in the LGN. We next asked whether this weak signal is broadly distributed across the LGN population or carried by a small subset of neurons. To address this, we first performed a “pooled” SVM analysis in which cell identity was erased. Pseudopopulations were generated as before, but the firing rate values for each feature were randomly drawn from the full pool of trials, independent of which neuron they came from. If the attentional signal was distributed across many neurons, this pooling procedure should have preserved classifier performance. However, it did not; the pooled model performed near chance (Fig. 9D; AUC = 0.507 ± 0.001), no better than the shuffled control (AUC = 0.501 ± 0.001; p = 0.4772). This was also the case when only parvocellular data (Fig. 9E; AUC = 0.506 ± 0.001; shuffle AUC = 0.500 ± 0.001; p = 0.4786) or magnocellular data (Fig. 9F; AUC = 0.503 ± 0.001; shuffle AUC = 0.500 ± 0.001; p = 0.4897) were used. This suggests that the attentional signal is concentrated in a minority of LGN neurons.
Figure 9.
LGN population activity is weakly modulated by spatial attention. SVM models were used to assess whether population-level activity in the LGN could predict the subject's attentional state. A–C, Distributions of classification performance (AUC) for SVM models trained on pseudopopulations that preserved cell identity (red), compared with shuffled-label controls (blue). D–F, Same analysis as in A–C but using “pooled” pseudopopulations in which cell identity was randomized. The sharp drop in performance suggests that the attentional signal is not broadly distributed across the population.
To further test this idea, we applied a “greedy ablation” SVM analysis. Here, we began with the full dataset and iteratively removed the most informative neuron, defined as the one with the largest absolute valued beta coefficient, with retraining and re-evaluating the SVM after each step. Classifier performance rapidly declined toward chance after the removal of only ∼10% of the neurons (Fig. 10A–C), supporting the conclusion that only a small subset of neurons carries the attentional signal. The neurons removed from the model to reach chance discrimination performance were proportionally balanced between the parvocellular and magnocellular pathways compared with random draws from the underlying populations (parvocellular ablations = 14/191; magnocellular ablations = 6/70; p = 0.8155).
Figure 10.
A minority of LGN neurons carry the attentional signal. SVM models were iteratively ablated to assess the relative contribution of individual neurons to the population-level attentional signal. A, “Greedy ablation” analysis: the most informative neurons were progressively removed from the model. AUC is plotted as a function of ablation number (0 = full dataset). Shaded areas show ±1 standard deviation across bootstrap iterations. B, AUC distribution for the full model (198 neurons). C, AUC distribution after 20 greedy ablations, demonstrating a drop to chance performance. D, “Reverse greedy ablation” analysis: the least informative neurons were progressively removed. Classifier performance increased as uninformative neurons were removed, peaking with ∼18% of the neurons remaining. E, AUC distribution for the full model. F, AUC distribution after 141 ablations, corresponding to peak model performance.
Conversely, we also performed a “reverse greedy ablation” analysis, in which the least informative neurons were progressively removed. In this case, classifier performance improved as uninformative neurons were removed, peaking at AUC = 0.859 ± 0.001 when ∼18% of the neurons remained (Fig. 10D–F). Together, these three complementary approaches strongly suggest that while an attentional signal is present in the LGN, it is not broadly distributed and is instead carried by a minority (∼10–20%) of the neuronal population.
Discussion
It is generally agreed that the central nervous system lacks the resources to process all visual information that falls on the retina. Instead, the processing of behaviorally relevant information is prioritized at the expense of other or less- or nonrelevant visual information. Spatial attention, the preferential processing of visual stimuli at a specific spatial location, is one mechanism that has a profound influence on what sensory information enters conscious perception. While early studies reported moderate attentional modulation in the LGN—including increased BOLD signal in humans and enhanced parvocellular/magnocellular firing rates in primates (O’Connor et al., 2002; McAlonan et al., 2008) —subsequent findings have indicated otherwise (Jiang et al., 2015; Shah et al., 2022). Notably, Shah et al. (2022) did not find effects of attention in the LGN, despite observing strong attentional modulation in V1. Interestingly, while measures of spatial attention on visual processing within the LGN have been inconsistent using electrophysiology in nonhuman primates, the effect has been consistently observed in humans using BOLD fMRI analyses (Schneider and Kastner, 2009; Schneider, 2011; Ling et al., 2015; Poltoratski et al., 2019). Possible reasons for these differences are discussed below.
While the results reported here confirm that spatial attention does influence LGN activity (Figs. 2, 6), a deeper analysis of the data indicates that these effects are weak and inconsistent (Figs. 3–5, 9, 10). The observed firing rate increases (on average, ∼1 spike/s) corresponded to only a 1–1.8% contrast change, and ROC analysis showed that an ideal observer could barely distinguish attend-toward from attend-away trials (53% accuracy). Furthermore, burst firing, a key indicator of thalamic network state, remained unchanged. Finally, using SVM analysis of pseudopopulations constructed from our sequentially recorded LGN neurons, we found that attentional modulation was detectable in the population response patterns. However, this signal was limited to a small subset of LGN neurons and did not generalize across the entire sample. Together, these findings suggest that while spatial attention exerts a statistically significant influence on LGN processing, its functional impact is weak.
Given its position between the retina and cortex (Usrey and Alitto, 2015), the LGN might seem like an ideal site for attentional gating. Its small RFs could, in theory, support a highly retinotopically precise spotlight of attention, which becomes harder to achieve in the higher-order cortex where RFs are larger. However, this is not how the visual system operates. Instead, attentional modulation is strongest in higher-order areas (e.g., IT, MT; Connor et al., 1997; Seidemann and Newsome, 1999; Treue and Maunsell, 1999; Ramezanpour and Fallah, 2022) and weakest at the early stages of processing (Luck et al., 1997; Shah et al., 2022).
One possible reason is that early visual areas serve as a preprocessing hub, establishing a basis set for the encoding of natural images that is robust against changes in basic stimulus properties such as luminance and contrast (Shapley and Victor, 1979; Heeger, 1992; Olshausen and Field, 1996; Alitto and Usrey, 2004; Bonin et al., 2005; Priebe and Ferster, 2012). When visual information reaches the cortex, V1 acts as a distribution hub sending visual information down a variety of parallel pathways, each of which builds upon the relatively simple, spatially compact RFs found in the early visual system, ultimately resulting in a unified perception of the visual world (Felleman and Van Essen, 1991). Strong attentional modulation at the LGN stage might introduce undesirable, nonspecific filtering, affecting all downstream visual pathways indiscriminately. A more efficient strategy is to reserve strong attentional modulation for higher-order areas where RFs are already task-tuned, for example, MT during motion discrimination or FFA during face recognition. Thus, in the context of spatial attention, the role of the LGN is to maintain a stable, unbiased flow of retinal input to V1, where visual information is distributed across parallel processing streams before attentional filtering occurs at later stages.
A parallel can be drawn to arousal-driven LGN modulation, which globally enhances or suppresses visual signals depending on behavioral state (Molnár et al., 2021; Crombie et al., 2024). Unlike spatial attention, arousal-based modulation affects all LGN neurons indiscriminately, ensuring coordinated gain control rather than interfering with specific spatial and/or feature-processing streams. Similarly, LGN activity is suppressed during modality-specific attention to nonvisual tasks, a scenario where broad suppression is beneficial rather than disruptive (Wimmer et al., 2015).
Animal and human experimental models often provide complementary evidence that, when combined, help build a more complete understanding of brain function. In the case of spatial attention and neural processing in the LGN, the two models appear to provide unique insight into how attention affects different components of the neural circuitry. In humans, spatial attention has been reliably shown to modulate LGN activity as measured by BOLD fMRI (Schneider and Kastner, 2009; Schneider, 2011; Ling et al., 2015; Poltoratski et al., 2019). In contrast, similar experiments in nonhuman primates using extracellular recordings to measure spiking activity have yielded far more variable results, ranging from relatively robust modulation to none at all (McAlonan et al., 2008; Jiang et al., 2015; Shah et al., 2022).
The reasons for this discrepancy are unclear, but a key methodological difference lies in the fundamental unit of measurement accessible to each technique. The BOLD signal reflects changes in blood oxygenation and is sensitive to the activity of large neural populations—potentially hundreds of thousands of neurons within a single voxel—averaged over both space and time, which should improve the detection of small changes in the firing rate. Importantly, while the BOLD signal is influenced by both spiking and subthreshold synaptic activity, it is only weakly correlated with the spiking output of the underlying network. Thus, it should be viewed as primarily reflecting aggregate input and local processing (Logothetis et al., 2001; Logothetis, 2008).
In contrast, extracellular electrophysiology captures the spiking activity of individual neurons, is largely blind to subthreshold events, and provides a direct readout of the output of the local network to downstream targets. Traditional single-unit recordings, including those used in the present study, typically capture only one or two neurons at a time. Even with modern multielectrode arrays, the number of neurons recorded simultaneously is orders of magnitude smaller than the population contributing to a single fMRI voxel. Thus, while fMRI provides a population-level view of neural dynamics emphasizing presynaptic activity, extracellular recordings offer a highly resolved but sparse sampling of network output. This fundamental difference in spatial scale and sensitivity likely contributes to the apparent inconsistency in results across methodologies.
One potential path toward resolving this discrepancy is to apply analytical methods that characterize population dynamics rather than focusing solely on individual neuron activity. Such an approach, applied to a sufficiently large sample of LGN neurons, may more reliably reveal an underlying influence of spatial attention on LGN processing. Although the current dataset is not ideal for this type of analysis (e.g., neurons were not recorded simultaneously), we took steps in this direction by applying SVM classification to our sequentially recorded data. Overall, this analysis partially supports the view that a population code enriches the detectability of spatial attention in the LGN; however, the influence of spatial attention is not evenly distributed across our recording sample. Instead, the SVM models suggest that attentional modulation in the LGN is carried by ∼10–20% of the neurons in our sample. This is in line with our finding that 16.6% of LGN neurons had a statistically significant attention index. Of course, this conclusion does not account for possible temporal dynamics in population activity which could depend on how attention influences a variety of factors including network state dynamics (van Kempen et al., 2021; Shi et al., 2022) and noise correlations (Cohen and Maunsell, 2009; Ruff and Cohen, 2014).
If we are to conclude that only a small percentage of LGN neurons are modulated by attention, there are at least three possible scenarios regarding this subpopulation. First, the modulated neurons may correspond to a known LGN cell type, such as inhibitory interneurons or misclassified koniocellular neurons. Second, the modulated neurons may represent a previously unidentified functional subtype. Finally, it is possible that the modulated neurons do not form a distinct category at all. Instead, the attentional signal may be weak, temporally unstable, and only rarely sufficient to drive detectable changes in spiking activity of LGN neurons.
Finally, we would like to address how the results of the SVM analysis should be interpreted in the context of our data. While SVM classification can be informative, it may also miss the functional point of LGN activity during spatial attention. An SVM asks whether an ideal observer could accurately classify attentional state based on the joint activity of an n-dimensional neuronal population (Pooresmaeili et al., 2010). Any neuron whose firing rate is reliably modulated by attention—regardless of whether that modulation is an increase or a decrease—can contribute to building a linear decision boundary in this space. In this sense, both increases and decreases in the firing rate are equally useful to the classifier (Hastie et al., 2009).
While SVM classification is a valuable analytical tool, a literal interpretation of the results can be misleading. LGN neurons do not “encode” attentional state, nor is there reason to believe that downstream areas such as V1 are trying to “decode” attentional state based on LGN spiking activity. The LGN's functional role is to relay visual information from the retina to the cortex. Spatial attention, likely via nonretinal sources, modulates this relay by slightly prioritizing the transmission of signals that originate from attended regions of visual space. In this view, the attentional state is not encoded in the LGN per se but instead alters the gain or fidelity of visual signals being passed through it.
Synthesis
Reviewing Editor: Niko Busch, Westfalische Wilhelms-Universitat Munster
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Keith Schneider.
# Synthesis
Both reviewers find this to be a well-executed and valuable study that addresses an important question about the influence of covert attention on LGN responses. They agree that the methods are sound, the findings clearly presented, and the conclusions generally well justified. Accordingly, they both recommend the manuscript for publication pending minor revisions.
One point raised by Reviewer 1 deserves particular attention. The current version of the abstract and discussion concludes that the small attentional effects observed in the LGN are unlikely to have downstream efficacy. While this is a reasonable interpretation, it extends beyond the data directly reported in the study. To substantiate this claim, the authors are encouraged to complement the cell-by-cell ROC analysis with a multivariate analysis across neurons, for example using a simple classifier to assess whether attentional state can be reliably decoded from population activity. It would also be important to remind the reader explicitly that the present study did not directly assess downstream effects.
# Reviewer 1
This is an interesting study that investigates whether visual attention modulates the firing rate of neurons in the primate LGN. It is interesting because there may be a discrepancy between human and primate LGN in this regard, although the methodologies differ. Overall I think this study was well done, but I do have a few comments and questions:
1. In comparing the present primate physiology data to human fMRI data, some discussion of the methods is warranted. The fMRI signal most closely is represented by the LFP rather than the spiking activity. Was the LFP recorded/analyzed here?
2. As in other areas, it may be the case that not every neuron exhibits attentional modulations, to be able to preserve other aspects of the network code. The discussion and abstract state that a small attentional effect is unlikely to have any downstream efficacy. However, if you consider the population of neurons together, isn't there a highly reliable attentional signal present? Pooresmaeili et al (2010) showed, for example, that attention and contrast were separable codes in V1. Might this also be the case here? It would be interesting and would add to this study if you were to calculate the accuracy of a trained classifier using multiple neurons. (and compare differences in the M and P populations)
3. In the human fMRI literature, the spatial and even feature-based attentional modulations are require reliable and have been replicated a number of times (see for example Schneider &Kastner (2009) and Schneider (2011). These studies should be cited as well.
4. Were the attentional effect consistent across the range of contrasts tested? In humans perhaps the attentional effect is best described as an additive factor across contrasts, whereas single-unit studies have found various other effects.
5. Does attention have any effect on the timing of responses, on the individual neuron or population level?
# Reviewer 2
This study on covert attention's role in modulating LGN responses addresses an inconsistency in the field, namely that some previous work found that covert attention modulates LGN activity, while others fail to find this effect. This paper addresses this question by using a covert attention task in non-human primates and quantifying not only the presence or absence of an effect, but also how significantly it shapes visual responses. This better addresses if attention has a substantive role in shaping vision in the LGN, compared to the robust effects that attention plays in higher visual areas. This study found that while there is a statistically significant effect, it is of small amplitude, suggesting that the LGN generally is not a site for attention-dependent modulation of vision, but a site where visual features are more faithfully relayed from the retina to the visual cortex. This is an important finding and a carefully conducted work that will significantly add to the field, and thus I recommend it is accepted for publication in eNeuro with only minor revisions. I do not need to see further revisions of this manuscript, since the changes I request are minor enough for only further editorial oversight.
Minor revisions:
1) in Figure 1, it is unclear that "M1" or "M2" mean monkey 1 and monkey 2. While it is stated in the text, it should also be mentioned in the figure legend.
2) in Figure 1C and E, are the bars standard deviations, sem or confidence intervals? While this information is in the methods, it should also be in the figure legends. This also applies to Figure 4A and 5B.
3) Throughout figures, statistical significance shouldn't be only on the text, but also in the figures between compared conditions (with *, **, ***, and n.s), to improve rapid interpretability of the results.
4) There is a lack of clarity in the difference between the valid and invalid conditions of stimulation explained in figure 1A-B and "attending toward" and "attending away". Perhaps an illustration similar to A-B should be added to figure 1 showing how the later concept relates to the former for the subsequent experiments done in the paper. Additional text in the early results section further detailing the stimulation paradigm switch between figure 1 and 2 would also be welcome.
5) Bar graphs like in Figure 1E should be replaced with displays of data that better show the distribution of the underlying data, like a violin plot or box-and-whiskers, but ideally showing all data points if there aren't too many.
Author Response
We thank the reviewers and editors for their thoughtful and constructive feedback on our manuscript, "Spatial Attention Weakly Modulates Visual Responses in the Lateral Geniculate Nucleus." We appreciate the opportunity to revise our work and have made numerous changes to clarify key points, address specific concerns, and incorporate suggested analyses. Below, we provide detailed, point-by-point responses to each comment and indicate how the manuscript has been revised accordingly. All changes to the manuscript are highlighted in red text.
Response to Reviewers Reviewer 1:
This is an interesting study that investigates whether visual attention modulates the firing rate of neurons in the primate LGN. It is interesting because there may be a discrepancy between human and primate LGN in this regard, although the methodologies differ. Overall I think this study was well done, but I do have a few comments and questions:
1. In comparing the present primate physiology data to human fMRI data, some discussion of the methods is warranted. The fMRI signal most closely is represented by the LFP rather than the spiking activity. Was the LFP recorded/analyzed here? • We agree that the fMRI BOLD signal is more closely related to local field potentials (LFPs) than to spiking activity. We have added a section to the Discussion that addresses the relationship between extracellular physiology and fMRI (lines 562-589).
The present manuscript is focused exclusively on single-unit responses, and we have chosen not to include LFP analyses here in order to maintain a clear focus. That said, we have collected LFP data from the same recording sessions and we explores these signals in detail in a separate manuscript. In that work, we examine how oscillatory activity in the LGN and V1 is modulated by visual stimulation and behavioral state, including a modest but statistically significant suppression of LGN beta-band oscillations when spatial attention is directed toward an LGN receptive field. This manuscript is available as a preprint and has been submitted for publication and it currently under review.
2. As in other areas, it may be the case that not every neuron exhibits attentional modulations, to be able to preserve other aspects of the network code. The discussion and abstract state that a small attentional effect is unlikely to have any downstream efficacy. However, if you consider the population of neurons together, isn't there a highly reliable attentional signal present? Pooresmaeili et al (2010) showed, for example, that attention and contrast were separable codes in V1. Might this also be the case here? It would be interesting and would add to this study if you were to calculate the accuracy of a trained classifier using multiple neurons. (and compare differences in the M and P populations) • We very much appreciate this suggestion and enjoyed exploring the use of linear classifiers on our data. Although our data represents a large population of sequentially recorded LGN neurons, we generated pseudo-populations and used support vector machines to address the modulation of LGN processing by spatial attention at the population level. To address these analyses, we have added large sections to the Methods (lines 214-241), Results (lines 444 - 501), and Discussion (lines 590-629), with more minor edits in the introduction.
3. In the human fMRI literature, the spatial and even feature-based attentional modulations require reliable and have been replicated a number of times (see for example Schneider &Kastner (2009) and Schneider (2011). These studies should be cited as well. • We have added text to the discussion section that cites these two papers, as well as two additional papers that also show modulation of LGN BOLD signal during spatial attention. In addition, we have added a section on comparing results between electrophysiological and fMRI studies (lines 562-589).
4. Were the attentional effect consistent across the range of contrasts tested? In humans perhaps the attentional effect is best described as an additive factor across contrasts, whereas single-unit studies have found various other effects. • Unfortunately, we only tested the effect at one contrast level for each cell we recorded.
We chose to use the contrast to evoke a half-maximum response (i.e., the cell's C50) to ensure each cell had the capacity to increase or decrease its firing rate with attention.
5. Does attention have any effect on the timing of responses, on the individual neuron or population level? • Our stimulus design incorporated a fixed phase at the time of the contrast change, but due to varying latencies between stimulus onset and the contrast change (necessary to prevent animals from anticipating the timing of contrast changes), the phase at stimulus onset varied across trials. This feature of the design precluded precise analysis of some temporal response properties, particularly response onset latency.
That said, we did examine other aspects of temporal dynamics, such as response duration, and found no significant differences between attention conditions. Thus, to maintain focus and clarity, we have decided not to report on these findings.
Reviewer 2:
This study on covert attention's role in modulating LGN responses addresses an inconsistency in the field, namely that some previous work found that covert attention modulates LGN activity, while others fail to find this effect. This paper addresses this question by using a covert attention task in non-human primates and quantifying not only the presence or absence of an effect, but also how significantly it shapes visual responses. This better addresses if attention has a substantive role in shaping vision in the LGN, compared to the robust effects that attention plays in higher visual areas. This study found that while there is a statistically significant effect, it is of small amplitude, suggesting that the LGN generally is not a site for attention-dependent modulation of vision, but a site where visual features are more faithfully relayed from the retina to the visual cortex. This is an important finding and a carefully conducted work that will significantly add to the field, and thus I recommend it is accepted for publication in eNeuro with only minor revisions. I do not need to see further revisions of this manuscript, since the changes I request are minor enough for only further editorial oversight.
Minor revisions:
1) in Figure 1, it is unclear that "M1" or "M2" mean monkey 1 and monkey 2. While it is stated in the text, it should also be mentioned in the figure legend. • We have altered the legend for figure 1 as suggested.
2) in Figure 1C and E, are the bars standard deviations, sem or confidence intervals? While this information is in the methods, it should also be in the figure legends. This also applies to Figure 4A and 5B. • We have added this information to the figure legends where needed.
3) Throughout figures, statistical significance shouldn't be only on the text, but also in the figures between compared conditions (with *, **, ***, and n.s), to improve rapid interpretability of the results. • The figures have been updated with the relevant p values.
4) There is a lack of clarity in the difference between the valid and invalid conditions of stimulation explained in figure 1A-B and "attending toward" and "attending away". Perhaps an illustration similar to A-B should be added to figure 1 showing how the later concept relates to the former for the subsequent experiments done in the paper. Additional text in the early results section further detailing the stimulation paradigm switch between figure 1 and 2 would also be welcome. • Figure 1 has been expanded to make the classification of "valid vs invalid" and "toward vs away trials" clearer. We have also added text to both the figure legend and Materials and Methods section to clarify this point.
5) Bar graphs like in Figure 1E should be replaced with displays of data that better show the distribution of the underlying data, like a violin plot or box-and-whiskers, but ideally showing all data points if there aren't too many. • We have altered the legend for figure 1 as suggested.
References
- Alitto HJ, Usrey WM (2004) Influence of contrast on orientation and temporal frequency tuning in ferret primary visual cortex. J Neurophysiol 91:2797–2808. 10.1152/jn.00943.2003 [DOI] [PubMed] [Google Scholar]
- Alitto H, Rathbun DL, Vandeleest JJ, Alexander PC, Usrey WM (2019) The augmentation of retinogeniculate communication during thalamic burst mode. J Neurosci 39:5697–5710. 10.1523/JNEUROSCI.2320-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Moore BD, Rathbun DL, Usrey WM (2011) A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. J Physiol 589:87–99. 10.1113/jphysiol.2010.190538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Balaram P, Kaas JH (2017) The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol 525:3207–3226. 10.1002/cne.24272 [DOI] [PubMed] [Google Scholar]
- Bonin V, Mante V, Carandini M (2005) The suppressive field of neurons in lateral geniculate nucleus. J Neurosci 25:10844–10856. 10.1523/JNEUROSCI.3562-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshra R, Kastner S (2022) Attention control in the primate brain. Curr Opin Neurobiol 76:102605. 10.1016/j.conb.2022.102605 [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S (2004) Attention alters appearance. Nat Neurosci 7:308–313. 10.1038/nn1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerkevich CM, Lyon DC, Balaram P, Kaas JH (2014) Distribution of cortical neurons projecting to the superior colliculus in macaque monkeys. Eye Brain 23:121–137. 10.2147/EB.S53613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12:1594–1600. 10.1038/nn.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Preddie DC, Gallant JL, Van Essen DC (1997) Spatial attention effects in macaque area V4. J Neurosci 17:3201–3214. 10.1523/JNEUROSCI.17-09-03201.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie D, Spacek MA, Leibold C, Busse L (2024) Spiking activity in the visual thalamus is coupled to pupil dynamics across temporal scales. PLoS Biol 22:e3002614. 10.1371/journal.pbio.3002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47. 10.1093/cercor/1.1.1 [DOI] [PubMed] [Google Scholar]
- Glenn LL, Steriade M (1982) Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci 2:1387–1404. 10.1523/JNEUROSCI.02-10-01387.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning, Ed 2. New York: Springer US. [Google Scholar]
- Heeger DJ (1992) Normalization of cell responses in cat striate cortex. Vis Neurosci 9:181–197. 10.1017/S0952523800009640 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yampolsky D, Purushothaman G, Casagrande VA (2015) Perceptual decision related activity in the lateral geniculate nucleus. J Neurophysiol 114:717–735. 10.1152/jn.00068.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara P, Reinagel P, Reid RC (2000) Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron 27:635–646. 10.1016/S0896-6273(00)00072-6 [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG (2000) Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23:315–341. 10.1146/annurev.neuro.23.1.315 [DOI] [PubMed] [Google Scholar]
- Lauritzen TZ, Ales JM, Wade AR (2010) The effects of visuospatial attention measured across visual cortex using source-imaged, steady-state EEG. J Vis 10:1–17. 10.1167/10.14.39 [DOI] [PubMed] [Google Scholar]
- Ling S, Pratte MS, Tong F (2015) Attention alters orientation processing in the human lateral geniculate nucleus. Nat Neurosci 18:496–498. 10.1038/nn.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453:869–878. 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM (1992) Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J Neurophysiol 68:2185–2198. 10.1152/jn.1992.68.6.2185 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R (1997) Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77:24–42. 10.1152/jn.1997.77.1.24 [DOI] [PubMed] [Google Scholar]
- Lynch JC, Graybiel AM, Lobeck LJ (1985) The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol 235:241–254. 10.1002/cne.902350207 [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J (2022) Visual attention in the prefrontal cortex. Annu Rev Vis Sci 8:407–425. 10.1146/annurev-vision-100720-031711 [DOI] [PubMed] [Google Scholar]
- Maunsell JHR (2015) Neuronal mechanisms of visual attention. Annu Rev Vis Sci 1:373–391. 10.1146/annurev-vision-082114-035431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC (2005) Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci 25:11023–11033. 10.1523/JNEUROSCI.2904-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH (2008) Guarding the gateway to cortex with attention in visual thalamus. Nature 456:391–394. 10.1038/nature07382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR (1992) A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68:1384–1400. 10.1152/jn.1992.68.4.1384 [DOI] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB (2015) Brain state dependent activity in the cortex and thalamus. Curr Opin Neurobiol 31:133–140. 10.1016/j.conb.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyyappan S, Rajan A, Yang Q, Mangun GR, Ding M (2025) Decoding visual spatial attention control. eNeuro 12:1–20. 10.1523/ENEURO.0512-24.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH (2007) Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55:131–141. 10.1016/j.neuron.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH (2009) Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63:879–888. 10.1016/j.neuron.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár B, Sere P, Bordé S, Koós K, Zsigri N, Horváth P, Lőrincz ML (2021) Cell type-specific arousal-dependent modulation of thalamic activity in the lateral geniculate nucleus. Cereb Cortex Commun 2:tgab020. 10.1093/texcom/tgab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni AM, Maunsell JHR (2019) Neuronal effects of spatial and feature attention differ due to normalization. J Neurosci 39:5493–5505. 10.1523/JNEUROSCI.2106-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S (2002) Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5:1203–1209. 10.1038/nn957 [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ (1996) Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381:607–609. 10.1038/381607a0 [DOI] [PubMed] [Google Scholar]
- Poltoratski S, Maier A, Newton AT, Tong F (2019) Figure-ground modulation in the human lateral geniculate nucleus is distinguishable from top-down attention. Curr Biol 29:2051–2057.e3. 10.1016/j.cub.2019.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooresmaeili A, Poort J, Thiele A, Roelfsema PR (2010) Separable codes for attention and luminance contrast in the primary visual cortex. J Neurosci 30:12701–12711. 10.1523/JNEUROSCI.1388-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI (1980) Orienting of attention. Q J Exp Psychol 32:3–25. 10.1080/00335558008248231 [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D (2012) Mechanisms of neuronal computation in mammalian visual cortex. Neuron 75:194–208. 10.1016/j.neuron.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM (2000) Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci 17:55–62. 10.1017/S0952523800171056 [DOI] [PubMed] [Google Scholar]
- Ramezanpour H, Fallah M (2022) The role of temporal cortex in the control of attention. Curr Res Neurobiol 3:100038. 10.1016/j.crneur.2022.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinagel P, Godwin D, Sherman SM, Koch C (1999) Encoding of visual information by LGN bursts. J Neurophysiol 81:2558–2569. 10.1152/jn.1999.81.5.2558 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L (2004) Attentional modulation of visual processing. Annu Rev Neurosci 27:611–647. 10.1146/annurev.neuro.26.041002.131039 [DOI] [PubMed] [Google Scholar]
- Ruff DA, Cohen MR (2014) Attention can either increase or decrease spike count correlations in visual cortex. Nat Neurosci 17:1591–1597. 10.1038/nn.3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S (2011) Cognitive and perceptual functions of the visual thalamus. Neuron 71:209–223. 10.1016/j.neuron.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AN, Alitto HJ, Rathbun DL, Fisher TG, Usrey WM (2023) Stimulus contrast modulates burst activity in the lateral geniculate nucleus. Curr Res Neurobiol 4:100096. 10.1016/j.crneur.2023.100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA (2011) Subcortical mechanisms of feature-based attention. J Neurosci 31:8643–8653. 10.1523/JNEUROSCI.6274-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Kastner S (2009) Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J Neurosci 29:1784–1795. 10.1523/JNEUROSCI.4452-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidemann E, Newsome WT (1999) Effect of spatial attention on the responses of area MT neurons. J Neurophysiol 81:1783–1794. 10.1152/jn.1999.81.4.1783 [DOI] [PubMed] [Google Scholar]
- Shah S, Mancarella M, Hembrook-Short JR, Mock VL, Briggs F (2022) Attention differentially modulates multiunit activity in the lateral geniculate nucleus and V1 of macaque monkeys. J Comp Neurol 530:1064–1080. 10.1002/cne.25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Victor JD (1979) Nonlinear spatial summation and the contrast gain control of cat retinal ganglion cells. J Physiol 290:141–161. 10.1113/jphysiol.1979.sp012765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2001) Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24:122–126. 10.1016/S0166-2236(00)01714-8 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW (2002) The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc London B Biol Sci 357:1695–1708. 10.1098/rstb.2002.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Usrey WM (2021) Cortical control of behavior and attention from an evolutionary perspective. Neuron 109:3048–3054. 10.1016/j.neuron.2021.06.021 [DOI] [PubMed] [Google Scholar]
- Shi Y-L, Steinmetz NA, Moore T, Boahen K, Engel TA (2022) Cortical state dynamics and selective attention define the spatial pattern of correlated variability in neocortex. Nat Commun 13:44. 10.1038/s41467-021-27724-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JH (1999) Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci 19:7591–7602. 10.1523/JNEUROSCI.19-17-07591.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Alitto HJ (2015) Visual functions of the thalamus. Annu Rev Vis Sci 1:351–371. 10.1146/annurev-vision-082114-035920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen J, Gieselmann MA, Boyd M, Steinmetz NA, Moore T, Engel TA, Thiele A (2021) Top-down coordination of local cortical state during selective attention. Neuron 109:894–904.e8. 10.1016/j.neuron.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM (2015) Thalamic control of sensory selection in divided attention. Nature 526:705–709. 10.1038/nature15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ (2012) Attention deficits without cortical neuronal deficits. Nature 489:434–437. 10.1038/nature11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, Desimone R (2016) Pulvinar-cortex interactions in vision and attention. Neuron 89:209–220. 10.1016/j.neuron.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]