Abstract
Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype involving alterations in both the cytoplasmic membrane and the cell wall and a requirement for divalent cations. In addition to a lower ratio of C15 to C17 fatty acids than in the wild-type strain (A. S. Mazzotta and T. J. Montville, J. Appl. Microbiol. 82:32–38, 1997), this nisin-resistant (Nisr) strain contained significantly more zwitterionic phosphatidylethanolamine and less anionic phosphatidylglycerol and cardiolipin. The extraction of cardiolipin was enhanced by a penicillin-lysozyme step to disrupt the cell wall. This study is the first to quantify the phosphatidylethanolamine component of the L. monocytogenes cytoplasmic membrane. While these cytoplasmic membrane changes were induced by nisin, the Nisr strain also showed altered sensitivities to cell wall-acting compounds, even when grown in the absence of nisin, suggesting a constitutive alteration in the strain’s cell wall. A model which integrates the roles of the cell membrane, cell wall, and divalent cations is presented. Finally, nisin resistance in L. monocytogenes ATCC 700302 conferred cross-resistance to the class IIa bacteriocin pediocin PA-1 and the class IV leuconocin S.
Listeria monocytogenes, the causative agent of listeriosis, has resulted in numerous major food-borne outbreaks worldwide (16). The ability of L. monocytogenes to grow at temperatures ranging from 1 to 45°C (19, 41, 42), its high tolerance for salt (11), and its ability to initiate growth at a relatively low pH (19) make this pathogen particularly difficult to control in food. A novel approach to controlling L. monocytogenes in food is the use of antimicrobial bacteriocins from lactic acid bacteria (36). Nisin, a lanthionine-containing peptide produced by certain strains of Lactococcus lactis (26) with antimicrobial activity against L. monocytogenes (2–4, 25), is a bacteriocin with many “generally recognized as safe” applications in the United States (12–14).
Nisin’s action stems from the disruption of the cell’s cytoplasmic membrane, as evidenced by the rapid efflux of small molecules from both whole cells and liposomes (1, 3, 18, 39, 44). As a result, nisin depletes the proton motive force (PMF) of sensitive cells and artificial liposomes (3, 17, 37, 39). Nisin acts through a multistep process which includes binding of nisin to the cell, insertion into the membrane, and pore formation (10, 18, 35, 40, 44). Anionic phospholipids play an important role in nisin’s interaction with membranes (9, 10, 30). On binding to anionic phospholipids, nisin causes a local perturbation of the lipid bilayer (10), followed by electrical potential (Δψ)- or pH gradient (ΔpH)-enhanced insertion into the membrane to form a wedge-like pore (35).
Nisin’s efficacy as an antilisterial agent would be compromised by the emergence of nisin-resistant L. monocytogenes in a food-processing situation. The generation of nisin-resistant (Nisr) L. monocytogenes mutants in the laboratory is easily achieved by exposure to high concentrations of nisin (21, 32, 33). Given the role of the cytoplasmic membrane in nisin’s mechanism of action, several researchers have examined membrane compositional changes to explain nisin resistance in L. monocytogenes. Resistance has been correlated with both an altered fatty acid composition (32, 33) and an altered phospholipid composition (34). When grown in the presence of nisin, the Nisr strain (32) investigated in this study contains a lower ratio of C15 to C17 fatty acids than the wild-type or Nisr strain grown in the absence of nisin. In addition to membrane compositional changes, the cell wall may also be involved in nisin resistance in L. monocytogenes (8).
The objectives of this research were to investigate the roles of both the cytoplasmic membrane and the cell wall in a single nisin-resistant strain of L. monocytogenes, to investigate whether nisin resistance results in intrinsic resistance to other antimicrobials, and to develop a model of nisin’s interaction with nisin-resistant L. monocytogenes.
MATERIALS AND METHODS
Bacterial cultures, growth media, and nisin stock preparations.
All bacterial stock cultures were maintained in their appropriate broth containing 20% glycerol at −80°C. L. monocytogenes Scott A was cultured in Trypticase soy broth without dextrose (BBL Microbiology Systems, Cockeysville, Md.), supplemented with 0.6% yeast extract (Difco Laboratories, Detroit, Mich.) and 0.5% glucose (Fisher Scientific Co., Fair Lawn, N.J.) (TSBYEG). The nisin-resistant strain of L. monocytogenes Scott A was isolated at 30°C after the wild-type strain was plated on TSBYEG agar plates containing 1,000 IU of nisin preparation per ml (32). This Nisr strain was recently deposited in the American Type Culture Collection with accession no. ATCC 700302. The Nisr strain was maintained and cultured in TSBYEG containing 1,000 IU of nisin preparation per ml. L. lactis ATCC 11454, L. lactis ATCC 21053, Leuconostoc paramesenteroides OX (28), and Pediococcus acidilactici PAC1.0 (provided by J. L. Swezey, USDA Agricultural Research Service Midwest Area, Peoria, Ill.) were cultured in Lactobacillus MRS (Difco) broth. Working cultures were maintained at 4°C on agar slants solidified with 1.5% Bacto-Agar (Difco). All cultures were grown at 30°C. TSBYEG diffusion agar contained 0.1% Tween 20 and was solidified with 1.5% Noble agar (Difco).
Nisin stock solutions were prepared from either pure nisin (a gift of Aplin and Barrett, Trowbridge, England) or nisin preparation (Sigma, St. Louis, Mo., or Aplin and Barrett Ltd.), as noted, in 0.2 N HCl–0.75% NaCl (nisin diluent) and autoclaved at 15 lb/in2 for 15 min.
Identification and quantification of membrane phospholipids.
Overnight cultures of wild-type cells and of Nisr cells grown in the presence of 1,000 IU of nisin preparation per ml were centrifuged; the cell pellets were resuspended in TSBYEG containing 20% (wt/vol) sucrose, 0.2% (wt/vol) MgSO4, 8 mg of lysozyme (Sigma) per ml, and 1,000 U of penicillin G (Sigma) per ml and incubated for another 2 h at 30°C. The lysozyme-penicillin treatment disrupted the cell wall, a step necessary for the complete extraction of cardiolipin from stationary-phase gram-positive bacteria (15). The cells were then pelleted again and the lipids were extracted as previously described (45).
The dried lipid extract was resuspended in chloroform and separated by high-performance thin-layer chromatography (HPTLC) (23) on Silica Gel 60 HPTLC plates with concentrating zones (E. Merck, Darmstadt, Germany). For two-dimensional analysis, the plates were developed first in chloroform-methanol-water (65:25:4, vol/vol) and then in chloroform-methanol-acetic acid-water (50:25:6:2, vol/vol). For one-dimensional analysis, the plates were developed with chloroform-methanol-acetic acid-water (50:25:6:2, vol/vol). Lipid spots were visualized by staining with iodine vapors. Phosphatidylglycerol and cardiolipin were identified on the basis of their relative mobilities compared with those of authentic standards, as well as by reaction with the specific detection reagents ninhydrin, Dragendorff, and molybdenum blue. The spot identified as phosphatidylethanolamine stained ninhydrin positive, Dragendorff negative, and molybdenum blue positive. In one-dimensional thin-layer chromatography (TLC), it migrated similarly to the amine-containing phospholipids phosphatidylserine, phosphatidylethanolamine, and phosphatidylmonomethylethanolamine. This spot was identified as phosphatidylethanolamine following extraction from a one-dimensional silica gel plate, acid hydrolysis to yield the free phospholipid head group base, and comparison to similarly prepared standards separated on a cellulose TLC plate (Merck) developed in phenol-ethanol-acetic acid (50:5:6, vol/vol) (6).
The phospholipids were quantified via radioactive counting of 32P-labeled phospholipids. Wild-type and Nisr cells were labeled by being grown overnight in TSBYEG containing 0.1 mCi of [32P]inorganic phosphorus (New England Nuclear). The lipid extracts were prepared and separated by two-dimensional HPTLC as described above. Spots identified by autoradiography were scraped off, and radioactivity was measured with a Beckman LS6500 multipurpose scintillation counter. The phospholipid concentrations were calculated by assuming that each phospholipid contains one phosphate group, with the exception of cardiolipin, which contains two phosphate groups, and were expressed as percentages of total phospholipid.
Lysozyme sensitivity.
Overnight cultures of wild-type cells, Nisr cells, and Nisr cells grown with 1,000 IU of nisin preparation per ml were inoculated at 1% (vol/vol) into fresh TSBYEG containing 10 mM MgCl2 and 4 mg of lysozyme (Sigma) per ml. The cultures were incubated at 30°C, and absorbances at 620 nm were monitored spectrophotometrically.
MIC determinations.
The MICs of antimicrobials for the wild-type and Nisr strains were determined by a spiral gradient agar dilution technique as previously described (32). This method is based on the establishment of a spiral gradient of antimicrobial in an agar plate by using a Spiral Plater (model D; Spiral Biotech, Inc.). Antimicrobial stock solutions were spiral plated over 20 ml of TSBYEG agar, and the plates were held at room temperature for 4 h. Overnight cultures of wild-type cells, Nisr cells, or Nisr cells grown in the presence of 1,000 IU of nisin preparation per ml were then radially swabbed across the antimicrobial gradient, from low to high concentrations, with a sterile swab. The plates were incubated at 30°C for 48 h. The growth endpoint was measured and the MIC was calculated. Stock solutions consisted of gramicidin S, chloramphenicol, and tetracycline in methanol; nigericin and erythromycin in acetone; benzylpenicillin, ampicillin, gentamicin, cycloserine, streptomycin, and vancomycin in water; and nisin in nisin diluent. Control plates were prepared with either methanol, acetone, distilled water, or nisin diluent. All antimicrobials other than nisin were obtained from Sigma.
Transmission EM.
Electron microscopy (EM) was utilized to visualize the cell wall of wild-type and Nisr L. monocytogenes. Mid-log-phase wild-type cells, Nisr cells, and Nisr cells grown with 500 IU of nisin per ml were prepared for EM by resuspending cell pellets in TSBYEG broth containing 2% (vol/vol) glutaraldehyde (Sigma). The cells were then again pelleted and shipped under the glutaraldehyde broth supernatant. The EM work was kindly conducted by Peter Cooke at the USDA Eastern Regional Research Center in Philadelphia, Pa.
Determination of the PMF, pH gradient, and Δψ.
The PMF, an electrochemical gradient of protons across the cytoplasmic membrane, is required to drive a number of bacterial energy-requiring processes, such as ATP synthesis, protein phosphorylation, flagellar rotation, and active transport. The PMF is composed of a Δψ and a ΔpH. These two components of the PMF were determined as previously described (4). The pH potential is calculated as ZΔpH. Factor Z, used to convert pH units to millivolts, represents 2.3 RT/F, where R is the universal gas constant, T is temperature in degrees kelvin, and F represents Faraday’s constant. The value of Z is 59 mV at 25°C. The results are calculated as the means of three independent experiments. Variances were analyzed and compared by a general linear model procedure using SAS (SAS Institute Inc., Cary, N.C.).
The degree of lethality caused by nisin under the conditions of the PMF assay was determined. Cells were treated as in the PMF assay except for the absence of the radiolabeled probes, diluted in 0.1% peptone water, and spiral plated on TSBYEG agar plates. The plates were incubated at 30°C, and colonies were counted after 24 h.
Divalent-cation requirement of Nisr L. monocytogenes.
Overnight cultures of wild-type or Nisr cells were harvested by centrifugation, washed once with phosphate-buffered saline, and resuspended in 50 mM MES (morpholineethanesulfonic acid) buffer (pH 6.5) containing either divalent cations alone, divalent cations plus EDTA, EDTA alone, or none of these. The metal ions tested, all at a final concentration of 10 mM, were MgSO4, MgCl2, CaCl2, MnSO4, BaCl2, NaCl, and KCl. The final concentration of EDTA was 20 mM. The cells were then treated with a final concentration of 60 IU of nisin per ml for 20 min. Control samples were not treated with nisin. The cells were then diluted in 0.1% peptone water and spiral plated on TSBYEG agar plates. The plates were incubated at 30°C, and colonies were counted with a laser colony counter after 48 h. The results were reported as log reductions in cell viability relative to an untreated control.
The effect of MgSO4 concentration on the killing action of 1,000 IU of nisin per ml against wild-type and Nisr cells was determined. Overnight cultures of wild-type cells and Nisr cells grown with 1,000 IU of nisin preparation per ml were centrifuged, washed with 50 mM MES buffer (pH 6.5), and resuspended in MES buffer. The cells were energized with a final glucose concentration of 10 mM for 15 min at room temperature and centrifuged. The cells were then resuspended in various concentrations of MgSO4 in MES buffer and treated with a final concentration of 1,000 IU of nisin per ml for 15 min. Controls were treated with nisin alone or MgSO4 alone or were left untreated. The samples were diluted in 0.1% peptone water and spiral plated on TSBYEG agar plates. The colonies were counted after 24 h. Results were expressed as the log reduction in cell numbers for a given treatment relative to the untreated control.
Determination of bacteriocin cross-resistance.
The sensitivities of wild-type and Nisr L. monocytogenes to bacteriocins produced by different lactic acid bacteria were determined. MRS agar containing 0.5% glucose was prepared in MES buffer at pH 6.5 to neutralize acid production. Aliquots (20 μl) of overnight lactic acid bacteria cultures were dispensed in 6.8-mm-diameter wells cut into a 15-ml MRS agar layer. After a 48-h incubation at 30°C, the plates were overlaid with 10 ml of TSBYEG agar with 0.1% Tween 20 containing either wild-type or Nisr L. monocytogenes. The L. monocytogenes inocula were obtained by adjusting the absorbance at 660 nm of overnight cultures to 0.4 and adding 1 μl/ml to the TSBYEG agar to yield 105 CFU/ml. The plates were incubated at 30°C, and the inhibition zones were measured from the edge of the well containing the lactic acid bacteria to the edge of the inhibition zone.
RESULTS
Phospholipid composition.
The Nisr cells, when grown in the presence of 1,000 IU of nisin preparation per ml, contained significantly (P < 0.05) more phosphatidylethanolamine than either wild-type cells or Nisr cells grown in the absence of nisin (Table 1). The Nisr cells also contained less cardiolipin and phosphatidylglycerol. These decreases were not statistically significant. However, when cardiolipin and phosphatidylglycerol were combined to give the total anionic phospholipids, this value was significantly decreased for Nisr cells grown with nisin. No significant differences between wild-type cells and Nisr cells grown in the absence of nisin for any of the phospholipids or between any of the strains for an unidentified amine-containing phospholipid and two other minor phospholipids were found.
TABLE 1.
Phospholipid compositions of wild-type cells and Nisr cells grown in the absence of nisin and Nisr cells grown with nisin
| Phospholipid | % of total phospholipids fora:
|
||
|---|---|---|---|
| Cells grown without nisin
|
Nisr cells grown with nisinb | ||
| Wild type | Nisr | ||
| Phosphatidylglycerol | 28.6 ± 5.8A | 26.8 ± 4.3A | 24.6 ± 3.2A |
| Cardiolipin | 21.8 ± 5.8A | 23.3 ± 1.9A | 18.3 ± 5.6A |
| Phosphatidylethanolamine | 8.7 ± 3.7A | 7.5 ± 0.8A | 16.2 ± 3.4B |
| Amino-containing phospholipids | 23.4 ± 6.1A | 25.8 ± 2.5A | 26.7 ± 6.7A |
| Unidentified phospholipid | 8.2 ± 1.4A | 7.21 ± 1.6A | 6.0 ± 0.3A |
| Unidentified phospholipid | 9.7 ± 6.0A | 9.5 ± 5.8A | 6.9 ± 4.0A |
| Anionic phospholipids (phosphatidylglycerol + cardiolipin) | 50.4 ± 7.7A | 50.1 ± 4.2A | 41.4 ± 5.5B |
Values are means ± standard deviations from at least three independent experiments. Different letters indicate statistically significant (P < 0.05) differences between strains for a given phospholipid.
Nisin preparation (1,000 IU/ml).
Lysozyme sensitivity.
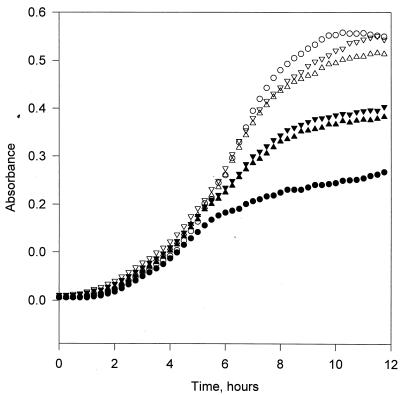
In the absence of lysozyme, the wild-type and Nisr strains exhibited similar growth characteristics (Fig. 1), attaining a final optical density at 660 nm of approximately 0.5. In the presence of 4 mg of lysozyme per ml, the Nisr strain reached a higher final optical density of approximately 0.4, compared to 0.25 for the wild-type strain.
FIG. 1.
Growth of wild-type (circles) and Nisr (triangles) strains of L. monocytogenes in TSBYEG at 30°C. The Nisr culture inoculum was grown either in the absence (▵ and ▴) or in the presence (▿ and ▾) of 1,000 IU of nisin preparation per ml. The cells were treated with 4 mg of lysozyme at time zero (closed symbols) or left untreated (open symbols).
MIC determinations.
The MICs of all the antibiotics tested for Nisr cells grown in the absence of nisin (data not shown) were not significantly different (P < 0.05) from those for the Nisr strain grown in the presence of 1,000 IU of nisin preparation per ml (Table 2). The MIC of nisin for the Nisr strain was 1,423 ± 310 IU/ml, compared to 240 ± 31 IU/ml for the wild-type strain (means ± standard deviations). The MICs of benzylpenicillin and ampicillin for the Nisr strain were approximately 10-fold lower than those for the wild-type strain (significant at P < 0.001). The Nisr strain was also slightly more sensitive to cycloserine (significant at P < 0.001). In addition, small but significant (P < 0.001) increases in the MICs of gramicidin S and gentamicin for the Nisr strain were observed. No significant differences in the MICs of any of the other antibiotics tested were found.
TABLE 2.
MICs of antimicrobials for wild-type L. monocytogenes grown in the absence of nisin and Nisr L. monocytogenes grown in the presence of nisin
| Antimicrobial | Site of action | MIC, μg/mla
|
|
|---|---|---|---|
| Wild type | Nisin resistant | ||
| Benzylpenicillin | Cell wall synthesis | 0.26 ± 0.04A | 0.03 ± 0.00B |
| Ampicillin | Cell wall synthesis | 0.33 ± 0.08A | 0.05 ± 0.00B |
| Cycloserine | Cell wall synthesis | 26.18 ± 4.01A | 14.81 ± 2.47B |
| Vancomycin | Cell wall synthesis | 0.63 ± 0.11A | 0.59 ± 0.11A |
| Gramicidin S | Cytoplasmic membrane | 2.07 ± 0.25A | 3.27 ± 0.64B |
| Nigericin | Cytoplasmic membrane | 0.09 ± 0.02A | 0.09 ± 0.01A |
| Gentamicin | Protein synthesis (30S) | 2.79 ± 0.30A | 6.38 ± 1.26B |
| Tetracycline | Protein synthesis (30S) | 1.31 ± 0.57A | 0.98 ± 0.30A |
| Streptomycin | Protein synthesis (30S) | 10.89 ± 1.33A | 13.58 ± 2.68A |
| Erythromycin | Protein synthesis (50S) | 0.39 ± 0.07A | 0.39 ± 0.10A |
| Chloramphenicol | Protein synthesis (50S) | 3.57 ± 0.77A | 3.27 ± 0.98A |
Values are means ± standard deviations. Different letters for the wild-type and Nisr strains for a given antimicrobial indicate statistically significant differences (P < 0.001).
Transmission EM.
No significant morphological differences between wild-type and Nisr cells were observed by transmission EM at ×70,000 magnification. Wild-type and Nisr cells contained cell walls of comparable thicknesses (approximately 360 nm).
PMF measurements.
The PMFs of wild-type and Nisr L. monocytogenes were measured both in the absence and in the presence of nisin. Basal PMFs of −122 ± 11 and −117 ± 13 mV (means ± standard deviations) were measured for the wild-type and Nisr strains, respectively (Fig. 2). These values were not significantly different (P < 0.05). The addition of 60 IU of nisin per ml to the wild-type strain caused a significant (P < 0.05) dissipation of the PMF, to −15.0 ± 5.3 mV. Treatment of the Nisr strain with the same concentration of nisin caused the PMF to be lowered by only 21%, to −100.3 ± 9.8 mV. The partially depleted PMF of the Nisr strain was significantly lower (P < 0.05) than that strain’s resting PMF and significantly higher (P < 0.05) than the wild-type strain’s depleted PMF. Under the conditions of the PMF assay, nisin resulted in a 3.2-log reduction in wild-type cell numbers and a 0.5-log reduction in Nisr cells.
FIG. 2.
Total PMF values (means and standard deviations), represented as the sum of Δψ and ZΔpH, for wild-type and Nisr L. monocytogenes in the absence and presence of 60 IU of nisin per ml. Mean values for Δψ and ZΔpH appear to the right of each bar. Different letters indicate statistically significant differences (P < 0.05) for the total PMF.
Divalent-cation requirement of Nisr L. monocytogenes.
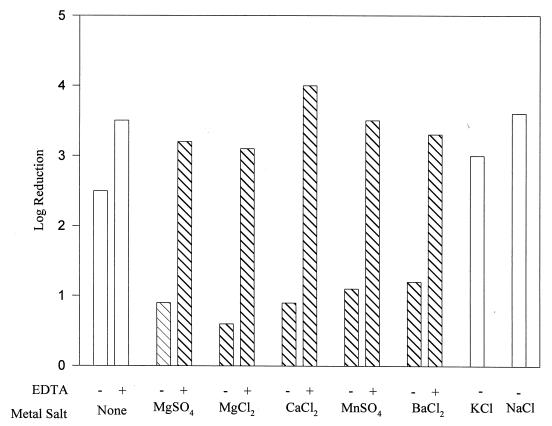
In the course of this study, we realized that while the Nisr strain was able to resist the effects of nisin either in broth or on agar plates, it was unable to do so when suspended in buffer. A major component of growth media which could be required by the Nisr strain to resist nisin is divalent cations. Therefore, the nisin sensitivity of the Nisr strain in the absence and presence of different divalent cations was assessed. The viability of Nisr cells suspended in MES buffer and treated with 60 IU of nisin per ml was reduced by 2.5 log cycles (Fig. 3). Inclusion of either 10 mM MgSO4, MgCl2, CaCl2, MnSO4, or BaCl2 reduced the lethality caused by nisin to an approximately 1-log reduction or less. In the absence of nisin, the divalent cations had no effect on cell viability. This effect was attributed to the divalent cation, as opposed to the anion, on the basis of the similar results obtained with MgSO4 and MgCl2 and the lack of effect of KCl and NaCl. The effect was confirmed to be due to the divalent cation by experiments involving EDTA, a chelator of divalent cations. Inclusion of 20 mM EDTA in any of the systems containing divalent cations increased the lethality caused by nisin to a 3- to 4-log reduction. In a parallel experiment with wild-type cells, nisin was equally lethal regardless of the presence or absence of divalent cations (data not shown). The metal salts and EDTA, either alone or in combination, had no effect on the viability of either strain.
FIG. 3.
Log reduction in cell viability of Nisr L. monocytogenes by 60 IU of nisin per ml in the absence (−) and presence (+) of 20 mM EDTA and 10 mM metal salts.
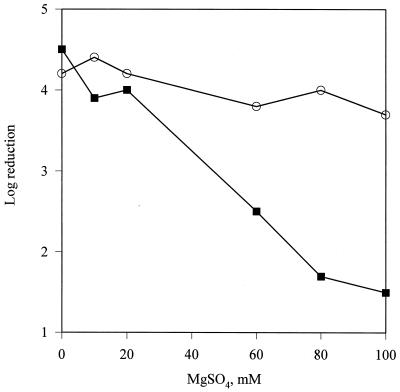
A concentration dependence of the divalent cation magnesium on the ability of the Nisr strain to resist nisin was demonstrated. Nisin, in the absence of MgSO4, caused an approximately 4.5-log reduction in both wild-type and Nisr cell numbers (Fig. 4). For the wild-type strain, the log reduction caused by nisin remained constant at approximately 4, even in the presence of concentrations of MgSO4 up to 100 mM. With the Nisr strain, as the concentration of MgSO4 was increased, the lethality caused by nisin decreased to approximately 1.5 log at 80 mM MgSO4.
FIG. 4.
Concentration dependence of MgSO4 on the log reduction in cell viability caused by 1,000 IU of nisin per ml against wild-type (○) and Nisr (▪) L. monocytogenes.
Determination of bacteriocin cross-resistance.
The sensitivity of the Nisr strain to other lactic acid bacteria bacteriocins was assessed. Neither the wild-type nor the Nisr strain was inhibited by L. lactis ATCC 21053, the control strain which does not produce bacteriocin (Table 3). As expected, the Nisr strain was not inhibited by L. lactis ATCC 11454, the nisin-producing strain. The Nisr strain was less sensitive to pediocin PA-1 and leuconocin S than the wild-type strain.
TABLE 3.
Deferred antagonism of wild-type and nisin-resistant L. monocytogenes by bacteriocin-producing lactic acid bacteria
| Test strain | Bacteriocin produced | Inhibitiona of:
|
||
|---|---|---|---|---|
| Cells grown without nisin
|
Nisr cells grown with nisin | |||
| Wild type | Nisr | |||
| L. lactis | ||||
| ATCC 21053 | None | 0 | 0 | 0 |
| ATCC 11454 | Nisin | 2.77 ± 0.24 | 0 | 0 |
| P. acidilactici PAC1.0 | Pediocin PA-1 | 8.94 ± 0.32 | 1.79 ± 0.45 | 2.01 ± 0.27 |
| Leuconostoc paramesenteroides S | Leuconocin OX | 8.61 ± 1.05 | 1.78 ± 0.22 | 1.41 ± 0.34 |
Radius of inhibition zone from edge of the well to the edge of the L. monocytogenes lawn, in millimeters (means ± standard deviations).
DISCUSSION
Nisin resistance in L. monocytogenes has been correlated with altered cytoplasmic membrane fatty acids (32, 33) and phospholipid compositions (34) and with alterations in the cell wall (8). This study identifies all of these changes, in addition to a requirement for divalent cations, in a single nisin-resistant strain, demonstrating that resistance to nisin in L. monocytogenes occurs via a complex mechanism.
Alterations in the cytoplasmic membrane composition of nisin-resistant L. monocytogenes were first reported by Ming and Daeschel (33, 34). Their nisin-resistant mutant contained more straight-chain and fewer branched-chain fatty acids (33) and less phosphatidylglycerol and cardiolipin (34) than the wild type. Similar changes in membrane fatty acid composition, including increased long-chain fatty acids, decreased short-chain acids, and a lower C15/C17 ratio, were found for Nisr L. monocytogenes ATCC 700302 (32). Both laboratories concluded that the observed fatty acid composition changes were consistent with a less fluid cytoplasmic membrane and that this increase in rigidity might prevent nisin from inserting into the membrane. In addition, we report here that the Nisr strain ATCC 700302 contained more of the zwitterionic phospholipid phosphatidylethanolamine and less of the anionic phospholipids phosphatidylglycerol and cardiolipin than the wild-type cells. A major difference between the Nisr strain ATCC 700302 and the nisin-resistant strain studied by Ming and Daeschel (34) is that phosphatidylethanolamine was a major component of the Nisr ATCC 700302 strain. While this study confirmed previous reports that the major phospholipids in L. monocytogenes are phosphatidylglycerol and cardiolipin (27, 31), it is the first to identify and quantify the phosphatidylethanolamine component of the cytoplasmic membrane of L. monocytogenes. Given the role that anionic phospholipids play in nisin’s interaction with membranes (10, 18, 30), a decrease in the net negative charge of the lipid bilayer might hinder nisin’s ability to bind and interact with the membrane.
Both the fatty acid composition change (32) and the alteration in membrane phospholipids were observed only when Nisr L. monocytogenes ATCC 700302 was grown in the presence of nisin, indicating that nisin induced these changes. This suggested that the cytoplasmic membrane alterations might not be the cell’s primary defense against nisin. We therefore looked for changes in the cell wall of the Nisr strain by evaluating the strain’s sensitivity to cell wall-acting compounds. The Nisr strain was more resistant than the wild type to lysozyme, which catalyzes the hydrolysis of the β-1,4 glycosidic bond between N-acetylmuramicglucosamine and N-acetylglucosamine of cell wall peptidoglycan, and more sensitive to the cell wall-acting antibiotics benzylpenicillin and ampicillin, which block the cross-linking reaction of peptidoglycan synthesis. These altered sensitivities suggest compositional changes in the cell wall of the Nisr strain. The nature of these compositional changes remains to be determined. In addition, small but significant (P < 0.001) increases in the MICs of gramicidin S and gentamicin for the Nisr strain were observed. The sites of action of gramicidin S and gentamicin are the cytoplasmic membrane and protein synthesis, respectively. Given that no other changes in sensitivities to other membrane-acting or protein synthesis-disrupting compounds were observed, the increases in resistance to gramicidin S and gentamicin might result from an alteration in the cell wall which prevents these compounds from reaching their targets. The altered sensitivities to lysozyme and the penicillins were observed even when the Nisr strain was cultured in the absence of nisin. Therefore, unlike the fatty acid composition change, the cell wall alterations are constitutive. No gross morphological changes in the cell wall of the Nisr strain were apparent by EM. The cell wall has also been implicated in nisin-resistant Listeria innocua, in which altered sensitivities to cell wall-acting antibiotics and enzymes and a thickened cell wall were observed (29). In addition, removal of the cell wall from nisin-resistant L. monocytogenes F6861 resulted in the loss of nisin resistance, suggesting that differences in the cell wall were responsible for resistance in this strain (7).
Next, to determine whether nisin causes significant membrane disruption in the Nisr strain, the cell’s PMF was measured. In the absence of nisin, the wild-type and Nisr strains of L. monocytogenes maintained similar basal PMFs of around −120 mV, indicating that the mutation to nisin resistance did not affect the Nisr strain’s ability to generate and maintain a PMF. Addition of nisin at 60 IU/ml to wild-type cells caused a 3-log reduction in cell viability and depleted the PMF by 88%. In the Nisr strain, nisin at 60 IU/ml reduced the PMF by only 21%. While this PMF reduction was statistically significant, the remaining PMF was still within the range required to support bacterial growth (22). The reduction of PMF in the Nisr strain indicates that some membrane disruption occurred in this strain, but this decrease was not sufficient to cause significant lethality.
In addition to changes in the cytoplasmic membrane and cell wall, the Nisr strain of L. monocytogenes required divalent cations to resist the inhibitory effect of nisin. The effect was dependent on the concentration of divalent cations. Abee et al. (1) found that di- and trivalent cations (Mg2+, Ca2+, and Gd3+) decreased the nisin Z-induced rate of K+ efflux from whole cells of L. monocytogenes Scott A. They suggested that di- and trivalent cations might inhibit the electrostatic interactions between the positive charges on the nisin molecule and negatively charged phospholipid head groups. Alternatively or additionally, the neutralization of the negative head group charges may induce a condensation of these phospholipids, resulting in a more rigid membrane (1). In our study, divalent cations did not prevent nisin from killing wild-type cells. As for the role of divalent cations in protecting Nisr L. monocytogenes from nisin, it seems unlikely that the cations are interfering only with the electrostatic binding of nisin to the anionic phospholipids. If this were simply the case, divalent cations would also be expected to prevent binding to and subsequent killing of wild-type cells. However, Nisr cells also have reduced amounts of the anionic phospholipids phosphatidylglycerol and cardiolipin in their membrane, as well as an altered fatty acid composition consistent with a more rigid membrane. These two membrane composition changes alone were still insufficient to prevent nisin from interacting with the Nisr cells in the absence of divalent cations. Perhaps divalent cations are required to sufficiently stabilize the altered Nisr cell’s cytoplasmic membrane against disruption by nisin. This stabilization might involve interfering with nisin’s binding or a specific interaction of the cations with membrane components or some combination of the two. Alternatively, the divalent cations may also play a role in the altered cell wall of the Nisr strain. Divalent cations interact extensively with cell wall components, especially the negatively charged teichoic acids. Perhaps the change in the cell wall of the Nisr strain requires additional divalent cations to either stabilize extra negative charge or prevent nisin from binding to anionic sites.
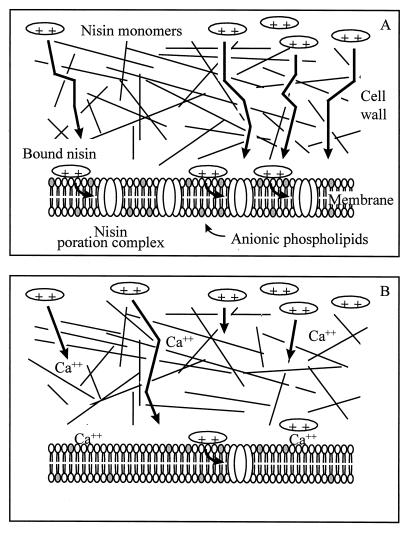
A model illustrating nisin’s interaction with wild-type L. monocytogenes and how this interaction might be disrupted in the Nisr strain is presented in Fig. 5. With wild-type cells, nisin passes through the cell wall; binds to the cytoplasmic membrane, probably via electrostatic interactions with the anionic phospholipids phosphatidylglycerol and cardiolipin; and disrupts the membrane through the formation of pores. In the Nisr strain, cell wall alterations may prevent nisin from interacting with the cytoplasmic membrane. Nisin which does reach the membrane interacts with it to induce the changes in fatty acid and phospholipid composition. The bound nisin probably forms a limited number of pores, since the PMF of the Nisr strain was partially depleted by nisin. These pores, however, are not sufficient to cause significant lethality. Nisin’s ability to bind to the cytoplasmic membrane of the Nisr strain may be hindered by the decrease in net negative charge of the membrane surface, and its ability to insert may be hampered by a decrease in membrane fluidity. Divalent cations, which are required by the Nisr strain to resist the effects of nisin, may play a role in stabilizing the cell wall and/or the cytoplasmic membrane or may prevent nisin from binding to the cell wall and/or the membrane. This model is consistent with data from a variety of sources and provides a conceptual framework for more-detailed studies of various aspects of the Nisr phenotype.
FIG. 5.
Model, as described in Discussion, for the action of nisin against wild-type (A) and Nisr (B) L. monocytogenes.
Finally, the Nisr strain of L. monocytogenes was also cross-resistant to the class IIa bacteriocin pediocin PA-1 and the class IV leuconocin S. Pediocin PA-1 is a 44-amino-acid protein whose sequence has been determined (24) and modeled into a three-dimensional structure (5) which predicts that initial binding to membranes is through electrostatic interactions. Leuconocin S is a small (molecular weight, <10,000) glycoprotein (28). Both leuconocin S and pediocin PA-1, like nisin, act against L. monocytogenes by depleting the PMF (4). L. monocytogenes mutants resistant to mesenterocin 52, curvaticin 13, and plantaricin were each also cross-resistant to the other bacteriocins (38). In addition, piscicolin 126-resistant mutants of L. monocytogenes which emerged in cheese made from milk containing the bacteriocin were also resistant to pediocin P02 (43). These reports of cross-resistance indicate that the use of multiple bacteriocins to achieve greater antibacterial efficacy (20) might not be feasible. The development of resistance to one of the bacteriocins in the combination might render the organism resistant to the others.
ACKNOWLEDGMENTS
Research in our laboratory and preparation of the manuscript were supported by state appropriations, U.S. Hatch Act Funds, and U.S. Department of Agriculture CSRS NRI Food Safety Program (no. 94-37201-0994).
We sincerely thank Peter Cooke for the EM work and George Carman, Richard Ludescher, Karen Schaich, and Judith Storch for helpful discussion and comments.
Footnotes
Manuscript D-10974-2-97 of the New Jersey Agricultural Experiment Station.
REFERENCES
- 1.Abee T, Rombouts F M, Hugenholtz J, Guihard G, Letellier L. Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol. 1994;60:1962–1968. doi: 10.1128/aem.60.6.1962-1968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benkerroum R, Sandine W E. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci. 1988;71:3237–3245. doi: 10.3168/jds.S0022-0302(88)79929-4. [DOI] [PubMed] [Google Scholar]
- 3.Bruno M E, Kaiser A, Montville T J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno M E C, Montville T J. Common mechanisms of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1993;59:3003–3010. doi: 10.1128/aem.59.9.3003-3010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Shapira R, Eisenstein M, Montville T J. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relation to a predicted tertiary structure. Appl Environ Microbiol. 1997;63:524–531. doi: 10.1128/aem.63.2.524-531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels L, Hansen R S, Phillips J A. Chemical analysis. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 511–554. [Google Scholar]
- 7.Davies E A, Adams M R. Resistance of Listeria monocytogenes to the bacteriocin nisin. Int J Food Microbiol. 1994;21:341–347. doi: 10.1016/0168-1605(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 8.Davies E A, Falahee M B, Adams M R. Involvement of the cell envelope of Listeria monocytogenes in the acquisition of nisin resistance. J Appl Bacteriol. 1996;81:139–146. doi: 10.1111/j.1365-2672.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 9.Demel R A, Peelen T, Siezen R J, De Kruijff B, Kuipers O P. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity. A monolayer study. Eur J Biochem. 1996;235:267–274. doi: 10.1111/j.1432-1033.1996.00267.x. [DOI] [PubMed] [Google Scholar]
- 10.Driessen A J M, van den Hooven H W, Kuiper W, van de Kamp M, Sahl H-G, Konings R N H, Konings W N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 11.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federal Register. Nisin preparation: affirmation of GRAS status as a direct human food ingredient. Fed Regist. 1988;53:11247–11251. [Google Scholar]
- 13.Federal Register. M. G. Waldbaum Co.; filing of petition for affirmation of GRAS status. Fed Regist. 1994;59:42277–42278. [Google Scholar]
- 14.Federal Register. Aplin & Barrett Ltd.; filing of petition for affirmation of GRAS status. Fed Regist. 1995;60:64167. [Google Scholar]
- 15.Filgueiras M H, op den Kamp J A F. Cardiolipin, a major phospholipid of gram-positive bacteria that is not readily extractable. Biochim Biophys Acta. 1980;620:332–337. doi: 10.1016/0005-2760(80)90215-5. [DOI] [PubMed] [Google Scholar]
- 16.Gahan C G M, Collins J K. Listeriosis: biology and implications for the food industry. Trends Food Sci Technol. 1991;2:89–93. [Google Scholar]
- 17.Gao F J, Abee T, Konings W N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991;57:2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcera J J G, Elferink G L, Driessen J M, Konings W N. In vitro pore-forming activity of the lantibiotic nisin. Role of PMF and lipid composition. Eur J Biochem. 1993;212:417–422. doi: 10.1111/j.1432-1033.1993.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 19.George S M, Lund B M, Brocklehurst T T. The effect of pH and temperature on initiation of growth of Listeria monocytogenes. Lett Appl Microbiol. 1988;6:153–156. [Google Scholar]
- 20.Hanlin M B, Kalchayan N, Ray B. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J Food Prot. 1993;56:252–255. doi: 10.4315/0362-028X-56.3.252. [DOI] [PubMed] [Google Scholar]
- 21.Harris L J, Daeschel M A, Stiles M E, Klaenhammer T R. Antimicrobial activity of lactic acid bacteria against Listeria monocytogenes. J Food Prot. 1989;52:384–387. doi: 10.4315/0362-028X-52.6.384. [DOI] [PubMed] [Google Scholar]
- 22.Hellingwerf K J, Konings W N. The energy flow in bacteria: the main free energy intermediates and their regulatory role. Adv Microb Physiol. 1985;26:125–154. doi: 10.1016/s0065-2911(08)60396-3. [DOI] [PubMed] [Google Scholar]
- 23.Henderson J R, Tocher D R. Thin-layer chromatography. In: Hamilton R J, Hamilton S, editors. Lipid analysis: a practical approach. Oxford, United Kingdom: IRL Press; 1992. pp. 65–112. [Google Scholar]
- 24.Henderson J T, Chopko A L, van Wassenaar P D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Arch Biochem Biophys. 1992;295:5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- 25.Hugenholtz J, de Veer G J C. Applications of nisin A and Z in dairy technology. In: Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 440–447. [Google Scholar]
- 26.Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. [Google Scholar]
- 27.Kosaric N, Carroll K K. Phospholipids of Listeria monocytogenes. Biochim Biophys Acta. 1971;239:428–442. doi: 10.1016/0005-2760(71)90035-x. [DOI] [PubMed] [Google Scholar]
- 28.Lewus C B, Sun S, Montville T J. Production of an amylase-sensitive bacteriocin by an atypical Leuconostoc paramesenteroides strain. Appl Environ Microbiol. 1992;58:143–149. doi: 10.1128/aem.58.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisnier-Patin S, Richard J. Cell wall changes in nisin-resistant variants of Listeria innocua grown in the presence of high nisin concentrations. FEMS Microbiol Lett. 1996;140:29–35. doi: 10.1111/j.1574-6968.1996.tb08310.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin I, Ruysschaert J-M, Sander D, Giffard C J. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur J Biochem. 1996;239:156–164. doi: 10.1111/j.1432-1033.1996.0156u.x. [DOI] [PubMed] [Google Scholar]
- 31.Mastronicolis S K, German J B, Smith G M. Diversity of the polar lipids of the food-borne pathogen Listeria monocytogenes. Lipids. 1996;31:635–640. doi: 10.1007/BF02523834. [DOI] [PubMed] [Google Scholar]
- 32.Mazzotta A, Montville T J. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10°C and 30°C. J Appl Microbiol. 1997;82:32–38. doi: 10.1111/j.1365-2672.1997.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 33.Ming X, Daeschel M A. Nisin resistance of foodborne bacteria and the specific resistance response of Listeria monocytogenes Scott A. J Food Prot. 1993;56:944–948. doi: 10.4315/0362-028X-56.11.944. [DOI] [PubMed] [Google Scholar]
- 34.Ming X, Daeschel M A. Correlation of cellular phospholipid content with nisin resistance of Listeria monocytogenes Scott A. J Food Prot. 1995;58:416–420. doi: 10.4315/0362-028X-58.4.416. [DOI] [PubMed] [Google Scholar]
- 35.Moll G N, Clark J, Chan W C, Bycroft B W, Roberts G C K, Konings W N, Driessen A J M. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179:135–140. doi: 10.1128/jb.179.1.135-140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muriana, P. M. 1996. Bacteriocins for control of Listeria spp. in food. J. Food Prot. 1996(Suppl.):54–63. [DOI] [PubMed]
- 37.Okereke A, Montville T J. Nisin dissipates the proton motive force of the obligate anaerobe Clostridium sporogenes PA 3679. Appl Environ Microbiol. 1992;58:2463–2467. doi: 10.1128/aem.58.8.2463-2467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rekhif N, Atrih A, Lefebvre G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr Microbiol. 1994;28:237–241. [Google Scholar]
- 39.Ruhr E, Sahl H-G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985;27:841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahl H G. Pore formation in bacterial membranes by cationic lantibiotics. In: Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 347–358. [Google Scholar]
- 41.Sorrells K M, Enigl D C, Harfield J R. Effect of pH, acidulant, time and temperature on the growth and survival of Listeria monocytogenes. J Food Prot. 1989;52:571–573. doi: 10.4315/0362-028X-52.8.571. [DOI] [PubMed] [Google Scholar]
- 42.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 43.Wan J, Harmark K, Davidson B E, Hillier A J, Gordon J B, Wilcock A, Hickey M W, Coventry M J. Inhibition of Listeria monocytogenes by piscicolin 126 in milk and Camembert cheese manufactured with a thermophilic starter. J Appl Microbiol. 1997;82:273–280. doi: 10.1046/j.1365-2672.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- 44.Winkowski K, Bruno M E C, Montville T J. Correlation of bioenergetic parameters with cell death in Listeria monocytogenes cells exposed to nisin. Appl Environ Microbiol. 1994;60:4186–4188. doi: 10.1128/aem.60.11.4186-4188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkowski K, Ludescher R D, Montville T J. Physicochemical characterization of the nisin-membrane interaction using liposomes derived from Listeria monocytogenes. Appl Environ Microbiol. 1996;62:323–327. doi: 10.1128/aem.62.2.323-327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]