Abstract
Oxidation of nitrite to nitrate in aquaria is typically attributed to bacteria belonging to the genus Nitrobacter which are members of the α subdivision of the class Proteobacteria. In order to identify bacteria responsible for nitrite oxidation in aquaria, clone libraries of rRNA genes were developed from biofilms of several freshwater aquaria. Analysis of the rDNA libraries, along with results from denaturing gradient gel electrophoresis (DGGE) on frequently sampled biofilms, indicated the presence of putative nitrite-oxidizing bacteria closely related to other members of the genus Nitrospira. Nucleic acid hybridization experiments with rRNA from biofilms of freshwater aquaria demonstrated that Nitrospira-like rRNA comprised nearly 5% of the rRNA extracted from the biofilms during the establishment of nitrification. Nitrite-oxidizing bacteria belonging to the α subdivision of the class Proteobacteria (e.g., Nitrobacter spp.) were not detected in these samples. Aquaria which received a commercial preparation containing Nitrobacter species did not show evidence of Nitrobacter growth and development but did develop substantial populations of Nitrospira-like species. Time series analysis of rDNA phylotypes on aquaria biofilms by DGGE, combined with nitrite and nitrate analysis, showed a correspondence between the appearance of Nitrospira-like bacterial ribosomal DNA and the initiation of nitrite oxidation. In total, the data suggest that Nitrobacter winogradskyi and close relatives were not the dominant nitrite-oxidizing bacteria in freshwater aquaria. Instead, nitrite oxidation in freshwater aquaria appeared to be mediated by bacteria closely related to Nitrospira moscoviensis and Nitrospira marina.
The oxidation of nitrite to nitrate by chemolithoautotrophic nitrite-oxidizing bacteria (NOB) in fish culture systems, ranging from home aquaria to commercial aquaculture systems, is an important process. The accumulation of high concentrations of nitrite, which is toxic to fish and other aquatic organisms, is prevented by active nitrite removal by nitrifying microorganisms. Nitrite is formed in aquarium systems from the oxidation of ammonia, the principal nitrogenous waste of teleosts, by autotrophic ammonia-oxidizing bacteria (AOB). Thus, closed aquatic filtration systems usually provide a solid substratum, which is termed a biological filter or biofilter, to promote the growth of AOB and NOB. A variety of materials can form the substratum of a biofilter, ranging from gravel to specially engineered molded plastics. Biofilters can be submerged in the flow path of the filtration system or can be located such that the water trickles or percolates through a medium situated in the atmosphere outside the aquarium, before flowing back into the tank.
Traditionally, the bacteria responsible for the oxidation of ammonia and nitrite in aquaria were considered to be Nitrosomonas europaea and Nitrobacter winogradskyi or their close relatives, respectively (17, 18). However, there is some indication that both N. europaea and N. winogradskyi may not be predominant components of actively nitrifying freshwater aquaria (9). In seawater aquaria, however, N. europaea and close relatives do appear to comprise a significant proportion of the total eubacterial community, but N. winogradskyi was below detection limits (9).
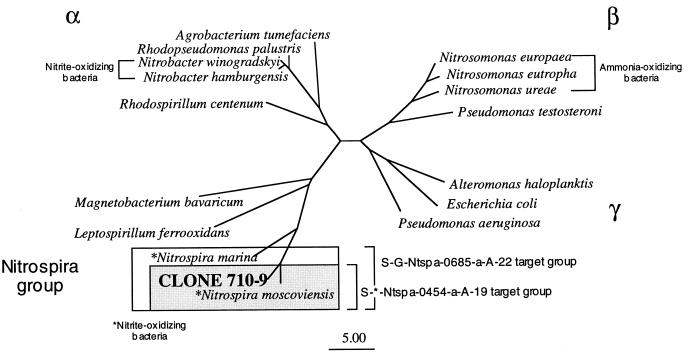
Chemolithoautotrophic NOB are phylogenetically diverse, occurring in several subdivisions of the class Proteobacteria (Fig. 1). The most well-studied members of this group of organisms (i.e., N. winogradskyi and close relatives) belong to the α subdivision of the class Proteobacteria (16). Nitrospina gracilis and Nitrococcus mobilis, which were first isolated by Watson and Waterbury (16), were determined to be members of the δ and γ subdivisions of the class Proteobacteria, respectively (14). Another NOB, Nitrospira marina, is phylogenetically affiliated with non-NOB such as Leptospirillum ferrooxidans (7, 14, 16). Based on phylogenetic analysis of 16S rRNA sequences, Erlich et al. (7) proposed a new phylum within the domain Bacteria for these organisms (Fig. 1). A newly discovered NOB from a freshwater environment (a corroded iron pipe in a heating system), Nitrospira moscoviensis, was recently found to be phylogenetically related to N. marina (7).
FIG. 1.
Phylogenetic relationships of autotrophic NOB in the α subdivision of the class Proteobacteria and the Nitrospira group. Clone 710-9, an rDNA clone originating from aquaria with active NOB populations, is most similar to NOB of the Nitrospira group. The specificities of two oligonucleotide probes designed for Nitrospira spp. are indicated by the boxed sections.
Whether in pure culture or on biofilters, NOB are slowly growing organisms with doubling times from 12 to 32 h (3, 5, 7). Therefore, in newly set up aquaria, ammonia and nitrite can reach concentrations toxic to fish before a sufficient biomass of AOB and NOB becomes established. To reduce the length of time for establishment of NOB on biofilters, commercial preparations of these organisms, in various forms of preservation, are available to seed the aquarium environment. These preparations range from essentially pure cultures of Nitrobacter species to mixed cultures of autotrophic AOB and NOB organisms and to products which combine autotrophic nitrifying bacteria with various species of heterotrophic bacteria. Past studies have generally shown these mixes to be ineffectual but have not elucidated specific reasons for their poor performance (4, 15).
In this study, we observed that Nitrospira-like species rather than Nitrobacter species appeared responsible for oxidation of nitrite to nitrate in freshwater aquaria. A combination of methods was used to investigate concurrently the appearance of NOB on biofilters and the oxidation of nitrite to nitrate. Oligonucleotide probes, which target Nitrospira and close relatives, were developed and used to quantify this group at different times during the establishment of nitrification. Denaturing gradient gel electrophoresis (DGGE) was used to monitor the appearance of Nitrospira-like bacteria during the onset of nitrite oxidation. The effectiveness of a commercial mix of AOB and NOB was also evaluated.
MATERIALS AND METHODS
Nucleic acid sampling and extraction.
For rRNA extractions from aquarium gravel, individual gravel samples (10 g) were placed in a polypropylene tube and covered with 2.5 ml of low-pH buffer (50 mM sodium acetate, 10 mM disodium EDTA) and processed as previously described (9). For DNA extraction, gravel samples were resuspended in cell lysis buffer (40 mM EDTA, 50 mM Tris-HCl, 0.75 M sucrose) and processed as described previously (9). Samples were stored at −20°C until extraction.
DNA was quantified by Hoechst type 33258 dye binding and fluorometry (DynaQuant 200; Hoefer Pharmacia Biotech, Inc., San Francisco, Calif.). rRNA was quantified by measuring A260 (Lambda 3B; Perkin Elmer), assuming that 1 A260 U corresponds to 40 μg of RNA per ml.
Clone libraries of PCR-amplified rRNA genes.
Clone libraries were derived from nucleic acid extracts of aquarium samples. Bacterial rRNA gene fragments were amplified with the primers S-D-Bact-0011-a-S-17 (8f; GTT TGA TCC TGG CTC AG) and 1492r (eubacterial; GGT TAC CTT GTT ACG ACT T) or S-*-Univ-0519-a-A-18 (519r; GWA TTA CCG CGG CKG CTG). PCR conditions, cycle parameters, and reaction components were as previously described (6). PCR products were evaluated by agarose gel electrophoresis. PCR fragments were cloned with a TA cloning kit (Invitrogen, Carlsbad, Calif.), as previously described (6).
DGGE analysis and profiling.
For DGGE analysis, ribosomal DNA (rDNA) fragments were amplified with the forward primer 358f (eubacterial; CCT ACG GGA GGC AGC AG) with a 40-bp GC clamp on the 5′ end as described by Murray et al. (11) and the reverse primer S-*-Univ-0519-a-A-18 (519r; GWA TTA CCG CGG CKG CTG). PCR was performed on a Stratagene Robocycler Gradient 96 (La Jolla, Calif.) with the manufacturer’s reagents. PCR conditions included a hot start (80°C) and a touchdown procedure (11). Initial denaturation at 94°C for 3 min was followed by a denaturation at 94°C for 1 min, a touchdown annealing from 65 to 55°C for 1 min and 29 s (the annealing time during the touchdown increased by 1.4 s per cycle), and primer extension at 72°C for 56 s (the extension time was increased 1.4 s per cycle). The final temperature series of the above thermal cycle was repeated for 20 total cycles, followed by a final extension at 72°C for 5 min. Amplicons were examined by agarose gel electrophoresis.
DGGE was performed with a Bio-Rad D-GENE System (Bio-Rad Laboratories, Hercules, Calif.). All gels were 8.5% acrylamide–bis with Bio-Rad reagents (D-GENE Electrophoresis Reagent kit). Gel gradients were poured with Bio-Rad reagents (D-GENE Electrophoresis Reagent kit) with a denaturing gradient of 20 to 60% (where 100% denaturant is a mixture of 40% deionized formamide and 7 M urea) and the Bio-Rad gradient delivery system (model 475; Bio-Rad). All gels were run at 200 V for 6 h. The gels were visualized in one of two ways. For visualization and recovery of discrete DNA bands, the gels were first stained for 10 min in 250 ml of 1× Tris-acetate-EDTA (TAE) buffer, in which 100 μl of ethidium bromide (1 mg/ml) was added, and then were washed for 10 min in 1× TAE buffer. For documentation purposes, some gels were stained in Vistra Green (diluted 1:10,000) (Molecular Dynamics, Sunnyvale, Calif.) for 20 min, followed by a 20-min wash in 1× TAE buffer, and then were scanned with a FluorImager SI (Molecular Dynamics).
Individual bands were excised from the DGGE gels with alcohol-sterilized scalpels. Extraction of DNA from the gel followed the methods of Ferris et al. (8). The excised band was placed in a sterile 2-ml screw-cap tube with 500 μl of sterile deionized water. The tubes were half filled with glass beads (catalog no. 11079-101; BioSpec Products, Inc., Bartlesville, Okla.) and placed in a mechanical bead beater (Mini-beadbeater-8; BioSpec Products) for 3 min at the highest setting. The processed DNA remained in the tubes at 4°C overnight. After overnight storage, the tubes were centrifuged at 3,200 × g for 8 min at 4°C to concentrate the gel fragments. The supernatant was transferred to a clean Eppendorf tube.
To check the extraction efficiency, the supernatant was reamplified with the DGGE primers and reanalyzed by DGGE. An extraction was considered acceptable if it yielded a single band in DGGE analysis which comigrated with the original DGGE band in the mixed population sample.
Oligonucleotide probe development and hybridization procedures.
Two oligonucleotide probes were designed which specifically hybridize with N. marina, N. moscoviensis, and the Nitrospira-like rRNA gene sequence isolated in this study from biofilters. One probe (S-G-Ntspa-0685-a-A-22) targets the biofilter-derived Nitrospira-like bacterium and both N. marina and N. moscoviensis. The second probe (S-*-Ntspa-0454-a-A-19) targets the biofilter-derived Nitrospira-like bacterium and its closest cultivated relative, N. moscoviensis (Fig. 1). Probe matches were initially screened by BLAST (2) and CHECK_PROBE (10). The probes were synthesized by Operon Tech, Inc. (Alameda, Calif.). The nucleotide sequences and positions of the probes are shown in Table 1.
TABLE 1.
The nucleotide sequences and positions of oligonucleotide probes for NOB
| Probea | Position (nucleotides)b | Base sequence (5′ to 3′) | Td (°C)c | Wash temp (°C) | Targeted group | Nontarget bacteria with exact match to probe sequence |
|---|---|---|---|---|---|---|
| S-G-Ntspa-0685-a-A-22 | 664–685 | CAC CGG GAA TTC CGC GCT CCT C | 63.0 | 60.0 | N. moscoviensis, N. marina, and 710-9 clone | None |
| S-*-Ntspa-0454-a-A-19 | 435–454 | TCC ATC TTC CCT CCC GAA AA | 58.5 | 56.0 | N. moscoviensis 710-9 clone | None |
Probe names designated by the standard proposed by Alm et al. (1).
E. coli numbering.
Td, temperature at which 50% of the bound probe is released from the homologous hybrid.
Since no pure rRNA of the biofilter-derived Nitrospira-like bacterium is yet available, in vitro-transcribed 16S rRNA was used as a template for temperature of dissociation (Td) determinations and as a control in hybridization experiments. In vitro-transcribed 16S rRNA was synthesized as described by Polz and Cavanaugh (12).
The Tds of the oligonucleotide probes were determined by measuring the amounts of probe eluted over a series of increasing wash temperatures (13). For these tests, 200 ng of template was immobilized on a nylon membrane (Hybond-N; Amersham) and hybridized overnight at 45°C with 32P-labelled probe. After hybridization, the membrane was washed at room temperature in 1× SET (150 mM NaCl, 1 mM EDTA, 20 mM Tris; pH 7.8)–1% sodium dodecyl sulfate (SDS) for 30 min on a shaker table. Individual filter strips were then placed in a 0.5-ml Eppendorf tube containing 500 μl of 1× SET–1% SDS preheated to the initial test temperature. The Eppendorf tubes were placed in a thermal cycler (Perkin-Elmer) and incubated for 30 min. The membrane was transferred to a new Eppendorf tube containing 1× SET–1% SDS, and the temperature was increased and maintained at the elevated temperature for 30 min. After each wash, the wash buffer was transferred to a scintillation vial containing 3 ml of scintillation cocktail (Liquiscint; National Diagnostics, Atlanta, Ga.) and was mixed, and radioactivity was quantified by liquid scintillation counting. Each profile was performed in duplicate.
rRNA from aquaria was slot blotted and quantified with nucleic acid probes developed in this and an earlier study (9) under conditions previously described (9). The methods for determining the relative amounts of rRNA-specific hybridization signal from each probe were the same as those previously described (9).
Sequencing.
Sequencing of SSU rDNA excised from DGGE gels or clones was performed directly with Sequenase 2.0 (U.S. Biochemicals, Cleveland, Ohio).
Experimental aquarium systems.
Three sets of experiments in aquaria were run to (i) study the establishment of nitrifying bacteria and (ii) determine the effect of a bacterial additive. New aquaria, filter systems, and gravel were used for each test. Samples of aquarium water for the three tests were analyzed for ammonia (gas diffusion membrane method), nitrite (azo dye method), and nitrate (cadmium reduction-azo dye method) by flow injection analysis as previously described (9).
(i) Bacterial additive test.
Six all-glass aquaria were established with an airlift undergravel filtration system (model KF720; Neptune Products, Moorpark, Calif.) in a temperature-controlled laboratory (mean air temperature, 26.0 ± 1.5°C). The aquaria were covered with glass lids but were not illuminated other than by room ceiling lights which were on a 14- and 10-h light and dark cycle, respectively. A 6.8-kg amount of natural aquarium gravel (Kaytee Products, Irwindale, Calif.) was placed on top of the filtration plate. A 30-liter volume of city tap water, passed through activated carbon, was added to each aquarium. Filtered air was supplied to each aquarium from a common air source. Six fish (Danio aequipinnatus) were placed in each aquarium and fed 0.5 g of fish feed (Aquarian; Kal Kan Foods, Vernon, Calif.) daily over two feedings. Three of the aquaria (the treatment group) were each given doses of 8 ml of bacterial additive (Cycle; Rolf C. Hagen Inc., Mansfield, Mass.) on the first day and once every 7 days afterwards for an additional 3 weeks. The other three aquaria were the control group and did not receive an additive.
Two samples of 10 g of gravel were collected from each aquarium on a weekly basis, and nucleic acids were extracted and analyzed as described above.
(ii) Time of NOB appearance.
Three all-glass aquaria were established as described above. A 34-liter sample of city tap water, which was passed through activated carbon, was added to each aquarium, which contained 4.53 kg of gravel. Initially, 0.71 mmol of filter-sterilized (0.2-μm-pore-size filter) ammonium chloride was added to each tank, followed by an additional dosing of 5.0 mmol of NH4Cl on the fourth day. On days 10, 15, 18, 23, and 30, further ammonia additions of 8.9 mmol were made to each aquarium. During the test, a total of 50.4 mmol of ammonia was added to each aquarium. Water samples were collected daily.
Two 10-g samples of gravel were collected from each aquarium daily for 33 days. To one sample, 2 ml of lysis buffer was added and the sample was frozen (−20°C) until rDNA was extracted by previously described methods. rDNA was subjected to DGGE after undergoing PCR with the primers and conditions described above. The other sample was preserved with 2 ml of bead beating buffer.
(iii) Time series.
Three aquaria were set up as previously described with 4.53 kg of gravel and were filled with 30 liters of city water which had been passed through activated carbon. The test was run for 138 days, during which the aquaria were individually dosed with 8.9 mmol of filter-sterilized (0.2 μm) ammonia (as ammonium chloride) on the first and second days of the test. From days 12 to 78 of the test, further additions of 8.9 mmol of ammonia were done on average every 3 days. A total of 246 mmol of ammonia was added to each tank during the test. The water was sampled three times a week for chemical analysis. The aquaria were run for 80 days with freshwater, at which time the water was switched to seawater (32 ppt) by draining and refilling with water mixed with artificial sea salts (Marineland Commercial Aquariums, Moorpark, Calif.). After the switch, the testing continued for an additional 57 days.
Nucleotide sequence accession no.
The nucleotide sequence reported in this paper for clone 710-9 has been deposited in the GenBank database under accession no. AF035813.
RESULTS
Isolation of putative NOB.
Two approaches were taken to identify NOB in aquarium samples. The first approach was to develop clone libraries from gravel samples from an aquarium at several times during the establishment of nitrification. Samples were taken 17 and 31 days after the aquarium establishment and ammonia additions started. A third library was constructed from DNA extracted from the material of a commercial biofilter constructed of thermoplastic material (model CBW-1; Aquaria, Inc.). This filter had been set up for 109 days in a system with daily dosing of ammonium chloride.
The second approach used to monitor and identify nitrifying microorganisms was DGGE. The DNA extracted from aquarium gravel samples taken during the establishment of nitrification was subjected to DGGE to produce a pattern of discrete bands. The banding patterns were compared to each other and to band patterns produced by a mix of known nitrifiers. Unique bands were excised from the gels and sequenced.
The sequences from the clone libraries and DGGE were compared to bacterial sequences found in public databases (BLAST [2] and RDP [10]). Some clones, which showed a close similarity to those of known nitrite-oxidizing organisms, were more completely sequenced.
Identification of putative Nitrospira-like NOB.
Five samples were screened for NOB by either clone library development or DGGE. A total of 96 clones or excised bands were partially sequenced. Of these, 11 were highly similar to members of the Nitrospira group but none were similar to Nitrobacter spp. The partial sequences were most highly similar to those of N. marina and N. moscoviensis (data not shown). The 16S rDNA of a representative clone which contained the Nitrospira-like rDNA was fully sequenced, and a phylogenetic tree was inferred. Phylogenetic analysis indicated a high similarity between this cloned rDNA (710-9) and members of the Nitrospira group, N. moscoviensis and N. marina (Fig. 1). The rDNA contained in clone 710-9 was 96.1% similar to that of N. moscoviensis and 87.4% similar to that of N. marina (Table 2).
TABLE 2.
Similarity ranking for clone 710-9 isolated from freshwater aquaria and members of the Nitrospira group
| rDNA source | % Similarity to rDNA of:
|
|||||
|---|---|---|---|---|---|---|
| 710-9 sequence | N. moscoviensis | N. marina | Leptospirillum sp. | L. ferrooxidans | M. bavaricum | |
| 710-9 sequencea | ||||||
| N. moscoviensis | 96.1 | |||||
| N. marina | 87.4 | 87.6 | ||||
| Leptospirillum sp. | 79.9 | 80.3 | 80.2 | |||
| L. ferrooxidans | 78.1 | 78.4 | 77.9 | 91.0 | ||
| Magnetobacterium bavaricum | 78.2 | 77.9 | 79.7 | 78.3 | 77.0 | |
Positions 24 to 1284 of 710-9 (E. coli numbering).
Oligonucleotide probe specificity.
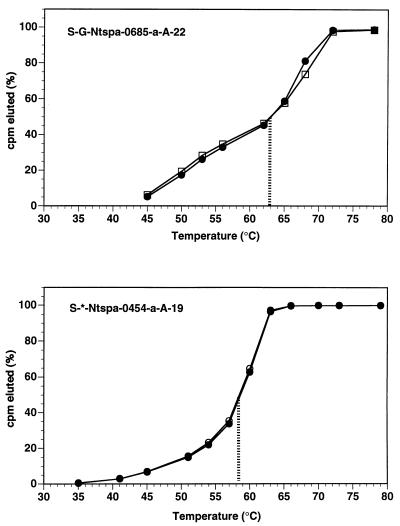
Oligonucleotide probe sequences, positions (Escherichia coli numbering), Tds, wash temperatures and target groups for the probes are indicated in Table 1. For probe S-*-Ntspa-0454-a-A-19, the Td was 58.5°C, while the Td was 63.0°C for the S-G-Ntspa-0685-a-A-22 probe (Fig. 2).
FIG. 2.
Results of the Td experiments for the probes S-G-Ntspa-0685-a-A-22 and S-*-Ntspa-0454-a-A-19, with the 50% Td indicated by the vertical line. □, rRNA of N. marina; • and ○, transcribed RNA of clone 710-9.
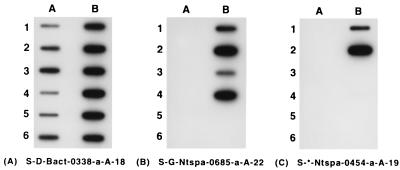
Slot blot experiments confirmed that the probe S-G-Ntspa-0685-a-A-22 was specific to the known NOB of the Nitrospira group, as well as to the clone 710-9 (Fig. 3). As predicted, probe S-*-Ntspa-0454-a-A-19 hybridized to clone 710-9, but not N. marina. Furthermore, experiments demonstrated that neither probe hybridized with NOB which are members of the α or δ subdivisions of the class Proteobacteria (Fig. 3).
FIG. 3.
Specificities of the oligonucleotide probes targeting NOB of the Nitrospira group and the 710-9 clone identified in this study. Probe order was eubacterial probe S-D-Bact-0338-a-A-18 (A), Nitrospira-like NOB probe S-G-Ntspa-0685-a-A-22 (B), and Nitrospira-like NOB probe S-*-Ntspa-0454-a-A-19 (C), with rRNA, transcribed RNA (trRNA), or PCR-amplified rDNA, in the following arrangement: slot A-1, Comamonas testosteroni; slot A-2, Alcaligenes eutrophus; slot A-3, Alcaligenes faecalis; slot A-4, Comamonas acidovorans; slot A-5, N. winogradskyi (rDNA); slot A-6, Nitrobacter agilis (rDNA); slot B-1, clone 710-9 (rDNA); slot B-2, clone 710-9 (trRNA); slot B-3, N. marina (rDNA); slot B-4, N. marina (trRNA); slot B-5, N. gracilis; slot B-6, Shewanella putrefaciens. See text for description of methods.
Detection of NOB in aquaria.
Table 3 summarizes the results from the probing of several aquarium biofilms with the NOB probes. Probe S-G-Ntspa-0685-a-A-22 yielded a positive signal with all freshwater and saltwater aquaria tested. The probe S-*-Ntspa-0454-a-A-19 detected Nitrospira-like bacteria in all freshwater aquaria, but not in all the saltwater aquaria (Table 3). There were no cases of positive detection by a probe which targets α proteobacterial Nitrobacter species (Table 3).
TABLE 3.
Results of probing rRNA extracted from aquarium biofilms with nucleic acid probes for NOB
| Sample label | Aquarium environmenta | Biofilm substrateb | Daily ammonia amtc | Ammonia sourced | Signal with the following oligonucleotide probese,f:
|
|||
|---|---|---|---|---|---|---|---|---|
| S-D-Bact-0338-a-A-18 | NOB
|
|||||||
| S-*-Nbac-1017-a-A-20 | S-G-Ntspa-0685-a-A-22 | S-*-Ntspa-0454-a-A-19 | ||||||
| 710r | Freshwater | Polyfiber | 32.1 mM | NH4Cl | + | − | + | + |
| 711r | Freshwater | Polyfiber | 32.1 mM | NH4Cl | + | − | + | + |
| T825 | Freshwater | Polypp | 0.8 g | Fish | + | − | + | + |
| T825 | Freshwater | Gravel | 0.8 g | Fish | + | − | + | + |
| WDF1036 | Freshwater | Polypp | 3.2 g | Fish | + | − | + | + |
| WDF1036 | Freshwater | Gravel | 3.2 g | Fish | + | − | + | + |
| WDF1026 | Freshwater | Polypp | 2.0 g | Fish | + | − | + | + |
| WDF1039 | Freshwater | Gravel | 3.2 g | Fish | + | − | + | + |
| WDF1038 | Freshwater | Sponge | 2.0 g | Fish | + | − | + | + |
| WDF1035 | Freshwater | Polypp | 2.0 g | Fish | + | − | + | + |
| FLRT6 | Freshwater | Gravel | 2.0 g | Fish | + | − | + | + |
| EXP8B | Freshwater | Polypp | 1.4 g | Fish | + | − | + | + |
| FWSW4 | Freshwater | Polypp | 5 mM | NH4Cl | + | − | + | + |
| FWSW6 | Freshwater | Polypp | 5 mM | NH4Cl | + | − | + | + |
| BC2-8 | Freshwater | Gravel | 5 mM | NH4Cl | + | − | + | + |
| BC2-10 | Freshwater | Gravel | 5 mM | NH4Cl | + | − | + | + |
| BC2-12 | Freshwater | Gravel | 5 mM | NH4Cl | + | − | + | + |
| BC2-13 | Freshwater | Gravel | 5 mM | NH4Cl | + | − | + | + |
| BC2-16 | Freshwater | Gravel | 5 mM | NH4Cl | + | − | + | + |
| BC2-4 | Freshwater | Gravel | 2.0 g | Fish | + | − | + | + |
| BC2-16a | Freshwater | Gravel | 2.0 g | Fish | + | − | + | + |
| 714r | Seawater | Polyfiber | 714 mM | NH4Cl | + | − | + | − |
| 715r | Seawater | Polyfiber | 714 mM | NH4Cl | + | − | + | + |
| FWSW2 | Seawater | Polypp | 5 mM | NH4Cl | + | − | + | − |
| FWSW3 | Seawater | Polypp | 5 mM | NH4Cl | + | − | + | − |
| FWSW8 | Seawater | Polypp | 5 mM | NH4Cl | + | − | + | − |
| FWSW9 | Seawater | Polypp | 5 mM | NH4Cl | + | − | + | − |
Type of aquarium water.
Media from which the bacterial cells were extracted, Polypp, polypropylene.
Fish, the aquarium had a fish population and ammonia was generated by the fish; NH4Cl, the tank had no fish and the ammonia was from daily dosing with ammonium chloride.
Values in grams are the amounts of fish feed put into the aquarium each day; molar or millimolar values are the concentrations of ammonia added to the aquarium or system in which the biofilter was located each day.
+, signal detected by probe; −, no signal detected.
S-*-Nbac-1017-a-A-20 was originally called NBAC2 (9) and targeted N. winogradskyi and N. agilis. Probe S-G-Ntspa-0685-a-A-22 targeted N. marina, N. moscoviensis, and clone 710-9. Probe S-*-Ntspa-0454-a-A-19 targeted N. moscoviensis and clone 710-9.
Time series.
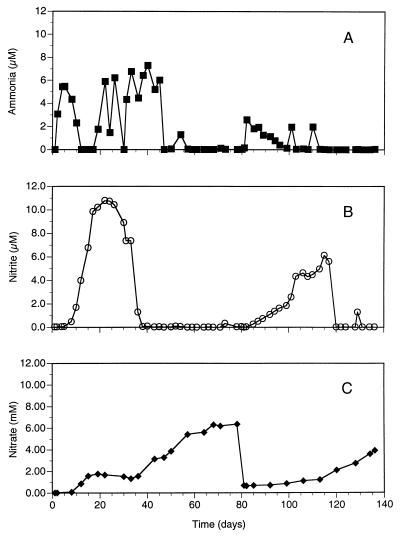
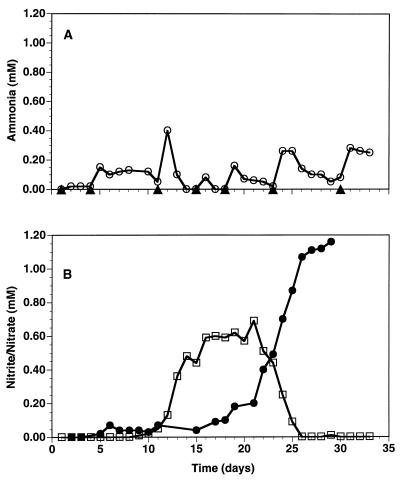
The ammonia, nitrite, and nitrate values for a representative test aquarium dosed with ammonium chloride for 138 days are shown in Fig. 4. The data show the expected pattern for the establishment of nitrification in aquaria. Initially, the concentration of ammonia increased and then decreased to undetectable levels by day 12 (the saw-toothed pattern of the ammonia values is the result of the increasing frequency of ammonia additions). By day 12, the amount of nitrite increased, reaching its maximum value on day 22. By day 38, the amount of nitrite was essentially 0 and that of nitrate was steadily increasing (Fig. 4). The change from freshwater to seawater at day 80 resulted in an immediate increase in the amounts of ammonia and, subsequently, nitrite. It took nearly 20 days for ammonia oxidation to become reestablished. Reestablishment of nitrite oxidation took approximately 40 days.
FIG. 4.
Ammonia (A), nitrite (B), and nitrate (C) chemistry for an aquarium from startup through 138 days. The saw-toothed pattern for ammonia is the result of the increasing frequency of dosing with ammonium chloride as nitrification was being established. The water was switched from freshwater to seawater on day 80.
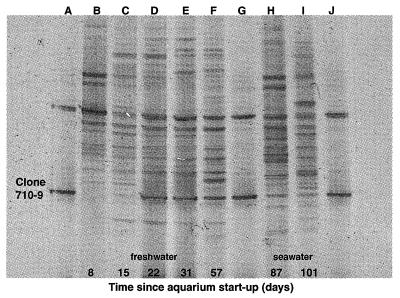
A DGGE profile for selected days over the first 101 days for this aquarium shows that the Nitrospira-like rDNA sequence appeared faintly on day 15, corresponding to the onset of nitrite oxidation (Fig. 5). By day 22, the band corresponding to the Nitrospira-like rDNA sequence increased in relative intensity and remained intense over the next two sampling dates. After the switch to seawater, the relative intensity of the Nitrospira-like band diminished. The general band pattern also changed qualitatively between freshwater and seawater sampling dates. The banding pattern for day 87 (7 days after the switch) appeared to more closely resemble the pattern for day 57 (freshwater) than the pattern for day 101 (seawater) (Fig. 5).
FIG. 5.
DGGE time series profile from a biofilm of a freshwater aquarium during the establishment of nitrification. The aquarium water was switched to seawater on day 80. Lanes A, G, and J contain two clones, including clone 710-9, a putative NOB showing close similarity to the Nitrospira group. The band corresponding to this organism first appears with significant intensity on day 22. Lanes B, C, D, E, and F are sampling dates before the switch to seawater. Lanes H and I are sampling dates after the switch to seawater. The water chemistry for various forms of nitrogen in this aquarium is indicated in Fig. 4.
Time of Nitrospira-like bacterial appearance.
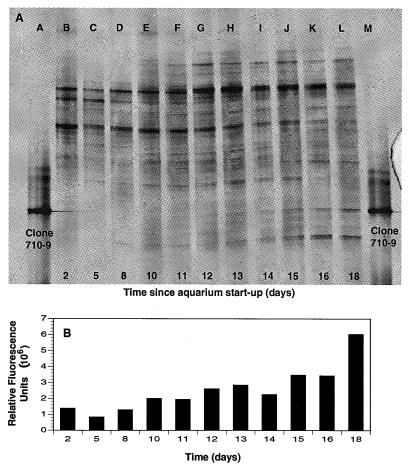
The daily concentrations of ammonia, nitrite, and nitrate over the first 33 days after setup of a new aquarium are presented in Fig. 6. The trends were as expected, with ammonia peaking about day 12. Nitrite values increased starting at day 12, peaked at day 21, and decreased to below detection limits by day 26. Nitrate values steadily increased from about day 15 onwards. DGGE showed that the band corresponding to clone 710-9, the putative NOB, first appeared on day 12, with the relative intensity of the 710-9 band increasing daily based on relative fluorescence units of rDNA amplicons (Fig. 7).
FIG. 6.
Inorganic nitrogen values for a newly established freshwater aquarium dosed with ammonia chloride over 33 days. (A) Ammonia (○) values along with dates of ammonia additions (▴); (B) nitrite (□) and nitrate (•) values for the same aquarium. A DGGE profile of the nitrifying assemblage associated with this aquarium is presented in Fig. 7.
FIG. 7.
(A) DGGE of select dates during the first 18 days after the startup of a freshwater aquarium, during which time nitrification became established. Clone 710-9, a Nitrospira-like putative NOB, can be seen to appear starting at about day 12 (lane G). (B) Relative intensities of the band for clone 710-9 at each sampling date. Associated water chemistry data for this aquarium are presented in Fig. 6.
Commercial additive.
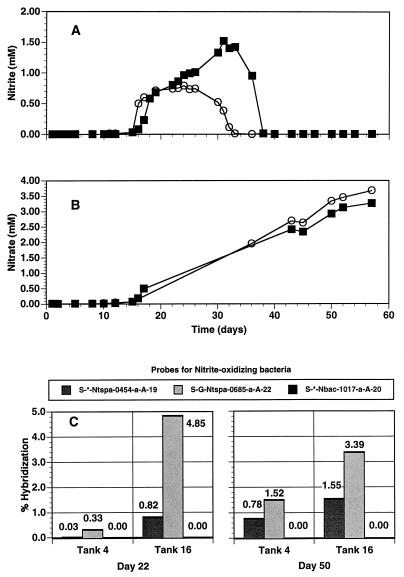
The addition of a commercial bacterial mixture which contained Nitrobacter sp., but not Nitrospira sp., did not result in the detection of Nitrobacter species by oligonucleotide probe hybridization experiments (Fig. 8). However, a band which comigrated with a control derived from pure Nitrobacter DNA could be detected in the original commercial mixture by DGGE analysis (data not shown). Nitrospira-like rRNA was readily detected in the aquarium. Nitrospira group-specific probes indicated that the tank which received the additive had a significantly greater percentage of the Nitrospira species rRNA (Fig. 8). By day 16, approximately 5% of the eubacterial rRNA hybridized with the general Nitrospira group-specific probe, compared to only 0.33% of the eubacterial rRNA in the tank which did not receive an additive (Fig. 8). By day 50, the values were 3.39 and 1.52% for the additive and nonadditive aquaria, respectively (Fig. 8).
FIG. 8.
Water chemistry data and nucleic acid probe hybridization results for a freshwater aquarium during the first 57 days after startup. Nitrite (A) and nitrate (B) are for two tanks, i.e., tank 4 (▪), which did not receive a commercial bacterial mixture, and tank 16 (○), which received weekly additions of a commercial bacterial mixture for the first 4 weeks. Problems with nitrate analysis equipment resulted in no data for days 18 through 40. (C) Percent hybridization (relative to that of a eubacterial probe, S-D-Bact-0338-a-A-18) to probes specific for NOB. Probes S-G-Ntspa-0685-a-A-22 and S-*-Ntspa-0454-a-A-19 target Nitrospira spp., while probe S-*-Nbac-1017-a-A-20 is for NOB of the α subdivision of the class Proteobacteria.
Nitrite concentrations in the two aquaria decreased as the relative percentages of Nitrospira-like rRNA increased. By day 22, the nitrite value had reached a maximum in the tank which received the additive. Nitrite concentrations reached maxima in the nonadditive aquarium on about day 32. By day 38, the nitrite levels in both aquaria were essentially below our limits of detection, and nitrate levels were equivalent in the treated and nontreated aquaria (Fig. 8).
DISCUSSION
Our results from DGGE analysis, rRNA probing, and sequencing generally indicate that Nitrospira-like bacteria are the most likely candidates responsible for nitrite oxidation in freshwater aquaria. The combined use of molecular phylogenetic techniques and monitoring of water chemistry suggested a correspondence between changes in the biofilm microbial community which coincided with the onset of ammonia and nitrite oxidation. The commencement of nitrite oxidation coincided with the appearance of the putative nitrite-oxidizing Nitrospira-like bacterium. The results lend support to the conclusion of an earlier study, which suggested that α subdivision proteobacterial NOB (Nitrobacter types) were not major components of nitrite oxidation bacterial populations in freshwater or marine aquaria (9).
Results regarding the beneficial effects of the addition of a bacterial additive containing Nitrobacter species were equivocal. While nitrite levels in treated aquaria decreased earlier than those in nontreated aquaria, there was no evidence that Nitrobacter species were actively growing in these aquaria. It is possible that the levels of Nitrobacter species were below the limits of detection of our techniques. However, since Nitrospira-like bacteria were readily detected and that their establishment coincided with nitrite oxidation we postulate that Nitrospira-like organisms, and not Nitrobacter species, are the major nitrite oxidizers in the freshwater aquarium environment. It is possible that the addition of bacterial mixtures supplies vitamins and other nutrients which generally stimulate the growth of the nitrifying assemblages, fostering their growth and development and indirectly stimulating nitrite oxidation.
In the present study, we identified Nitrospira-like putative NOB by amplification of rDNA with general bacterial PCR primers and DGGE analyses. We chose to use universal and domain primers rather than group-specific primers, since previous analysis suggested that nitrite oxidizers other than Nitrobacter might be involved in nitrification in aquaria (9). Combined monitoring of environmental conditions (water chemistry) with bacterial assemblage analysis (DGGE) allowed us to detect a correspondence between nitrite oxidation and Nitrospira-like rRNA. By monitoring samples over time, changes in the microbial assemblage were evident. This approach permitted a more focused effort in the search for links between environmental processes and the microbes which mediate them.
When comparing biofilters, researchers in the past have been generally limited to assessing mainly water chemistry changes, such as ammonia disappearance and nitrate appearance. The use of molecular probes for the relevant nitrifying bacteria in different systems should provide a more detailed understanding of the interaction between the biology and chemistry of the systems. This in turn provides information relevant to better filter design and may allow the effects of various conditions to be assessed with respect to their effects on the biology as well as the chemistry of the system.
ACKNOWLEDGMENTS
We thank Ellen Ko, Quynh Lu, and Michelle Waugh for helpful assistance and Alison Murray for assisting with the DGGE. We also thank Julia Sears-Hartley, Melissa Lokken, and Les Wilson for water chemistry analysis.
This work was supported in part by National Science Foundation grants OCE95-29804 and OPP94-18442 to E.F.D. and by assistance from Aquaria, Inc. to T.A.H.
REFERENCES
- 1.Alm E W, Oerther D B, Larsen N, Stahl D A, Raskin L. The oligonucleotide probe database. Appl Environ Microbiol. 1996;62:3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Belser L W, Schmidt E L. Diversity in the ammonia-oxidizing nitrifier population of a soil. Appl Environ Microbiol. 1978;36:584–588. doi: 10.1128/aem.36.4.584-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower C E, Turner D T. Accelerated nitrification in new seawater culture systems: effectiveness of commercial additives and seed media from established systems. Aquaculture. 1981;24:1–9. [Google Scholar]
- 5.Carlucci A F, Strickland D H. The isolation, purification and some kinetic studies of marine nitrifying bacteria. Exp Mar Biol Ecol. 1968;2:156–166. [Google Scholar]
- 6.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrich S, Behrens D, Lebedeva E, Ludwig W, Bock E. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch Microbiol. 1995;164:16–23. doi: 10.1007/BF02568729. [DOI] [PubMed] [Google Scholar]
- 8.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined population inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovanec T A, DeLong E F. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl Environ Microbiol. 1996;62:2888–2896. doi: 10.1128/aem.62.8.2888-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray A L, Hollibaugh J T, Orrego C. Phylogenetic compositions of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl Environ Microbiol. 1996;62:2615–2620. doi: 10.1128/aem.62.7.2676-2680.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polz M F, Cavanaugh C M. A simple method for quantification of uncultured microorganisms in the environment based on in vitro transcription of 16S rRNA. Appl Environ Microbiol. 1997;63:1028–1033. doi: 10.1128/aem.63.3.1028-1033.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raskin L, Stromley J M, Rittmann B E, Stahl D A. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol. 1994;60:1232–1240. doi: 10.1128/aem.60.4.1232-1240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teske A, Alm E, Regan J M, Toze S, Rittmann B E, Stahl D A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmermans J A, Gerard R. Observations sur l’utilisation en étangs de suspensions bactériennes du commerce. Bull Fr Péche Piscicult. 1990;316:28–30. [Google Scholar]
- 16.Watson S W, Waterbury J B. Characteristics of two marine nitrite oxidizing bacteria, Nitrospina gracilis nov. gen. nov. sp. and Nitrococcus mobilis nov. gen. nov. sp. Arch Mikrobiol. 1971;77:203–230. [Google Scholar]
- 17.Wheaton F W. Aquacultural engineering. New York, N.Y: John Wiley & Sons, Inc.; 1977. [Google Scholar]
- 18.Wheaton F W, Hochheimer J, Kaiser G E. Fixed film nitrification in filters for aquaculture. In: Brune D E, Tomasso J R, editors. Aquaculture and water quality. Baton Rouge, La: The World Aquaculture Society; 1991. pp. 272–303. [Google Scholar]