Abstract
Additions of ammonium and non-ammonium salts inhibit atmospheric methane consumption by soil at salt concentrations that do not significantly affect the soil water potential. The response of soils to non-ammonium salts has previously raised questions about the mechanism of ammonium inhibition. Results presented here show that inhibition of methane consumption by non-ammonium salts can be explained in part by ion-exchange reactions: cations desorb ammonium, with the level of desorption varying as a function of both the cation and anion added; differential desorption results in differential inhibition levels. Differences in the extent of inhibition among ammonium salts can also be explained in part by the effects of anions on ammonium exchange. In contrast, only minimal effects of cations and anions are observed in liquid cultures of Methylosinus trichosporium OB3b. The comparable level of inhibition by equinormal concentrations of NH4Cl and (NH4)2SO4 and the insensitivity of salt inhibition to increasing methane concentrations (from 10 to 100 ppm) are of particular interest, since both of these patterns are in contrast to results for soils. The greater inhibition of methane consumption for NH4Cl than (NH4)2SO4 in soils can be attributed to increased ammonium adsorption by sulfate; increasing inhibition by non-ammonium salts with increasing methane concentrations can be attributed to desorbed ammonium and a physiological mechanism proposed previously for pure cultures.
A number of factors, including gas transport, soil water content, water stress, and temperature, limit atmospheric methane consumption by soils (1, 6, 7, 9, 19, 26, 30, 31). In addition, nitrogen mineralization and ammonium constrain methanotrophic activity (see e.g., references 1, 2, 18, 20–22, 24, 25, 28, and 32). The effects of added ammonium are usually substantial and persistent (see, e.g., references 14, 15, 20–22, and 24). King and Schnell (20, 21) have proposed a model of ammonium inhibition based on the physiological characteristics of known methanotrophic bacteria. This model includes a parabolic inhibition response as a function of methane concentrations and is consistent with observations for forest soils.
Inhibition of methane consumption by non-ammonium salts has also been observed in field and laboratory studies (see, e.g., references 1, 8, 12, 16, and 17), raising questions about the specificity of ammonium and the mechanism of ammonium inhibition. To address these questions, we have compared the responses of a methanotrophic culture (Methylosinus trichosporium OB3b) and atmospheric methane consumption by soils to a variety of ammonium and non-ammonium salts. M. trichosporium OB3b has been used previously as a model for understanding the physiology of ammonium inhibition. In this study, culture responses have been assayed at low headspace methane concentrations (10 to 100 ppm) and low to modest salt concentrations (0.5 to 8 mM). Salts have been added to soils at levels (e.g., ≤1 μmol g [fresh weight]−1) that do not significantly affect the total soil water potential and that are comparable to those used in previous studies. We have also examined the effect of various salts on ammonium desorption and adsorption. Our results indicate that many cations desorb ammonium and inhibit methane consumption, as expected from ion-exchange chemistry. In addition, some anions (e.g., nitrate and sulfate) promote ammonium absorption while others (e.g., chloride) promote desorption, further complicating the interpretation of salt effects. The results also support previously proposed mechanisms for ammonium inhibition and suggest that non-ammonium salts cannot be used unequivocally as controls for solute addition.
MATERIALS AND METHODS
Culture assays.
M. trichosporium OB3b was grown in batch culture with Higgins nitrate mineral salts (NMS) as described previously (see, e.g., references 19 and 21). The cells were harvested by centrifugation (10,000 × g at 4°C) after reaching an absorbance at 600 nm of 0.2 to 0.3 (early log phase), washed twice with 10 mM phosphate buffer, and resuspended in a modified NMS medium containing no NaCl. Replicate 100-ml cultures were incubated in sealed 500-ml Erlenmeyer flasks with rotary shaking (200 rpm) at 30°C with headspace methane concentrations of 10 or 100 ppm (about 14.7 and 147 nM, respectively) and various concentrations of either NaCl, KCl, or NH4Cl (0, 0.5, 2.0, or 8.0 mM); (NH4)2SO4 was added to parallel cultures at 0, 0.25, 1.0, or 4 mM. Methane uptake was determined by removing headspace subsamples (0.3 cm3) with a needle and syringe at intervals for assay by flame ionization gas chromatography (21). Uptake rate constants for duplicates of each treatment were estimated from a regression analysis of the exponential decrease in methane concentration over time.
Soil analyses.
The effect of various ammonium and non-ammonium salts on atmospheric methane consumption was determined with sieved soils (2-mm mesh) from the 6- to 10-cm layer of 6.5-cm-inner-diameter cores obtained from a mixed coniferous-hardwood forest at the Darling Marine Center. The 6- to 10-cm layer is the most active for methane uptake; the site has been characterized previously (1, 25, 26). Soil samples (10 g [fresh weight]) were transferred to jars with a headspace of about 110 cm3. The soil water contents varied between 25 and 30%, a range previously shown to represent a broad optimum for methane consumption (26); the water contents were determined by drying soils for 24 h at 105°C. Salts dissolved in deionized water were added to replicate jars by carefully pipetting 1-ml volumes onto soils and gently mixing them. The final salt concentrations were 1 μmol g (fresh weight)−1 for cations unless otherwise indicated. Deionized water with no added salts served as a control. The jars were sealed with butyl rubber stoppers that did not release methane or other organic gases. For most assays, the jars contained atmospheric methane (1.7 to 1.8 ppm; equivalent to 2.5 to 2.6 nM in soil solution), but in some cases methane was added to give initial headspace concentrations of 100 ppm. Methane uptake was determined by removing headspace subsamples (0.3 cm3) with a needle and syringe for processing as above. Uptake rate constants were estimated from triplicate determinations for each treatment by regression analysis of the exponential decrease in methane concentration over time.
The effect of inorganic salts on ammonium desorption was determined by adding salt solutions to 10 g (fresh weight) of soil (final concentrations, 1 μmol of N g [fresh weight] of soil−1). The soils were equilibrated for 1 to 2 h, and then ammonium was extracted by adding deionized water (1 ml g [fresh weight] of soil−1). The soil slurry was vortexed briefly and then centrifuged. Supernatant ammonium concentrations were assayed colorimetrically (3). Similarly, ammonium salts were added to a parallel set of soils that were extracted to determine the extent to which ammonium salt counterions affect adsorption.
Ammonium desorption and adsorption were also examined by suspending 50 g (fresh weight) of sieved soil from the 6- to 10-cm depth in 200 ml of deionized water. The suspension was mixed continuously with a magnetic stirrer while concentrated solutions of LiCl, KCl, or CsCl were added incrementally in fixed volumes to increase the ionic strength. Subsamples of the suspension (2 ml) were obtained after each incremental addition of salt for the ammonium assay as described above.
The soil water potential was measured with a Wescor dew point psychrometer as described by Schnell and King (26). The total water potential was calibrated by using a series of NaCl solutions with known molality. Molality was converted to potential by using the following relationship: ψs = RT ln (aw), where aw is the weight-based mole fraction of water in a solution, corrected for nonideal solute behavior.
RESULTS
Cultures.
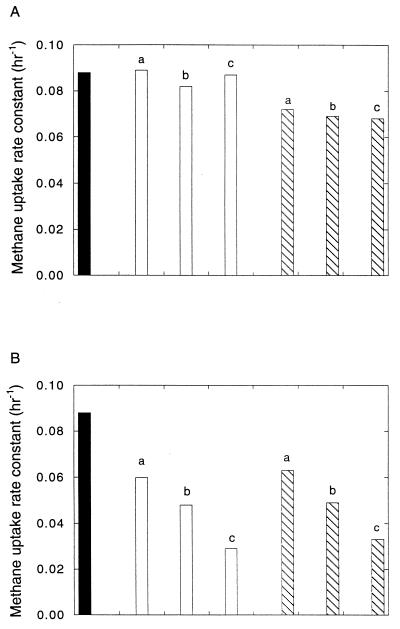
Methane uptake by M. trichosporium OB3b did not differ over a range of added NaCl or KCl concentrations from 0.5 to 8 mM in NMS (Fig. 1). However, uptake was consistently higher by about 10% for cultures with NaCl and lower by about 10% for cultures with KCl relative to controls. In contrast, NH4Cl was inhibitory relative to the alkaline metal salts and unamended controls (Fig. 1). While uptake rate constants for KCl-treated cultures were slightly lower than for those treated with NaCl, no significant differences were observed between the level of inhibition for equinormal concentrations of NH4Cl and (NH4)2SO4. The results for cultures incubated with 100 ppm of methane and either KCl or NaCl were similar to those for cultures incubated with 10 ppm of methane (data not shown). The rate constants at 100 ppm for NaCl and KCl treatments were 93.4 and 114.3% of the values at 10 ppm, respectively. In contrast, inhibition by either NH4Cl or (NH4)2SO4 was greater at 100 ppm than at 10 ppm (NH4Cl, 48.9 and 38.3% inhibition for 100 and 10 ppm, respectively; (NH4)2SO4, 43.2 and 36.1% inhibition at 100 and 10 ppm, respectively).
FIG. 1.
(A) Methane uptake rate constants for M. trichosporium OB3b incubated with 10 ppm of methane and without added salts (solid bar) or with NaCl (open bars; a, 0.5 mM; b, 2 mM; c, 8 mM) or KCl (hatched bars; a, 0.5 mM; b, 2 mM; c, 8 mM). (B) Methane uptake rate constants for M. trichosporium OB3b incubated with 10 ppm of methane and without added salts (solid bar) or with NH4Cl (open bars; a, 0.5 mM; b, 2 mM; c, 8 mM) or (NH4)2SO4 (hatched bars; a, 0.25 mM; b, 1 mM; c, 4 mM). Data are means of duplicate determinations; the range for duplicates was less than 10% of the mean.
Soils.
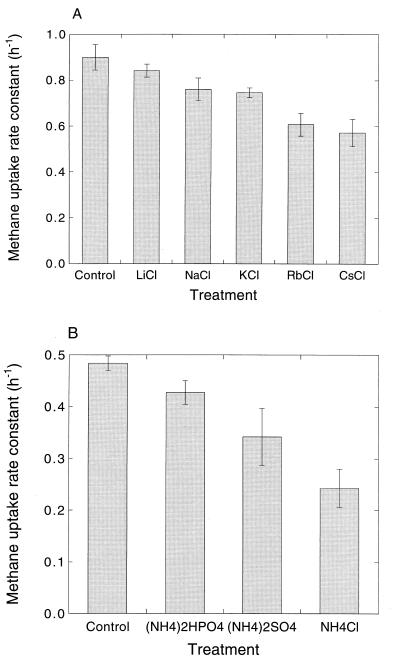
Salt additions at ≤1 μmol g (fresh weight) of soil−1 had little or no effect on the total soil water potential; all values were approximately ≥−0.05 MPa. Relative to deionized-water treatments, salt additions inhibited atmospheric methane consumption by sieved soils (Table 1). There was a trend for increasing inhibition from LiCl to CsCl (Fig. 2A), although the specific order of inhibition varied somewhat among different batches of soil; MgCl2 was typically more inhibitory than the alkaline metal chlorides. The extent of inhibition by salts increased during the first 24 h after addition but was relatively stable for 4 days thereafter (data not shown). The extent of inhibition by sodium and potassium salts varied as a function of the counteranion added (Table 1; Fig. 2A), with the nitrate, phosphate, and sulfate salts being less inhibitory than the chlorides.
TABLE 1.
Methane consumption by forest soils incubated with methane and various ammonium or non-ammonium saltsa
| Salt addedb | Uptake (nmol g [dry wt]−1 h−1) (% of control) in presence of methane atc:
|
|
|---|---|---|
| 1.7 ppm | 250 ppm | |
| Expt A | ||
| Controld | 0.41 | 2.69 |
| NaCl | 0.14 (34.5) | 0.62 (23.2) |
| Na2SO4 | 0.37 (89.5) | 2.77 (102.7) |
| NaH2PO4 | 0.38 (93.3) | 2.59 (96.3) |
| KCl | 0.15 (35.5) | 0.69 (25.8) |
| KNO3 | 0.23 (55.5) | 1.19 (44.2) |
| MgCl2 | 0.13 (32.4) | 0.74 (27.6) |
| Expt B | ||
| Control | 0.56 | 4.34 |
| NaCl | 0.30 (53.9) | 0.50 (11.4) |
| NH4Cl | 0.29 (52.3) | 0.39 (9.1) |
The methane was present at atmospheric concentrations (1.7 ppm) or at 250 ppm. The added salts were present at 1 μmol g (fresh weight)−1 (0.5 μ g−1 for Na2SO4). The total soil water potential for all samples was >−0.01 MPa.
Experiments A and B represent independent assays with similar soils.
Results are means of triplicate determinations.
Controls were treated with a volume of deionized water equal to that used for the salt additions.
FIG. 2.
(A) Atmospheric methane uptake rate constants for 10 g (fresh weight) of soil incubated with 2 μmol of the indicated salts g (fresh weight)−1. Data are means of triplicate determinations ± 1 standard error. (B) Atmospheric methane uptake rate constants for 10 g (fresh weight) of soil incubated with 1 μmol of N from the indicated ammonium salts g (fresh weight)−1. Data are means of triplicate determinations ± 1 standard error.
Inhibition by ammonium salts also varied as a function of the added counteranion, with the chloride salt being substantially more potent than the phosphate or sulfate salts (Fig. 2B). As with the alkaline metal salts, ammonium inhibition increased to a maximum about 24 h after addition and remained relatively stable subsequently for NH4Cl (data not shown). Although in some instances inhibition by various chloride salts slightly exceeded that by NH4Cl, the extent of inhibition was typically similar for salts used at equal concentrations (Fig. 3).
FIG. 3.
Atmospheric methane uptake rate constants for 10 g (fresh weight) of soil incubated with various concentrations of NH4Cl (•) or KCl (○). Data are means of triplicate determinations ± 1 standard error.
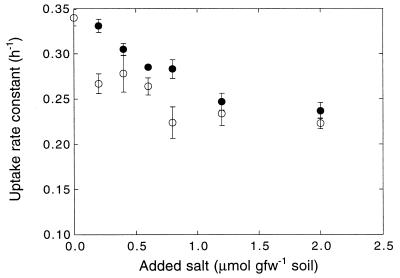
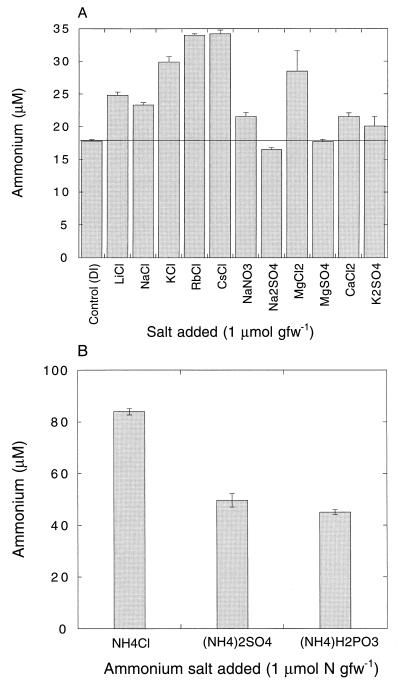
Salt additions altered ammonium concentrations in aqueous extracts of sieved soils (Fig. 4). The concentrations tended to increase with increasing atomic number for the alkaline metal chloride series (Li to Cs). Deionized-water-extractable ammonium levels in soils treated with MgCl2 exceeded those in soils treated with NaCl or CaCl2; the concentrations in extracts from soils treated with sulfate or nitrate salts were consistently lower than those in extracts from soils treated with the analogous chloride salts (Fig. 4). In some instances, extracts from soils treated with sulfate and nitrate salts were not statistically different from extracts from untreated soils. An analysis of ammonium desorption in soil slurries revealed a similar pattern, with desorption increasing in the order Li < K < Cs (Fig. 5). Ammonium concentrations in soils extracts also depended on the counteranion used for ammonium additions. The chloride salt resulted in higher water-extractable concentrations than did either the phosphate or sulfate salt, for which concentrations were similar (Fig. 4).
FIG. 4.
(A) Ammonium concentrations in aqueous extracts (1 ml of deionized water g [fresh weight] of soil−1 for soils treated with the indicated salts. (B) As in panel A but for additions of ammonium salts to soils. Data are means of triplicate determinations ± 1 standard error.
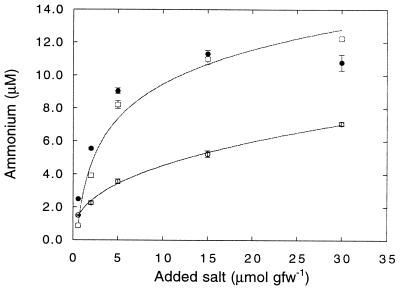
FIG. 5.
Ammonium concentrations in a soil slurry (250 ml of deionized water, 50 g [fresh weight] of soil) to which was progressively added increasing concentrations of LiCl (○), KCl (□), or CsCl (•). Data are means of triplicate determinations ± 1 standard error.
DISCUSSION
Rapid inhibition of atmospheric methane consumption by ammonium has been well documented (see, e.g., references 1, 2, 4, 5, 10, 15, 24, and 28) and attributed to the combined effect of substrate competition at the level of methane monooxygenase and toxicity of nitrite generated intracellularly as an ammonium oxidation end product (see, e.g., references 20, 21, and 25). However, the fact that non-ammonium salts inhibit methane consumption has led some researchers to speculate that ammonium inhibition may be due all or in part to nonspecific ionic or solute effects (see, e.g., references 8, 16, and 17). Non-ammonium salts have also been proposed as essential controls for partitioning ammonium inhibition between the nonspecific and methane monooxygenase-related mechanisms (17, 23). Results presented here support an enzyme-based model of ammonium inhibition and indicate that non-ammonium salts cannot be used unambiguously as controls to partition inhibition among multiple mechanisms.
Non-ammonium salts are unsuitable as controls for at least two reasons. First, non-ammonium cations desorb ammonium in soils by ion exchange. Ammonium desorption in general and increasing desorption with increasing cation radius in particular (Fig. 4 and 5) are well-known phenomena (see e.g., references 11 and 27) that apparently have not been considered in previous salt inhibition assays. Likewise, the effect of anions on ammonium exchange (Fig. 4) has not been considered previously. Differences in ammonium concentrations in soil solutions as a function of added anions most probably reflect the differential stability of various ion pairs, with increasing stability decreasing the ionic character of a given cation-anion series (29).
The collective observations described both here and previously provide a basis for understanding the effects of ammonium and nonammonium salts on soil methane uptake. Uptake is lowest for cation and anion pairs that promote ammonium desorption (e.g., KCl and MgCl2), and greatest for pairs that limit desorption (e.g., LiCl and Na2SO4); (Table 1; Fig. 2). Likewise, uptake is lowest for ammonium salts that are minimally adsorbed (e.g., chloride) and greatest for sulfate and phosphate pairs that are more strongly adsorbed (Fig. 2). In contrast to the situation in soils, ammonium counterions do not affect inhibition in cultures (Fig. 1), since ion exchange is relatively unimportant in the distribution of ions in liquid media. The response of salt-amended soils to elevated methane concentrations (Table 1) can also be understood best in the context of ammonium desorption. Increased inhibition by non-ammonium salts at elevated methane concentrations occurs regardless of the cation-anion pair added to Maine forest soils, although the specific levels of inhibition at elevated methane concentrations vary among salts. This response is consistent with the combined effect of desorption and the mechanism for ammonium inhibition described previously (25).
Non-ammonium salts are also inappropriate controls for ammonium addition because they may have inhibitory effects unrelated to those of ammonium. For example, high concentrations of NaCl or KCl decrease methane oxidation in cultures due to physiological stresses that do not specifically involve methane monooxygenase (25). Furthermore, M. trichosporium OB3b consistently oxidizes less methane in media with dilute concentrations (0.5 to 8 mM) of KCl rather than NaCl. This suggests that methanotrophs may be differentially sensitive to potassium and perhaps to other cations. This sensitivity involves a mechanism different from that for ammonium, since ammonium inhibition in cultures increases with increasing methane concentrations from 10 to 100 ppm (Fig. 1) (21) while no such effect is observed with non-ammonium salts.
Since the responses of soils to certain ammonium and non-ammonium salts (e.g., KCl and NH4Cl [Fig. 3] and NaCl-RbCl [Fig. 2]) are similar while chloride salts are more inhibitory than their sulfate, nitrate, or phosphate analogs (Table 1; Fig. 2), it is tempting to speculate that chloride has an additional, complicating toxicity of its own (13). Although this possibility deserves further attention, the culture data presented here provide no indication that chloride might be inhibitory per se. No differences were observed for M. trichosporium OB3b incubated with increasing chloride concentrations (as the sodium or potassium salt) from 0.5 to 8 mM; equinormal NH4Cl and (NH4)2SO4 were similarly inhibitory (Fig. 1). Furthermore, there is no obvious mechanism by which chloride alone could account for increased inhibition in salt-amended soils incubated with increasing methane concentrations from 1.7 to 250 ppm (Table 1); this phenomenon is most reasonably attributed to an effect of added or desorbed ammonium.
Although the data here raise doubts about the efficacy of non-ammonium salts as controls for ammonium addition to soils, the relative response of atmospheric methane consumption to a variety of salts remains interesting in the context of inputs via wet deposition. Sodium and ammonium typically dominate wet deposition, usually dwarfing potassium (33); thus, potassium salts might be ill suited for assays involving nonagricultural soils. With the exception of the situation for coastal sites influenced by marine weather systems, sulfate and nitrate dominate wet deposition, with chloride usually being a minor component (about 10%) and perhaps unrepresentative as a counterion. However, in areas such as coastal Maine, chloride accounts for about 50% of the anions assayed routinely by the National Acid Deposition and Precipitation program (extensive data on the geographical and temporal distribution of acid rain chemistry from the NADP/NTN program are available online from the U.S. Geological Survey at http://btdqs.usgs.gov/acidrain/); in this and similar cases, chloride salts might prove more representative than other choices.
Finally, it should be emphasized that comparisons of the responses of different soils to ammonium and non-ammonium salts must be tempered by recognition of the enormous variation that exists among soils in basic physical-chemical parameters (e.g., pH, water content, ammonium content, mineralology, organic content, and dynamics of ammonia oxidation) that determine ion exchange and soil solution ammonium concentrations. Although physical and chemical diversity may complicate comparisons among soils, it is apparent that ammonium is an important determinant of current and future variations in atmospheric methane consumption by soils. Global eutrophication and conversion of forests and grasslands to agricultural use will continue to decrease the relative significance of the soil methane sink, thereby intensifying climate change. Ammonium inputs and dynamics in soils, as shown in the physiology of methanotrophic bacteria, will be a key component of these changes, with or without additional effects of non-ammonium salts.
ACKNOWLEDGMENTS
We acknowledge the support of USDA CSRS-CRP grant 94-37107-0488.
We thank K. Hardy for technical assistance, and we appreciate very helpful comments from two anonymous reviewers.
Footnotes
Contribution 310 from the Darling Marine Center.
REFERENCES
- 1.Adamsen A P S, King G M. Methane consumption in temperate and sub-Arctic forest soils: rates, vertical zonation, and response to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeckx P, Van Cleemput O. Methane oxidation in a neutral landfill cover soil: influence of moisture content, temperature, and nitrogen-turnover. J Environ Qual. 1996;25:178–183. [Google Scholar]
- 3.Bower C E, Holm-Hansen T. A salicylate-hypochlorite method for determining ammonia in seawater. Can J Fish Aquat Sci. 1980;37:794–798. [Google Scholar]
- 4.Bronson K F, Mosier A R. Suppression of methane oxidation in aerobic soil by nitrogen fertilizers, nitrification inhibitors, and urease inhibitors. Biol Fertil Soils. 1994;17:263–268. [Google Scholar]
- 5.Castro M S, Peterjohn W T, Mellilo J M, Steudler P A, Gholz H L, Lewis D. Effects of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can J For Res. 1994;24:9–13. [Google Scholar]
- 6.Castro M S, Steudler P A, Bowden R D. Factors controlling atmospheric methane consumption by temperate forest soils. Global Biogeochem Cycles. 1995;9:1–10. [Google Scholar]
- 7.Crill P M. Seasonal patterns of methane uptake and carbon dioxide release by a temperate woodland soil. Global Biogeochem Cycles. 1991;5:319–334. [Google Scholar]
- 8.Crill P M, Martikainen P J, Nykänen H, Silvola J. Temperature and N fertilization effects on methane oxidation in a drained peatland soil. Soil Biol Biochem. 1994;26:1331–1339. [Google Scholar]
- 9.Czepiel P M, Crill P M, Harriss R C. Environmental factors influencing the variability of methane oxidation in temperate zone soils. J Geophys Res Ser D. 1995;100:9359–9364. [Google Scholar]
- 10.Dunfield P F, Topp E, Archambault C, Knowles R. Effect of nitrogen fertilizers and moisture content on CH4 and N2O fluxes in a humisol: measurements in the field and intact soil cores. Biogeochemistry. 1995;29:199–222. [Google Scholar]
- 11.Grim R E. Clay mineralogy. 2nd ed. New York, N.Y: McGraw-Hill Book Co.; 1968. [Google Scholar]
- 12.Gulledge J, Doyle A P, Schimel J P. Different NH4+-inhibition patterns of soil CH4 consumption: a result of distinct CH4-oxidizer populations across sites? Soil Biol Biochem. 1997;29:13. [Google Scholar]
- 13.Gulldege J, Schimel J P. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Ammonium inhibition of atmospheric methane oxidation in soil: the physiological mechanism revisited, abstr. N-97. [Google Scholar]
- 14.Hütsch B W, Webster C P, Powlson D S. Long-term effects of nitrogen fertilization on methane oxidation in soils of the Broadbalk Wheat experiment. Soil Biol Biochem. 1993;25:1307–1315. [Google Scholar]
- 15.Hütsch B W, Webster C P, Powlson D S. Methane oxidation in soil as affected by land use, pH, and N fertilization. Soil Biol Biochem. 1994;26:1613–1622. [Google Scholar]
- 16.Hütsch B W, Russell P, Mengel K. CH4 oxidation in two temperate arable soils as affected by nitrate and ammonium application. Biol Fertil Soils. 1996;23:86–92. [Google Scholar]
- 17.Kightley D, Nedwell D B, Cooper M. Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl Environ Microbiol. 1995;61:592–601. doi: 10.1128/aem.61.2.592-601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King G M. Regulation of methane oxidation: contrasts between anoxic sediments and oxic soils. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 318–325. [Google Scholar]
- 19.King G M, Adamsen A P S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl Environ Microbiol. 1992;58:2758–2763. doi: 10.1128/aem.58.9.2758-2763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King G M, Schnell S. Enhanced ammonium inhibition of methane consumption in forest soils by increasing atmospheric methane. Nature. 1994;370:282–284. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King G M, Schnell S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microbiol. 1994;60:3508–3513. doi: 10.1128/aem.60.10.3508-3513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosier A, Schimel D, Valentine D, Bronson K, Parton W. Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature (London) 1991;350:330–332. [Google Scholar]
- 23.Nedwell D B. Methane production and oxidation in soils and sediments. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases: sources, sinks and global change processes. Berlin, Germany: Springer-Verlag KG; 1996. pp. 33–49. [Google Scholar]
- 24.Nesbit S P, Breitenbeck G A. A laboratory study of factors influencing methane uptake by soils. Agric Ecosyst Environ. 1992;41:39–54. [Google Scholar]
- 25.Schnell S, King G M. Mechanistic analysis of ammonium inhibition of atmospheric methane consumption in forest soils. Appl Environ Microbiol. 1994;60:3514–3521. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnell S, King G M. Responses of methanotrophic activity in soils and cultures to water stress. Appl Environ Microbiol. 1996;62:3203–3209. doi: 10.1128/aem.62.9.3203-3209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spositio G. The chemistry of soils. New York, N.Y: Oxford University Press; 1989. [Google Scholar]
- 28.Steudler P A, Bowden R D, Mellilo J M, Aber J D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature (London) 1989;341:314–316. [Google Scholar]
- 29.Stumm W, Morgan J J. Aquatic chemistry: chemical equilibria and rates in natural waters. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 30.Whalen S C, Reeburgh W S, Sandbeck K A. Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol. 1990;56:3405–3411. doi: 10.1128/aem.56.11.3405-3411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whalen S C, Reeburgh W S. Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol Biochem. 1996;28:1271–1281. [Google Scholar]
- 32.Willison T W, Cook R, Müller A, Powlson D S. CH4 oxidation in soils fertilized with organic and inorganic-N: differential effects. Soil Biol Biochem. 1996;28:135–136. [Google Scholar]