Abstract
Penicillium fellutanum is osmotolerant and xerotolerant when cultured in a low-phosphate medium containing 3 M NaCl. Glycerol and erythritol accumulated in cultures with NaCl concentrations up to 2 M; glycerol was the only detectable polyol in cultures containing 3 M NaCl. In cultures with 3 M NaCl, the intracellular levels of glycine betaine and choline-O-sulfate were 22- and 2.6-fold greater (70 and 46 mM), respectively, than those of cultures without added NaCl. The levels of glycine betaine and glycerol decreased in mycelia transferred from a medium containing 3 M NaCl into a fresh medium without added NaCl. NaCl at 3 M inhibited mycelial mass accumulation; this inhibition was partially corrected by supplementation of cultures with glycine betaine (2 mM) or choline-O-sulfate (10 mM). The presence of exogenous choline chloride (2 mM) in plate cultures protected the cells from stress from 3 M NaCl. The data suggest that glycine betaine and choline-O-sulfate are secondary osmoprotectants which are effective at the point that the cell is incapable of synthesizing more glycerol.
Cellular adaptation to extreme environmental conditions, such as high-saline environments, is a fundamental biological process needed for survival and growth of organisms (14, 25, 43). Osmotic stress is caused by large concentrations of either salts or nonionic solutes in the surrounding medium; this results in dehydration of cells (7, 30, 46, 50). Salinity is a major limiting factor in agricultural productivity (30) and is of concern in the food and medicinal industries (8, 17, 24, 28, 40, 42, 43, 48).
Fungi, including yeasts, are well known for their ability to adapt to environments of high osmolarity by intracellular accumulation of species of neutral, low-molecular-weight compounds which maintain positive turgor pressure (7, 11, 12). Fungal cells synthesize polyols even without osmotic stress and respond to osmotic stress by accumulation of predominantly polyols (7, 21). These compatible solutes can accumulate up to molar concentrations without greatly interfering with the normal functions of intracellular enzymes; these solutes may protect enzymes and other cellular components from high salt concentrations (4, 41, 49, 50).
The known osmoresponsive compatible solutes used by fungi are mainly polyhydroxy alcohols, trehalose, and K+. Glycerol is the predominant osmoprotectant (1, 3, 5, 7, 16, 19, 26, 31). Penicillium and Aspergillus species tested accumulate glycerol as a major, and erythritol as a minor, osmoregulatory solute (2, 16, 22, 23).
We reported that glycine betaine (GB) and choline-O-sulfate (COS) accumulate inside Penicillium fellutanum cultured in a low-phosphate medium (39). Phosphocholine of extracellular peptidophosphogalactomannan (pPxGM [where x is the number of phosphodiester residues]) is a precursor of these two intracellular choline derivatives. Experiments were performed to determine if these intracellular choline derivatives are involved in adaptation to osmotic stress in P. fellutanum. This paper reports that intracellular GB, COS, and exogenous choline are involved in osmoprotection of P. fellutanum under low-phosphate and high-osmolarity conditions.
MATERIALS AND METHODS
Chemicals and strain.
Sodium (trimethylsilane)-1-propanesulfonate (TSP) was obtained from Wilmad Glass Co., Buena, N.J. l-[methyl-13C]methionine and deuterium oxide (D2O) were purchased from Sigma, St. Louis, Mo. All other chemicals were reagent grade and were obtained from commercial sources. P. fellutanum (formerly Penicillium charlesii G. Smith [NRRL 1887]), was a gift from Kenneth Raper, Department of Bacteriology, University of Wisconsin, Madison.
Medium and growth conditions.
Conidiospore suspensions were prepared as described previously (39), stored at 4°C, and used for routine inoculation. For 13C nuclear magnetic resonance (NMR) analysis of intracellular solutes, cultures were grown in 200 ml of low-phosphate standard growth (LPSG) medium containing 2 mM phosphate in 500-ml notched wide-mouth Erlenmeyer flasks at 22°C under constant light and shaking at 40 rpm on a New Brunswick model G-10 shaker. l-[methyl-13C]methionine (50 mg) was added to each culture flask 30 h after inoculation. l-[methyl-13C]methionine was dissolved in double-distilled water (ddH2O) and passed through a sterile 0.22-μm-pore-size membrane filter (Millipore). The composition of LPSG medium is described elsewhere (47).
For salt stress experiments in liquid cultures, stated concentrations of sterile, solid NaCl were added directly to the cultures 60 h after inoculation, unless noted otherwise.
Preparation of extracts of mycelium.
Mycelium was separated from the culture medium by filtration in vacuo on either a C- or M-type sintered glass filter, followed by washing with ddH2O containing the same salt concentration as that of the parent culture to avoid any possible loss of intracellular solutes during washing of harvested mycelium. Throughout this study, 80% ethanol extraction was performed as described previously (39).
13C NMR analysis of intracellular solute pools.
To increase the sensitivity of the methyl amine-containing intracellular solutes to 13C NMR spectroscopy, cultures were enriched with 99 atom% l-[methyl-13C]methionine. 13C from l-[methyl-13C]methionine incorporated in vivo into methyl groups of intracellular choline derivatives was determined by proton-decoupled 13C NMR analysis with an NT-300 spectrometer with a 7-T Oxford magnet operating in the pulsed Fourier transform mode. For quantification of intracellular solutes by 13C NMR spectrometry, cultures were grown without added l-[methyl-13C]methionine. P. fellutanum was grown in 200 ml of LPSG medium that contained 20 mM NaCl. Sterile, solid NaCl (0.5, 1.0, 2.0, or 3.0 M) was added directly to each 200 ml of culture 72 h after inoculation. The mycelium was harvested on day 8. Four milliliters of each extract of mycelium was subjected to 13C NMR analysis with the addition of 0.5 ml of D2O and 0.51% of sodium TSP as an internal reference. Proton-decoupled 13C NMR spectra were recorded at 75 MHz as described previously (39). Data acquisition parameters were as follows: spectral width, 10,000 Hz; pulse width, 28 μs; acquisition time, 409.6 ms; spectrometer frequency, 75.457 MHz; number of acquisitions, 1,000; temperature, 10°C.
Osmotic downshift.
To monitor the fate of intracellular solutes produced in the presence of 3 M NaCl in LPSG medium, the mycelium was initially cultured with l-[methyl-13C]methionine as described above. The mycelium was harvested on day 8 and transferred aseptically to a fresh LPSG medium without added NaCl and l-[methyl-13C]methionine. As a control, mycelium was transferred separately to a fresh medium containing 3 M NaCl. Each mycelium was harvested 3 days after transfer and extracted with 80% ethanol, and the water-soluble portions of extracts were analyzed by 13C NMR spectrometry.
Identification of COS and GB.
Identification of intracellular GB and COS was performed by proton-decoupled 13C NMR spectrometry as described previously (39).
Identification of intracellular polyols.
The identification of polyhydroxy alcohols in the intracellular solute pools was performed by 13C NMR analysis and confirmed by paper chromatography. Ten microliters of each cell extract obtained from the cultures treated with NaCl (final concentration, 0.5, 1.0, 2.0, or 3.0 M) was spotted onto Whatman no. 3 paper. Lanes containing 10 μl of glycerol and erythritol (100 mM each) served as references. The chromatogram was developed in a descending manner with n-butanol–pyridine–ddH2O (6:4:3, vol/vol/vol) for 11 h at room temperature. The chromatogram was then visualized after treatment with an aqueous solution of 2 ml of 40% AgNO3 in 80 ml of acetone. After air drying, the chromatogram was treated with 0.2 M Na2S2O3 and then air dried in a hood.
Salt stress and influence of exogenous GB chloride, COS, or choline chloride.
P. fellutanum was cultured initially in LPSG medium (40 ml per flask). Either GB chloride (final concentration, 2 mM) or COS (final concentration, 10 mM) was added to the cultures 30 min before the addition of NaCl (3 M) at 60 h. GB chloride and COS were dissolved in ddH2O and added to the culture through a sterile 0.22-μm-pore-size membrane filter (Millipore). Growth was determined by measuring the mycelial dry weight (39). At selected intervals, the mycelium was recovered by filtration in vacuo on preweighed Whatman no. 1 paper filters supported by sintered glass and then dried at 100°C for 10 h.
The effect of exogenously added choline was examined by measuring the radial growth of the colonies on the surface of plates of C-source-limiting LPSG medium supplemented with 3 M NaCl. Choline chloride was dissolved in ddH2O and filter sterilized as described above and added (final concentration, 2 mM) to the agar medium before it became solidified. To decrease the accumulation of polyols and stimulate the accumulation of other osmoregulatory compatible solutes such as choline derivatives, only 1/10 of the C source (glucose) was used for these culture plates. The spore suspension was inoculated as a single spot with a diameter of 2.7 cm on the surface of agar plates containing 2 mM choline chloride. The diameter of the circular colonies was measured daily for 29 days.
Measurement of intracellular choline derivatives.
The amounts of intracellular GB and COS were determined by 13C NMR spectrometry with sodium TSP as a reference (39). For this purpose, the cultures were not enriched with l-[methyl-13C]methionine. The intensities of 13C-methyl signals of 13C NMR spectra of the mycelium extracts were integrated by using Felix for Windows 1.02 software (Molecular Simulations Inc.), and the integrated values were compared with that of sodium TSP.
RESULTS
Effect of osmotic upshock and downshift on intracellular choline derivatives in P. fellutanum.
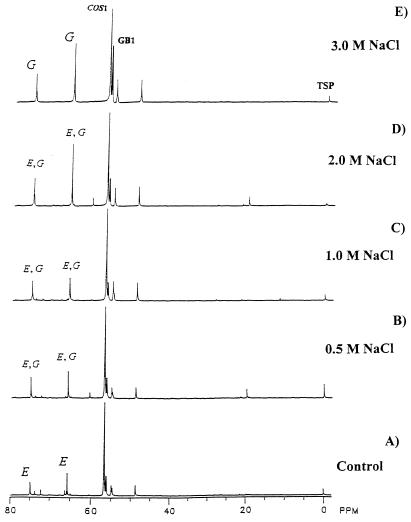
Cultures of P. fellutanum were enriched with l-[methyl-13C]methionine and NaCl at concentrations ranging from 0.5 to 3.0 M as described in Materials and Methods. The control contained no added NaCl. No major differences in the patterns and intensities of 13C NMR signals identified as COS1 and GB1 (39) were observed between cultures derived from mycelium intracellular pools in control medium lacking added NaCl (Fig. 1A) and those containing added 0.5 M (Fig. 1B) or 1.0 M (Fig. 1C) NaCl. [The signal at 56.76 ppm (designated COS1) represents -N+-(13CH3)3 of COS, and that at 56.23 ppm (designated GB1) represents -N+-(13CH3)3 of GB. The two signals upfield at 54.99 and 48.82 ppm were not of concern because they did not change intensity with increasing concentrations of NaCl.] The most prominent intracellular N-methyl solutes in P. fellutanum, grown for 8 days in LPSG medium with 3 M NaCl, were COS and GB (Fig. 1E). These results suggest that the level of GB is especially sensitive to high osmolarity.
FIG. 1.
Influence of NaCl concentration on intracellular solutes in P. fellutanum. Proton-decoupled 13C NMR spectra (proceeding from the bottom to the top) were recorded with the mycelial extracts obtained from cultures grown without added NaCl as control (A) or with NaCl at a concentration of 0.5 (B), 1.0 (C), 2.0 (D), or 3.0 (E) M. For simplicity, only the region from 40 to 80 ppm is shown. Peak symbols are described in the text. Chemical shifts for carbons in COS and GB were previously assigned (39).
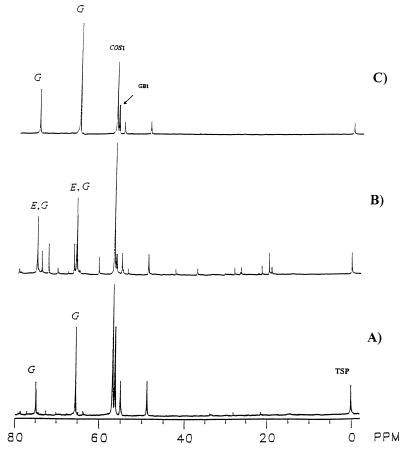
The levels of GB which accumulated under high-osmolarity conditions (Fig. 2A) significantly decreased when the salt stress was removed (Fig. 2B); however, the GB level remained approximately constant when similar 8-day cultures with 3 M NaCl were transferred to a fresh LPSG medium containing 3 M NaCl (Fig. 2C).
FIG. 2.
Influence of osmotic downshift on the levels of intracellular solutes. The mycelium grown for 8 days in LPSG medium containing 3 M NaCl and supplemented with l-[methyl-13C]methionine (A) were harvested and transferred aseptically to either a fresh LPSG medium without added NaCl (B) or with 3 M NaCl as a control (C). The transferred mycelia were harvested 3 days after transfer, and the extracts were analyzed with 13C NMR spectroscopy; 0.75% sodium TSP was used as a reference (chemical shift, 0.00). Peak symbols are described in the text, as are the acquisition parameters.
The GB quantity increased 22-fold (33 mg/g [dry weight] of mycelium) in LPSG medium supplemented to contain 3 M NaCl compared with that of unsupplemented controls (1.5 mg/g [dry weight] of mycelium) (Table 1). The data in Table 1 show that osmotic stress with 3 M NaCl in low-phosphate culture medium further increases the concentration of the solute GB in mycelium.
TABLE 1.
Influence of 3 M NaCl on accumulation of GB and COS in P. fellutanum
| LPSG medium supplement | Solute (mg/g [dry wt])a
|
Mycelium growth (g/200 ml)b | |
|---|---|---|---|
| COS | GB | ||
| None | 9.3 (17)c | 1.5 (3.0) | 3.5 ± 0.35 |
| NaCld | 25.0 (46) | 33.1 (70) | 1.8 ± 0.01 |
The quantity of each solute is provided as milligrams of COS or GB per gram (dry weight) of mycelium. The quantity of each solute was determined by integrating methyl carbon signals on 13C NMR spectra by using Felix for Windows 1.02 software (Molecular Simulations Inc.). Sodium TSP (0.51%) was used as a reference, and the data were acquired without nuclear overhauser effect enhancement.
Cultures were grown for 8 days in 200 ml each of LPSG medium in three separate flasks with 3 M NaCl and without added NaCl as a control. Growth was determined by measuring mycelium dry weight and by determining the means of the results of triplicate experiments ± standard deviations in grams (dry weight) per 200 ml of culture medium.
Values in parentheses represent the calculated millimolar concentration of the indicated solute. An approximate cell composition of 20% dry weight–80% water was assumed.
Sterile, solid NaCl (35.06 g) was added to 200 ml of culture.
The level of intracellular COS increased from 9.3 mg/g (dry weight) in unsupplemented LSPG medium to 25.0 mg/g (dry weight) in supplemented medium, which represents a 2.7-fold increase (Table 1). These values translate into combined concentrations of COS and GB in unsupplemented and supplemented mycelium of 80 and 464 μmol/g (dry weight), respectively.
Glycerol and erythritol as major osmoresponsive polyols in P. fellutanum.
The two signals (designated G and E in Fig. 1) are those of primary (chemical shift, 65.47 ppm) and secondary (74.95 ppm) alcohol carbons of glycerol and erythritol. Glycerol and erythritol were identified by 13C NMR spectroscopy and paper chromatography; the chemical shifts of these two signals exactly matched those of C-1 and C-3 (CH2OH) and C-2 (CHOH) of commercial glycerol and C-1 and C-4 (CH2OH) and C-2 and C-3 (CHOH) of commercial erythritol.
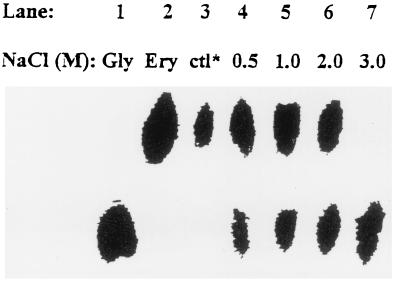
The mycelium grown without added NaCl, the control, contained mainly erythritol and a negligible amount of glycerol as observed from the ratio of the intensity of the signal at 65.47 to that of the signal at 74.95 ppm (Fig. 1A); paper chromatography (Fig. 3, lane 3) also showed erythritol as the major polyol in the mycelium grown without added NaCl. As the concentration of NaCl was increased to 3 M (Fig. 1A to E), the ratio of signal intensities at 65.47 and 74.95 ppm significantly increased, indicating that the level of glycerol gradually increased. The paper chromatogram (Fig. 3) showed that in mycelium grown in LPSG medium without added NaCl (Fig. 3, lane 3), i.e., the control, or supplemented with 0.5, 1.0, or 2.0 M NaCl (lanes 4 to 6, respectively), the level of erythritol increased with increasing concentrations of NaCl (up to 2 M) in the culture medium. The level of glycerol also increased with increasing concentrations of NaCl (lanes 3 to 7), although the mycelium grown with 0.5, 1, or 2 M NaCl (lanes 4 to 6, respectively) contained slightly more erythritol than glycerol. In contrast, in the mycelium from cultures grown with 3 M NaCl, glycerol was the major polyol detected (Fig. 1E); there was no significant spot equivalent to erythritol on the chromatogram (Fig. 3, lane 7). Both glycerol and erythritol were major polyols in mycelium cultured with 2 M NaCl (Fig. 1D and Fig. 3, lane 6).
FIG. 3.
Paper chromatographic separation and identification of glycerol and erythritol. The extracts were obtained from the mycelium grown in LPSG medium without added NaCl as a control (lane 3) or containing a final molar concentration of added NaCl of 0.5 (lane 4), 1.0 (lane 5), 2.0 (lane 6), or 3.0 (lane 7). Ten microliters of an appropriate cell extract was applied to lanes 3 to 7 on Whatman no. 3 paper. Ten microliters of 20 mM glycerol (lane 1) and 20 mM erythritol (lane 2) were used as reference solutes.
The level of glycerol previously accumulated in the mycelium grown with 3 M NaCl (Fig. 2A) significantly decreased, with a concomitant increase in erythritol level upon removal of salt stress (Fig. 2B). This suggests an osmoresponsive role of glycerol. In contrast, as shown in Fig. 2C, in the control mycelium, which was transferred to fresh medium also containing 3 M NaCl, the level of glycerol further increased, while levels of COS and GB did not change much compared to those in the parent mycelium (before transfer) (Fig. 2A).
Osmoprotection of mycelium by exogenous GB, COS, or choline.
To provide evidence of the ability of exogenous GB chloride or COS to protect the mycelium against osmotic stress, P. fellutanum was cultured in LPSG medium containing 3 M NaCl and with, or without, added GB chloride (2 mM) or COS (10 mM) (Table 2). Addition of 3 M NaCl to the cultures 60 h after inoculation caused a 33% decrease in mycelium dry weight within 12 h (on day 3); the dry weights of the cultures supplemented with either GB chloride (2 mM) or COS (10 mM) were unchanged or increased 25%, respectively. The dry weights of the mycelium in cultures with either GB or COS added were greater than those of control cultures at days 4, 6, and 8. After the initial stage of repressed growth, during the initial stage of salt stress, a parallel increase in dry weight occurred.
TABLE 2.
Effect of exogenous GB or COS on growtha of P. fellutanum stressed with 3 M NaCl
| Day | Mycelium growth (mg [dry wt])
|
||
|---|---|---|---|
| Control | GBb | COSb | |
| 1 | 9 | ||
| 2 | 30 | ||
| 2.5d | 60 | ||
| 3 | 40 | 60 (50)c | 75 (88) |
| 4 | 70 | 90 (29) | —e |
| 6 | 110 | 110 (27) | 120 (9) |
| 8 | 140 | 200 (43) | 180 (29) |
P. fellutanum was grown with or without added GB (2 mM) or COS (10 mM) in LPSG medium supplemented with 3 M NaCl. The growth was assessed by measuring the mycelium dry weight and determining the mean values of the results for duplicate samples. Dry weight was determined as described previously (39). The difference in dry weight between duplicate samples was less than 10%.
GB and COS were dissolved in ddH2O separately and filtered through a 0.22-μm-pore-size membrane.
Values in parentheses represent percentage increases as related to milligrams (dry weight) of mycelium in cultures with added GB or COS compared with that of control set at 100% on same day.
Time of addition of sterile solid NaCl; the final concentration of NaCl was 3 M. GB and COS were added 30 min before the addition of NaCl.
—, not measured.
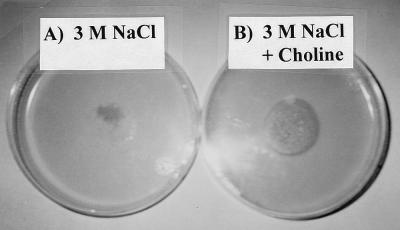
Enhanced osmotolerance by exogenously added choline chloride (2 mM) was also observed in the mycelium grown on low-glucose LPSG agar plates containing 3 M NaCl (Fig. 4). In this experiment, the LPSG agar medium was modified to contain 10-fold-less C source (glucose) than that of the standard LPSG medium; a decrease in the synthesis of glycerol and erythritol was observed by using 13C NMR spectroscopy (data not shown). The control plate without added choline showed only a faint smear of cells about 0.7 cm in diameter (Fig. 4A); spores, or the mycelium after germination, appeared lysed during 29 days of incubation under low-phosphate, C-source-limiting, and 3 M NaCl-stressed conditions. Although there was no hyphal growth, the colony on the plate containing 2 mM choline chloride had a diameter of about the same size (2.7 cm) as that of the inoculum and a dense and slimy appearance (Fig. 4B). The spores on the plates containing 2 mM choline germinated and survived the harsh environmental conditions better than those spores inoculated onto the control plates, which were not supplemented with choline chloride. These experiments demonstrated that exogenous GB, COS, and choline enhanced the tolerance of P. fellutanum toward the osmotic potential of 3 M NaCl.
FIG. 4.
Osmoprotection of P. fellutanum by exogenous choline. A spore suspension of P. fellutanum was inoculated as a single circular spot with a diameter of 2.7 cm on the center of the surface of C-source-limiting LPSG agar plates containing 3 M NaCl and without (A) or with (B) 2 mM choline chloride. The set of plates shown represents one of three replicate sets. The picture was taken on day 29 following inoculation.
DISCUSSION
Osmoregulatory compatible solutes have been identified and quantified by 13C NMR spectroscopy in bacteria (10, 32, 34), marine mollusc (36, 38), and fungi, including yeasts (6, 7, 10, 37, 44, 45). Polyhydroxy alcohols, such as glycerol, arabinitol, and erythritol, are the most predominant compatible solutes in fungi (7). The presence of GB and COS as osmoprotectants has not been reported for genera of Penicillium or Aspergillus.
It has been shown that P. fellutanum mycelium cultured in a medium containing 20 mM phosphate contained no detectable GB and 1.5-fold less COS than mycelium cultured in medium containing 2 mM phosphate (39). In contrast, modification of the high-phosphate medium with 3 M NaCl resulted in a large increase in glycerol in the mycelium but no significant increase in levels of COS and GB (data not shown).
The results of the current investigation suggest that intracellular GB and COS, as well as glycerol and erythritol, are important osmoregulators in P. fellutanum grown under low-phosphate and high-osmolarity conditions. GB, COS, and erythritol were found in the mycelium grown in low-phosphate medium with no NaCl supplementation. As the concentration of NaCl in the LPSG medium was increased from 2 to 3 M, GB and COS combined concentrations increased from approximately 20 mM to greater than 100 mM, assuming that 80% of the cell mass was water (Table 1). These data suggest that GB and COS are compatible solutes in P. fellutanum.
Nonspecific phosphocholine:phosphocholine hydrolase in LPSG cultures of P. fellutanum catalyzes the release of phosphocholine from its diester attachment as a component of extracellular pPxGM (39, 47). Thus, the choline moiety from released phosphocholine is the precursor of GB and COS that accumulates inside the mycelium as GB and COS (39). Utilization of GB and COS as osmoprotectants may thus be confined to those mycelia cultured in low-phosphate and high-osmolarity media and may be dependent on the presence of significant activity of extracellular pPxGM phosphocholine:phosphocholine hydrolase, which provides excess choline for its immediate needs compared with that for phosphate. This suggests a relationship between phosphate concentration and osmotic stress regulons in P. fellutanum. In recent reports, interesting connections are beginning to be revealed between the phosphate and osmotic stress regulons in bacteria (27). Whether the extracellular phosphocholine-containing polysaccharide pPxGM is actively involved in osmoprotection of this fungus and this process is governed by both phosphate and osmotic stress regulons remain open.
Furthermore, the data also suggest that the relative abundance of the C source (glucose) in fresh medium also influences which osmoprotectants are formed. For instance, the use of fresh LPSG medium containing 3 M NaCl, which contains 277 mM glucose, provided carbon for synthesis of additional glycerol (Fig. 2C). Thus, an additional quantity of glycerol precursor eliminated the need for GB and/or COS under continuing 3 M NaCl stress, and no further accumulation of GB and COS occurred.
The increase in the level of GB was about 10-fold greater than that of COS upon addition of 3 M NaCl to the LPSG culture medium (Table 1). This suggests that P. fellutanum preferentially uses GB rather than COS for protection against high osmotic stress. COS is known as a storage form of sulfate in Penicillium and Aspergillus spp. (33); this may account for the high level (17 mM) in cultures in LPSG medium lacking added NaCl. Although the total GB concentration in mycelium grown in LPSG medium that contained 3 M NaCl was nearly twice that of COS, accumulation of COS undoubtedly depends on the presence of adequate sulfate in the culture medium (unpublished data).
It is generally observed that Penicillium species survive in environments containing high concentrations of carbohydrates, protein degradation products, and salts. The data presented here show that P. fellutanum survives and grows at a reduced rate in an environment containing 3 M NaCl. Day-8 cultures have a limiting concentration of glucose; this limits the quantity of glycerol that it can generate for its osmoprotection. Products of proteolytic digestion will provide, in P. fellutanum, carbon skeletons for biosynthesis of choline, a precursor of GB and COS, in a natural environment with a limiting concentration of carbohydrate; however, the possibility that GB and COS are derived mostly from phosphocholine diester of pPxGM synthesized by N-methyl transfer to phosphoethanolamine phosphodiester of pPGM has not been eliminated. It has been shown that methyl groups derived from l-[methyl-13C]methionine (39) or from [C2-13C]glycine become incorporated into both COS and GB. Amino acids, betaines, and choline derivatives are known major osmoprotectants in bacteria (9, 13, 15, 18, 27, 29, 35), algae (8), plants (46), and animals (51). COS is an osmoregulator in marine algae, a few bacteria, some marine fungi, and halophytic plants (8, 20, 46). In Penicillium and Aspergillus species, choline and sulfate are stored as COS (33); however, the role of COS as an osmoprotectant in these species has not been reported.
ACKNOWLEDGMENTS
This work was supported by the Florida Agricultural Experiment Station and by a Research Project Enhancement Award from the Office of the Dean for Research, Institute of Food and Agricultural Sciences, University of Florida, Gainesville.
We thank Marian L. Buszko for assistance with NMR techniques and K. T. Shanmugam and R. R. Schmidt for helpful suggestions.
Footnotes
Journal Series No. RO5664 of the Florida Agricultural Experiment Station.
REFERENCES
- 1.Adler L, Gustafsson L. Polyhydric alcohol production and intracellular amino acid pool in relation to halotolerance of the yeast Debaryomyces hansenii. Arch Microbiol. 1980;124:123–130. [Google Scholar]
- 2.Adler L, Pedersen A, Tunblad-Johansson I. Polyol accumulation by two filamentous fungi grown at different concentrations of NaCl. Physiol Plant. 1982;56:139–142. [Google Scholar]
- 3.Andre L, Nilsson A, Adler L. The role of glycerol in osmotolerance of the yeast Debaryomyces hansenii. J Gen Microbiol. 1988;134:669–677. [Google Scholar]
- 4.Arakawa T, Timasheff S N. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beever R E, Laracy E P. Osmotic adjustment in the filamentous fungus Aspergillus nidulans. J Bacteriol. 1986;168:1358–1365. doi: 10.1128/jb.168.3.1358-1365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellinger Y, Larher F. A 13C comparative nuclear magnetic resonance study of organic solute production and excretion by the yeasts Hansenula anomala and Saccharomyces cerevisiae in saline media. Can J Microbiol. 1988;34:605–612. doi: 10.1139/m88-100. [DOI] [PubMed] [Google Scholar]
- 7.Bloomberg A, Adler A. Physiology of osmotolerance in fungi. Adv Microb Physiol. 1992;33:144–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- 8.Blunden G, Gordon S M. Betaines and their sulphonio analogues in marine algae. Prog Physiol Res. 1986;4:39–80. [Google Scholar]
- 9.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowitzka L J, Demmerle S, Mackay M A, Norton R S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980;210:650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- 11.Brown A D. Compatible solutes and extreme water stress in eukaryotic micro-organisms. Adv Microb Physiol. 1978;17:181–241. doi: 10.1016/s0065-2911(08)60058-2. [DOI] [PubMed] [Google Scholar]
- 12.Brown A D, Simpson J R. Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol. 1972;72:589–591. doi: 10.1099/00221287-72-3-589. [DOI] [PubMed] [Google Scholar]
- 13.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Costa M S, Duarte J C, Williams R A D, editors. Microbiology of extreme environments and its potential for biotechnology. Essex, England: Elsevier Science Publishers Ltd.; 1989. [Google Scholar]
- 15.Gadd G M, Chudek J A, Foster R, Reed R H. The osmotic response of Penicillium ochro-chloron: changes in internal solute levels in response to copper and salt stress. J Gen Microbiol. 1984;130:1969–1975. [Google Scholar]
- 16.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 17.Glaasker E, Konigs W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham J E, Wilkinson B J. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J Bacteriol. 1992;174:2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson L, Norkrans B. On the mechanisms of salt tolerance. Production of glycerol and heat during the growth of Debaryomyces hansenii. Arch Microbiol. 1976;110:177–183. doi: 10.1007/BF00690226. [DOI] [PubMed] [Google Scholar]
- 20.Hanson A D, Gage D A. Identification and determination by fast atom bombardment mass spectrometry of the compatible solute choline-O-sulfate in Limonium species and other halophytes. Aust J Plant Physiol. 1991;18:317–327. [Google Scholar]
- 21.Harris R F. Effect of water potential on microbial growth and activity. In: Parr J F, Gardner W R, Elliot L F, editors. Water potential relations in soil microbiology. Vol. 9. Madison, Wis: Soil Science Society of America; 1981. pp. 23–95. [Google Scholar]
- 22.Hocking A D. Effect of water activity and culture age on the glycerol accumulation of five fungi. J Gen Microbiol. 1986;132:269–275. [Google Scholar]
- 23.Hocking A D, Norton R S. Natural-abundance 13C nuclear magnetic resonance studies on the internal solutes of xerophilic fungi. J Gen Microbiol. 1983;129:2915–2925. [Google Scholar]
- 24.Hollaender A, Aller J C, Epstein E, San Pietro A, Zaborsky O R, editors. The biosaline concept: an approach to the utilization of under-exploited resources. New York, N.Y: Plenum Press; 1979. [Google Scholar]
- 25.Ingraham J. Effect of temperature, pH, water activity, and pressure on growth. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1543–1554. [Google Scholar]
- 26.Jennings D H. Polyol metabolism in fungi. Adv Microb Physiol. 1984;25:149–193. doi: 10.1016/s0065-2911(08)60292-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaenjak A, Graham J E, Wilkinson B J. Choline transport activity in Staphylococcus aureus induced by osmotic stress and low phosphate concentrations. J Bacteriol. 1993;175:2400–2406. doi: 10.1128/jb.175.8.2400-2406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landfald B, Strom A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Rudulier D, Strom A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 31.Luard E J. Accumulation of intracellular solutes by two filamentous fungi in response to growth at low steady state osmotic potential. J Gen Microbiol. 1982;128:2563–2574. [Google Scholar]
- 32.Mackay M A, Norton R S, Borowitzka L J. Marine blue-green algae have a unique osmoregulatory system. Mar Biol. 1983;73:301–307. [Google Scholar]
- 33.Markham P, Robson G D, Bainbridge B W, Trinci A P J. Choline: its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol Rev. 1993;104:287–300. doi: 10.1111/j.1574-6968.1993.tb05872.x. [DOI] [PubMed] [Google Scholar]
- 34.Martins L O, Carreto L S, Da Costa M S, Santos H. New compatible solutes related to di-myo-inositol-phosphate in members of the order Thermotogales. J Bacteriol. 1996;178:5644–5651. doi: 10.1128/jb.178.19.5644-5651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Measures J C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature (London) 1975;257:398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- 36.Norton R S. Identification of mollusc metabolites by natural abundance 13C-NMR studies of whole tissue and tissue homogenates. Comp Biochem Physiol B. 1979;63:67–72. [Google Scholar]
- 37.Norton R S. 13C-NMR studies of intact cells and tissue. Bull Magnetic Resonance. 1980;3:29–48. [Google Scholar]
- 38.Norton R S, de Rome P. 13C-NMR study of osmoregulatory metabolites in the marine mollusc Tapes watlingi. Experientia. 1980;36:522–532. [Google Scholar]
- 39.Park Y-I, Buszko M L, Gander J E. Utilization of phosphocholine from extracellular complex polysaccharide as a source of cytoplasmic choline derivatives in Penicillium fellutanum. J Bacteriol. 1997;179:1186–1192. doi: 10.1128/jb.179.4.1186-1192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitt J I. Xerophilic fungi and the spoilage of foods of plant origin. In: Duckworth R B, editor. Water relations of foods. London, United Kingdom: Academic Press Ltd.; 1975. pp. 273–307. [Google Scholar]
- 41.Pollard R, Wyn Jones R G. Enzyme activities in concentrated solutions of glycine betaine and other solutes. Planta. 1979;144:291–298. doi: 10.1007/BF00388772. [DOI] [PubMed] [Google Scholar]
- 42.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rains D W, Valentine R C, Hollanender A, editors. Genetic engineering of osmoregulation: impact on plant productivity for food, chemicals and energy. New York, N.Y: Plenum Press; 1980. [PubMed] [Google Scholar]
- 44.Reed R H, Chudek J A, Roster R, Gadd G M. Osmotic significance of glycerol accumulation in exponentially growing yeasts. Appl Environ Microbiol. 1987;53:2119–2123. doi: 10.1128/aem.53.9.2119-2123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed R H, Chudek J A, Foster R, Stewart W D P. Osmotic adjustment in cyanobacteria from hypersaline environments. Arch Microbiol. 1984;138:333–337. [Google Scholar]
- 46.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 47.Salt S D, Gander J E. Variations in phosphoryl substituents in extracellular peptidophosphogalactomannans from Penicillium charlesii G. Smith Exp Mycol. 1985;9:9–19. [Google Scholar]
- 48.Smith L T. Role of osmolytes in adaptation of osmotically stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl Environ Microbiol. 1996;62:3088–3093. doi: 10.1128/aem.62.9.3088-3093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyn Jones R G. Phytochemical aspects of osmotic adaptation. Recent Adv Phytochem. 1984;18:55–78. [Google Scholar]
- 50.Yancey P, Clark M, Hand S, Bowlus R, Somero G. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 51.Zablocki K, Miller S P F, Garcia-Perez A, Burg M B. Accumulation of glycerophosphocholine (GPC) by renal cells: osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci USA. 1991;88:7820–7824. doi: 10.1073/pnas.88.17.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]