Abstract
Two Acanthamoeba species, fed at three temperatures, expelled vesicles containing living Legionella pneumophila cells. Vesicles ranged from 2.1 to 6.4 μm in diameter and theoretically could contain several hundred bacteria. Viable L. pneumophila cells were observed within vesicles which had been exposed to two cooling tower biocides for 24 h. Clusters of bacteria in vesicles were not dispersed by freeze-thawing and sonication. Such vesicles may be agents for the transmission of legionellosis associated with cooling towers, and the risk may be underestimated by plate count methods.
In 1980, Rowbotham (10) demonstrated that Legionella spp. could reproduce within environmental isolates of amoebae; since then, there have been numerous laboratory studies of interactions between amoebae and Legionella spp., mainly in coculture experiments. Most studies concur that amoebae must play a major role in the epidemiology of legionellosis through their ability to amplify numbers of legionellae. Rowbotham (10, 11) has previously described the cycle of amplification of bacteria in amoeba cultures. At some stage after the ingestion of bacteria, amoebae produce vesicles that contain high numbers of legionellae. At 35°C, amoebae usually lyse, releasing numerous free bacteria or a few vesicles. Rowbotham (11) hypothesized that the inhalation of vesicles of tightly packed legionellae is dangerous when the particles are of respirable size (1 to 5 μm in diameter) and contain motile bacteria. He stated that such a particle, protected by a membrane, may reach the alveoli of the lungs and contain an infectious dose of legionellae.
The present work reports the production of vesicles from two species of amoebae fed a cooling tower isolate of Legionella pneumophila at temperatures found in cooling towers. Some temperatures allowed amoebae to remain viable and thereby able to serve as a continuous source of such vesicles filled with legionellae. Vesicles were also exposed to cooling tower biocides to determine whether bacteria could remain viable within vesicles after such treatment.
MATERIALS AND METHODS
Organisms and growth conditions.
Acanthamoeba polyphaga (ATCC 30461) and A. castellanii (ATCC 30010) were obtained from the American Type Culture Collection and were maintained axenically in plate count broth (PCB) (Difco, Detroit, Mich.) at 28°C. L. pneumophila (ATCC 33216) was obtained from the American Type Culture Collection and was originally isolated from a cooling tower. It was maintained on Legionella selective agar (Becton-Dickinson, Cockeysville, Md.) in a 5% CO2 atmosphere at 35°C. For feeding studies, bacteria (48-h old) were washed from agar plates with a Tris-buffered salts solution (TBSS) (2 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 1 mM Tris, [pH 6.8 to 7.2]). Escherichia coli (ATCC 25922) was used for a control as described below.
Feeding experiments.
Axenic amoeba trophozoites were washed by replacing the medium from their tissue culture flasks with TBSS. They were fed either live or heat-treated L. pneumophila cells in 4 ml of TBSS or in PCB. Heat-treated bacteria were exposed for 45 min at 85 to 90°C and then plated on buffered charcoal-yeast extract (BCYE) agar to determine whether they were killed. Previous treatment at 70°C was not effective in killing all bacteria. A multiplicity of infection (MOI) of 10,000 produced many vesicles; however, vesicles were also produced at MOIs of approximately 30 to 300. The numbers of vesicles per amoeba were determined for A. polyphaga at MOIs of 100, 400, 700, 1,000, and 10,000; since A. castellanii produced fewer vesicles per amoeba, MOIs of 1,000 and 10,000 were tested.
Amoebae were allowed to feed on L. pneumophila cells for 24 h in PCB or TBSS before the medium was examined for the presence of vesicles expelled from amoebae. Vesicle production was tested at 25, 30, and 35°C. Experiments in which the numbers of vesicles per amoeba were calculated were conducted only at 25 and 30°C, since amoebae lysed at 35°C when they were fed live L. pneumophila cells. Cellular debris from lysed amoebae (at 35°C) made the enumeration of small vesicles difficult, and the actual numbers of amoebae involved in vesicle production could not be determined. Samples of vesicles were examined by differential interference contrast microscopy for measurements of vesicle diameters and to confirm the presence of bacteria within vesicles. One hundred randomly selected vesicles from each species were measured. Other vesicle samples from amoebae fed live and killed L. pneumophila cells were treated with the respiratory indicator 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl tetrazolium chloride (INT) (Eastman Kodak, Rochester, N.Y.) at a final concentration of 0.05% to test the viability of bacteria within free vesicles. During active respiration, INT becomes reduced to an insoluble INT-formazan precipitate. The treatment of heat-killed bacteria served as a control for the performance of the INT technique, i.e., to show that formazan crystals would not form in the vesicles of amoebae that were fed dead bacteria. In addition, since free bacteria outside of vesicles had INT-formazan products after exposure of the suspension to biocides (see below), L. pneumophila suspensions were exposed in a separate test to up to 80 ppm of MBC 115 (MBC represents microbiocide) and to up to 200 ppm of MBC 215 for 5 h, after which they were plated on BCYE agar to show they were still viable and that the positive INT reaction was a valid indicator of viability.
Biocide treatment.
To test whether bacteria in free vesicles could survive exposure to cooling tower biocides, washed amoeba suspensions containing free vesicles were exposed separately to two biocides in wells of 96-well microplates after amoebae had fed on live Legionella cells for 4 h. The two cooling tower biocides were an isothiazolone derivative, MBC 215 (a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin [Nash-Chem, Nashville, Tenn.]), and a quaternary ammonium compound, MBC 115 {poly[oxyethylene (dimethyliminio)ethylene (dimethyliminio)ethylene dichloride] (Nash-Chem)}. Both biocides are registered with the U.S. Environmental Protection Agency under their MBC numbers. They were prepared by dilution with TBSS. Vesicles in TBSS were exposed to biocides for 4 h (a period of exposure in which biocide concentrations may remain effective in an actual cooling tower) and 24 h, after which they were treated with INT. Sterile PCB was added with INT as the substrate for respiration. Final concentrations of 15 ppm (vol/vol) of quaternary ammonium compound and 100 ppm of isothiazolone were used for exposure. These concentrations were selected on the basis of previous studies of amoeba tolerances to cooling tower biocides (15) and because they fell within the manufacturer’s recommended dosage for cooling tower maintenance. Samples of vesicles were examined at high magnifications with a Nikon Microphot microscope with differential interference contrast to confirm that vesicles contained bacteria.
Freeze-thawing.
To demonstrate that the presence of vesicles with high numbers of legionellae may result in underestimates of the total numbers of legionellae, based on CFU, vesicles were subjected to three cycles of freeze-thawing (−70 and +35°C) and sonication in an ultrasonic water bath to disperse bacteria. After such treatment, vesicles were examined microscopically to determine whether bacteria were released from vesicles.
Bacterial mixtures.
To determine whether amoebae select against L. pneumophila when they are presented with a choice of E. coli or L. pneumophila, a mixture of equal numbers of both bacteria were fed simultaneously to amoebae. After 24 h, free vesicles were enumerated and fluorescent antibodies to L. pneumophila were used to detect L. pneumophila cells within such vesicles. The numbers of vesicles produced by amoebae which fed on the mixture at 25 and 30°C were compared with the numbers produced when amoebae fed on each bacterial species separately. An MOI of 10,000 was used in these experiments. Vesicle diameters were determined from A. polyphaga and A. castellanii organisms fed E. coli cells alone and the mixture of L. pneumophila and E. coli cells.
Electron microscopy.
Vesicles from L. pneumophila-fed amoebae were fixed for electron microscopic examination in a solution of 3% glutaraldehyde and 0.5% (wt/vol) OsO4 in 0.05 M PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid] buffer at pH 7.1. The fixative was combined in a ratio of 2 parts fixative to 1 part vesicles suspended in TBSS. Fixation proceeded for 2 h at 4°C. After fixation, specimens were dehydrated in an ethanol series and embedded in Epon-araldite resin. Propylene oxide was used as a transition solvent. Microscopy was performed with a Zeiss 109 electron microscope at 50 kV.
Antibody treatment of vesicles.
As an additional treatment for the possible application of flow cytometry to detect such Legionella-packed vesicles, fluorescent antibodies to L. pneumophila (Genetic Systems Corp., Redmond, Wash.) were added to samples of vesicles to determine whether vesicles fluoresced. Controls consisted of vesicles from E. coli-fed amoebae treated in the same manner.
Data analysis.
Statistical analysis software was used to run an analysis-of-variance test, followed by Tukey’s multiple comparisons to determine significant differences between treatments in MOI and bacterial-mixture studies. Significant differences are reported at the P < 0.05 level.
RESULTS
Feeding experiments.
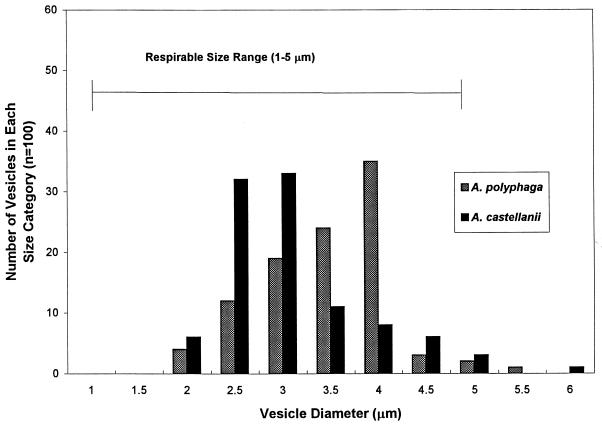
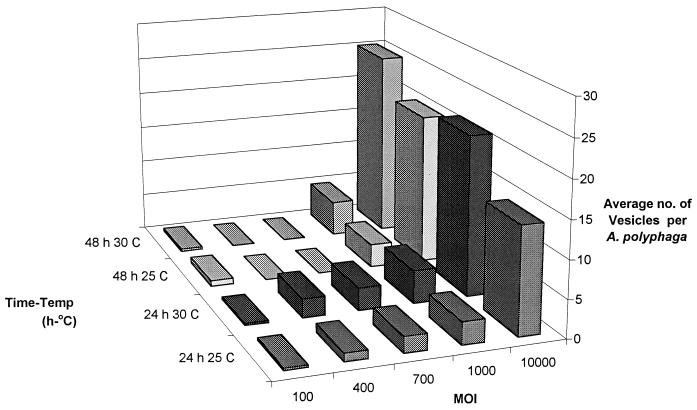
The results show that A. polyphaga and A. castellanii amoebae fed L. pneumophila cells produced vesicles ranging from 2.1 to 6.4 μm in diameter, with an average (±1 standard error) of 3.3 ± 0.08 to 3.6 ± 0.06, depending on the species (Fig. 1). Over 90% of vesicles from both species fell within the size range considered to be respirable. A large number of vesicles were released from amoebae after amoebae were fed live L. pneumophila cells (Fig. 2A), and these contained live L. pneumophila cells, based on INT reduction (Fig. 2C and D). Greater than 90% of vesicles had several cells with red formazan crystals inside, whereas vesicles from amoebae which fed on heat-treated bacteria rarely had any crystals inside vesicles (they were probably from bacteria that had not been killed by heat treatment). Legionellae exposed to biocides and plated onto BCYE agar formed colonies, indicating that a positive INT reaction after biocide exposure was a valid indicator of viability. No INT-formazan products were observed outside of cells, indicating that abiotic reduction of INT did not occur to create artifacts. Amoebae which fed on heat-treated L. pneumophila cells produced vesicles a day later than did those which fed on living bacteria. Amoebae produced vesicles with live L. pneumophila cells within 24 h at all three temperatures tested in PCB and TBSS. At 35°C in PCB and TBSS, amoebae did not appear to be healthy and many lysed, as reported by other investigators (1, 4, 6). Amoebae fed at 25 and 30°C produced normal viable cysts that were capable of excysting. The percentage of amoebae infected, i.e., having bacteria within a food vacuole, ranged from 73 to 98% for A. polyphaga, depending on the MOI and temperature, and from 96 to 100% for A. castellanii. The number of amoebae infected may have been slightly underestimated, since it was occasionally difficult to ascertain that there were no bacteria in any vesicle within an amoeba. A. polyphaga produced up to 25 vesicles per amoeba in 24 h under certain conditions (Fig. 3); however, the number of vesicles per amoeba was lower for A. castellanii, ranging from 0.08 to 0.11, depending on the temperature and MOI. In general, a higher MOI resulted in a higher number of vesicles produced.
FIG. 1.
Frequency distribution of vesicle sizes from A. polyphaga and A. castellanii. One hundred vesicles from each species were measured. Over 90% of vesicles from each species fell within the size range (1 to 5 μm) considered to be respirable.
FIG. 2.
Vesicles from amoebae. (A) Low magnification of numerous small vesicles among amoebae (three larger objects) photographed by differential interference contrast microscopy. (B) Higher magnification of three vesicles containing rods, observed by differential interference contrast microscopy. These vesicles were exposed for 3.5 h to MBC 215. (C) Vesicle containing formazan crystals. It was produced by A. polyphaga at 35°C. The light was altered to enhance the visualization of formazan. (D) Unusually large vesicle containing bacteria with formazan crystals. This vesicle was exposed for 4 h at 25°C to MBC 115 and then for 6 h to INT. Bar, 50 (A) and 20 (B through D) μm.
FIG. 3.
Numbers of vesicles produced per amoeba at various MOIs with respect to time and temperature. Data are averages from duplicate flasks. Error bars were omitted for visual clarity.
Biocide treatment.
The appearance of vesicles exposed to biocides was the same as that of controls, and these vesicles maintained their form with clusters of bacteria (Fig. 2B). The respiration of bacteria within vesicles was intense at 35°C, based on red-formazan precipitates associated with bacteria (Fig. 2C). After exposure to biocides, bacteria in vesicles contained formazan products (Fig. 2D).
Freeze-thawing.
The structural integrity of vesicles, based on differential interference microscopy, was apparent even after 3 months in culture flasks. The freeze-thawing treatment did not disperse bacteria clustered in vesicles, whereas amoeba trophozoites were completely destroyed. A count of intact vesicles before and after three cycles of freeze-thawing showed no significant change in the numbers of intact vesicles.
Electron microscopy.
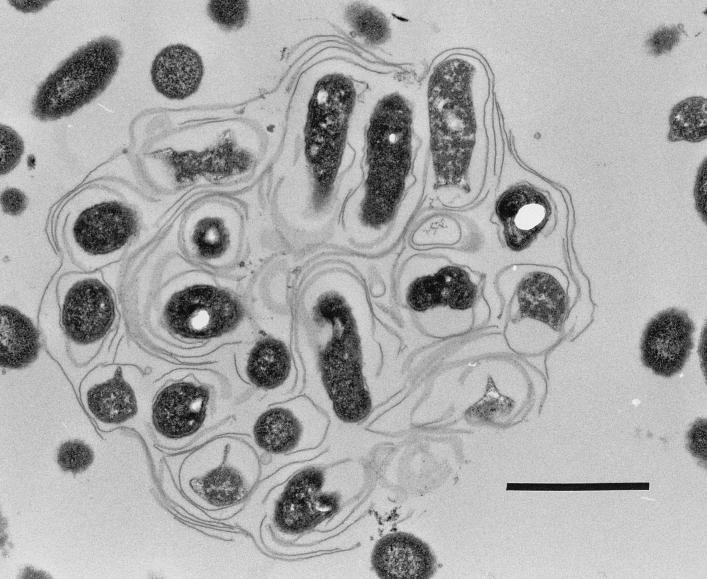
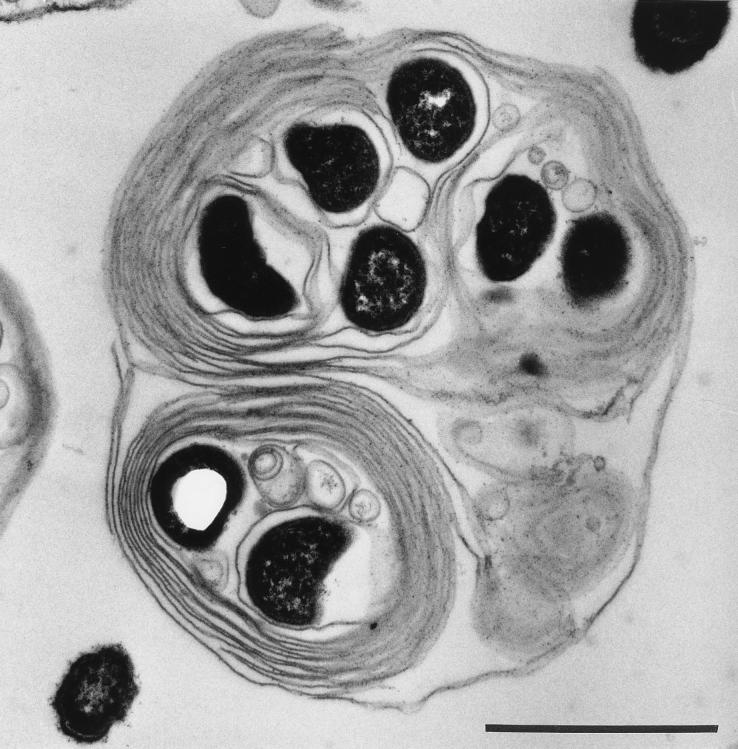
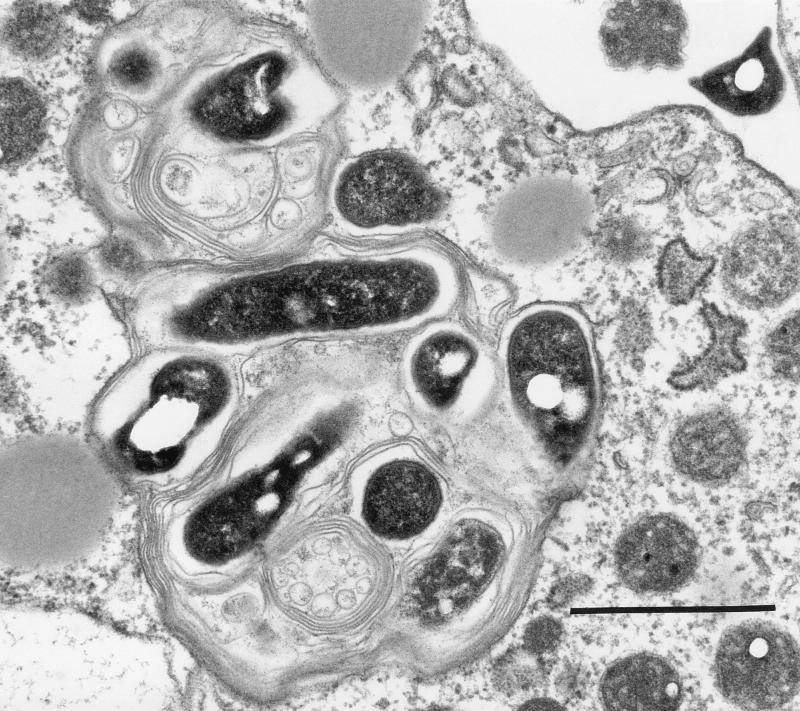
Electron microscopy revealed that bacteria in vesicles appeared to be wrapped in membranes with myelin forms (Fig. 4 and 5). These structures were present even within amoebae (Fig. 6).
FIG. 4.
Transmission electron micrograph of a vesicle expelled from A. castellanii. Bar = 1 μm.
FIG. 5.
Transmission electron micrograph of a vesicle expelled from A. castellanii and exposed to MBC 215 for 5 h. Bar = 1 μm.
FIG. 6.
Transmission electron micrograph of a vesicle within A. castellanii. Bar = 1 μm.
Bacterial mixtures.
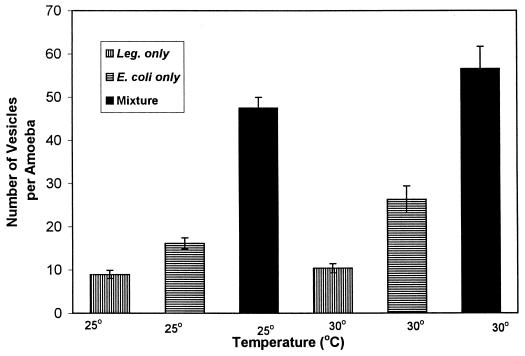
The number of vesicles produced when amoebae fed on a mixture of L. pneumophila and E. coli was significantly higher than when they fed on either one alone (Fig. 7). Fluorescent antibody treatment of vesicles from amoebae fed the bacterial mixture indicated that L. pneumophila cells were present in vesicles. Vesicles from E. coli-fed A. polyphaga amoebae averaged (±1 standard error) 2.9 ± 0.13 μm in diameter, and those from E. coli-fed A. castellanii amoebae averaged 2.14 ± 0.15 μm (Fig. 8). Vesicles from amoebae fed the mixture of bacteria appeared the same microscopically as did vesicles from amoebae fed L. pneumophila alone and averaged 3.65 ± 0.2 μm in diameter for A. polyphaga and 3.5 ± 0.2 μm in diameter for A. castellanii (Fig. 9). Vesicles from amoebae fed E. coli alone did not fluoresce with antibodies to L. pneumophila. Therefore, vesicles from amoebae fed the bacterial mixture contained L. pneumophila cells, indicating that amoebae did not select against legionellae when they were presented with a choice between L. pneumophila and E. coli. Vesicles from Legionella-fed amoebae contained brightly fluorescing rods after treatment with fluorescent antibodies to L. pneumophila, and vesicle membranes also fluoresced faintly. It was not possible to enumerate bacteria within vesicles, since they were packed in clusters.
FIG. 7.
Numbers of vesicles produced per amoeba within 24 h after feeding on E. coli, L. pneumophila (Leg.), or a mixture of both species at 25 and 30°C with an MOI of 10,000.
FIG. 8.
Size frequency distribution of vesicles from A. polyphaga amoebae fed E. coli alone or a mixture of E. coli and L. pneumophila (Lp and Ec).
FIG. 9.
Size frequency distribution of vesicles from A. castellanii amoebae fed E. coli alone or a mixture of E. coli and L. pneumophila (Lp and Ec).
DISCUSSION
Schuster (12) reported that an Acanthamoeba sp. expels food vacuoles prior to encystment rather than retaining them within the cyst. In the present study, most vesicles appeared in the medium just prior to encystment of the population. The addition of cooling tower biocides may induce encystment (16) and thereby facilitate the release of vesicles from amoebae. The fact that vesicles were produced much earlier when amoebae were fed live L. pneumophila cells may reflect the theory that live infectious legionellae resist digestion (5). Using the same A. polyphaga cultures as those employed in the present study, Newsome et al. (8) examined the acid phosphatase activities in amoeba fed live L. pneumophila cells, heat-killed L. pneumophila cells, live E. coli cells, and heat-killed E. coli cells. They found that amoebae fed live L. pneumophila cells had small acid phosphatase-positive vacuoles, presumably lysosomes, evenly distributed in the cytoplasm, whereas those fed heat-killed L. pneumophila cells or live or heat-killed E. coli cells had large acid phosphatase-positive vacuoles, presumably acidified food vacuoles. Others have previously reported the same phenomenon (2). Heat-treated legionellae may not contain the chemical signals for avoiding digestion; therefore, the vesicles produced a day later may have contained digested cell material. In addition, any control amoebae fed live E. coli cells produced smaller and clearer vesicles than did those containing L. pneumophila cells. Live E. coli cells may have been digested, although we did not test for this. The reason for amoebae to produce significantly more vesicles when they were fed the mixture of two bacterial species is not understood.
Calculating from the volume of a sphere and the estimated volume of a single bacterium by using the formula of Bratbak (3) [V = (π/4)w2(L − w/3), where w is the width and L is the length of the bacterium, we estimated that each vesicle could theoretically contain between 20 and 200 bacteria. Rowbotham (11) made similar calculations and reported that for a 5-μm-diameter vesicle, there may be a range of 365 to 1,483 bacteria for a vesicle that is 90% full, depending on the size of the bacterium. Such numbers may constitute an infectious dose (9, 11). Furthermore, Cirillo et al. (4) reported that L. pneumophila is more invasive, even for macrophages, after growth with amoebae; therefore, the infectious dose may be smaller for bacteria that have passed through amoebae.
Most investigations of the numbers of legionellae in the environment and in laboratory coculture experiments base such numbers on CFU on agar. Since 1 CFU may result from one vesicle, the risk for legionellosis may be underestimated severalfold. The fact that bacteria within vesicles were viable after biocide exposure and freeze-thawing underscores the significance of such vesicles in assessing the numbers of legionellae in environments where amoebae may be present and where many legionellae may be in vesicles. Amoebae from cooling towers have also previously been found to be resistant to cooling tower biocides (13–15).
These results demonstrate that respirable vesicles containing living legionellae can be produced by at least two species of amoebae at temperatures which allow the amoebae to carry out normal biological processes, such as encystment, excystment, and feeding. In a study of 40 cooling towers, Yamamoto et al. (17) found cooling tower temperatures to range from 8.3 to 35.2°C, depending on the season. Amoebae may therefore serve as continuous sources of such potentially infectious particles. In the present study, vesicles remained free in the medium, whereas amoeba trophozoites and cysts generally adhered tenaciously to the physical substrate. This indicates that such vesicles may be agents for the transmission of legionellosis from cooling towers, where aerosols may carry small respirable vesicles rather than amoebae or cysts. The existence of vesicles may help to explain some infectious-dose paradoxes (9), such as how certain cooling towers with relatively low numbers of legionellae can be sources of legionellosis and how aerosols can carry an infective dose miles from a cooling tower without bacteria dying from desiccation. There is a need to determine whether such vesicles can be found in environments which may be sources of Legionnaires’ disease. The fact that vesicles in the present study reacted with antibodies to L. pneumophila or with fluorescently tagged gene probes to L. pneumophila (7) indicates that flow cytometric analyses for such vesicles could be a tool in risk assessment.
Although the MOI used in the present study may be high, only bacteria which reach amoebae settled on the bottoms of flasks are encountered by amoebae. It is unknown how such numbers of bacteria relate to the numbers of legionellae attached to substrates and in biofilms, where amoebae most likely feed in cooling towers. In addition, the ratio of legionellae to amoebae on the surfaces of flasks may change as amoebae change their pattern of distribution on these surfaces. Ongoing studies in our laboratory aim to determine factors which lead to the production and release of vesicles containing legionellae. Such factors include the use of actual cooling tower water, the ratio of legionellae to other non-Legionella bacteria as food sources, and biofilms versus suspended bacteria. Also under investigation is the biocide resistance of vesicle-bound legionellae compared with that of legionellae free in medium.
ACKNOWLEDGMENTS
This work was supported in part by grant 1 R15 AI 31171-O1A1 BM to S.G.B. from the National Institute of Allergy and Infectious Diseases and by the Center for the Management, Utilization and Protection of Water Resources at Tennessee Technological University. The research described in this article was funded wholly or in part by the U.S. Environmental Protection Agency NCERQA environmental biology program through grant R 825352-01-0 to S.G.B.
REFERENCES
- 1.Barker J, Brown M R W. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology (Reading) 1994;140:1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 2.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratbak G. Microscope methods for measuring bacterial biovolume: epifluorescence microscopy, scanning electron microscopy, and transmission electron microscopy. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 309–317. [Google Scholar]
- 4.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–4261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields B S. Legionella and protozoa: interaction of a pathogen and its natural host. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 129–136. [Google Scholar]
- 6.Fields B S, Shotts E B, Jr, Martin W T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984;47:467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilly J A, Berk S G. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Use of rRNA-targeted oligonucleotide probes for detection of Legionella in amoebae and vesicles released by amoebae, abstr. D-109; p. 261. [Google Scholar]
- 8.Newsome A L, Seeman C, Ting R S, Wilson G A, Berk S G. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Acid phosphatase localization in Acanthamoeba feeding on Legionella and Escherichia coli, abstr. N-167; p. 351. [Google Scholar]
- 9.O’Brien S J, Bhopal R S. Legionnaires’ disease: the infective dose paradox. Lancet. 1993;342:5–6. doi: 10.1016/0140-6736(93)91877-o. [DOI] [PubMed] [Google Scholar]
- 10.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowbotham T J. Current views on the relationships between amoebae, Legionellae and man. Israel J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 12.Schuster F L. Small amebas and amoeboflagellates. In: Levandowsky M, Hutner S H, editors. Biochemistry and physiology of protozoa. Vol. 1. New York, N.Y: Academic Press; 1979. pp. 215–285. [Google Scholar]
- 13.Srikanth S, Berk S G. Stimulatory effect of cooling tower biocides on amoebae. Appl Environ Microbiol. 1993;59:3245–3249. doi: 10.1128/aem.59.10.3245-3249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikanth S, Berk S G. Adaptation of amoebae to cooling tower biocides. Microb Ecol. 1994;27:293–301. doi: 10.1007/BF00182412. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland E E. M.S. thesis. Cookeville: Tennessee Technological University; 1993. [Google Scholar]
- 16.Sutherland E E, Berk S G. Abstracts of the 92nd General Meeting of the American Society for Microbiology 1992. Washington, D.C: American Society for Microbiology; 1992. Biocide-induced encystment of protozoa from cooling towers, abstr. Q-14; p. 337. [Google Scholar]
- 17.Yamamoto H, Sugiura M, Kusunoki S, Ezaki T, Ikedo M, Yabuuchi E. Factors stimulating propagation of legionellae in cooling tower water. Appl Environ Microbiol. 1992;58:1394–1397. doi: 10.1128/aem.58.4.1394-1397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]