Abstract
In the open ocean, where turbidity is very low, UV radiation may be an important factor regulating interactions among planktonic microorganisms. The effect of exposure to UV radiation on grazing by a commonly isolated marine heterotrophic nanoflagellate, Paraphysomonas bandaiensis, on two strains of the cyanobacteria Synechococcus spp. was investigated. Laboratory cultures were exposed to a range of irradiances of artificially produced UV-B (290 to 319 nm) and UV-A (320 to 399 nm) for up to 10 h. At a UV-B irradiance of 0.19 W m−2, but not 0.12 W m−2, grazing mortality of Synechococcus spp. and nanoflagellate-specific grazing rates were reduced compared to mortality and grazing rates with UV-A treatment. Within 6 h of exposure, UV-A alone suppressed grazing mortality at irradiances as low as 3.02 W m−2. The extent to which grazing mortality and nanoflagellate-specific grazing rates were suppressed by UV-A increased with both irradiance and duration of exposure. Over a 6-h exposure period, differences in grazing mortality were largely attributable to differential survival of nanoflagellates. Over a longer period of exposure, there was impairment by UV-A alone of nanoflagellate-specific grazing rates. Rates of primary productivity of Synechococcus spp. were also reduced by UV-A. The extent to which Synechococcus productivity was reduced, compared to the reduction in Synechococcus grazing mortality, depended on the duration of UV-A exposure. These results support the hypothesis that UV-A alone influences the composition and biomass of marine microbial communities by affecting predator-prey interactions and primary production.
Away from coastal regions, production and consumption of organic matter in the ocean are dominated by prokaryotic and eukaryotic organisms with diameters of <2 to 3 μm, or picoplankton (28, 30, 37, 40, 41). Heterotrophic picoplankton are primarily bacteria. Phototrophic picoplankton encompass a wide variety of cyanobacteria and eukaryotic algae. The most well studied of the phototrophic picoplankton are single-celled cyanobacteria in the genus Synechococcus. Synechococcus spp., and other phototrophic picoplankton, constitute more than 50% of the autotrophic biomass and total primary production in the euphotic zone of the open ocean (16, 40, 44). Much of this production is consumed by heterotrophic nanoflagellates, flagellated protozoa less than 20 μm in diameter, which may occur in open-ocean plankton in densities exceeding 1,000 ml−1 (3, 5, 40).
Photosynthesis of some strains of marine Synechococcus spp. is saturated at low light levels (33), but maximum numbers of Synechococcus spp. are often found close to the surface, where the degree of solar irradiance is relatively high (24, 44). Photosynthetically active solar radiation (PAR) includes wavelengths of 400 nm and longer. Wavelengths between 290 and 400 nm are UV radiation and include UV-A (320 to 399 nm) and UV-B (290 to 319 nm). Atmospheric ozone strongly absorbs UV-B but not UV-A (17). Seasonal damage to the stratospheric ozone layer in the last 2 decades, probably resulting from air pollution, has led to enhanced penetration of UV-B at high and mid-latitudes (6, 38).
In response to the thinning of the ozone layer, more attention has been paid recently to the effects on biological processes of UV-B than to those of UV-A. However, there is abundant evidence that solar UV-A alone can impair biological processes, including nutrient assimilation (9), photosynthesis (7, 21, 26, 31), and motility (10), of marine and freshwater phytoplankton. Recently, Sommaruga et al. (39) reported that the rate of grazing of Bodo saltans, a heterotrophic nanoflagellate, on freshwater bacterioplankton is inhibited by both UV-B and UV-A alone. UV-A, relative to UV-B, may be particularly important in aquatic ecosystems, where, as a function of the concentration of seston and dissolved organic matter, UV-B is more rapidly attenuated with depth than is UV-A (1, 32).
In this study, we had two primary objectives. The first was to determine if UV-A alone, compared to treatment with both UV-A and UV-B, would suppress grazing of Paraphysomonas bandaiensis, a marine heterotrophic nanoflagellate, on Synechococcus spp. Our second objective was to compare the effect of UV-A on the impact of nanoflagellate grazing on a Synechococcus population with the effect of UV-A on the rate of Synechococcus primary production. As pointed out by Bothwell et al. (2), to predict the net effects of UV on the growth rate or population size of an organism, it is necessary to consider not just the direct effects of UV on the organism but also the responses of predators or symbionts of the organism to UV exposure. Here, we describe the effects of a range of UV-A irradiances on nanoflagellate grazing and on rates of primary production of two strains of Synechococcus spp.
MATERIALS AND METHODS
Synechococcus strains, nanoflagellates, and media.
We used two strains of Synechococcus spp. in these experiments. Synechococcus sp. strain WH8012 is coccoid with a diameter of between 1 and 1.5 μm. Synechococcus sp. strain WH7803 is ovoid with a lengthwise diameter of about 2 μm. Both strains were maintained in SN medium (44) prepared with Instant Ocean artificial seawater (composition per liter: 18.7 kg of Cl−, 10.4 kg of Na−, 2.6 kg of SO42−, 1.3 kg of Mg2+, 0.4 kg of Ca2+, 0.4 kg of K+, 0.2 kg of HCO3−, 0.006 kg of B, and 0.008 kg of Sr2+). The nanoflagellate used in all experiments was P. bandaiensis, a colorless chrysomonad commonly isolated from open ocean plankton samples (4) which readily consumes Synechococcus spp. in culture (34). P. bandaiensis was maintained on a mixed bacterial assemblage growing in 0.01% yeast extract made with artificial seawater and amended with rice grains. All organisms were cultured at 20°C under a light-dark cycle (12 h of light:12 h of darkness) at a PAR irradiance of 30 μmol s−1 m−2.
UV and visible irradiance.
The UV source was a Psoralite series 2400 light system fitted with F24T12BL/HO UV-A fluorescent lamps situated behind an acrylic shield. The spectrum of UV irradiance to which organisms were exposed during experiments (Fig. 1) was determined by a model 742 Optronics spectroradiometer calibrated with an Optronics OL-200H calibration standard. UV irradiance was regulated by the use of various numbers of neutral-density screens situated between the UV source and the samples. A Plexiglas sheet was used to remove both UV-A and UV-B. To remove UV-B selectively, we used two sheets of Mylar 500D, which reduced the irradiance of wavelengths below 320 nm by more than 99.5%. The ranges of irradiances of UV-A and UV-B used were 0.00 to 9.48 and 0.00 to 0.20 W m−2, respectively. In all experiments, continuous PAR of approximately 40 μmol s−1 m−2 was provided by a bank of fluorescent lights (tube no. RB15T8) positioned on the side of the samples opposite that of the UV source. The PAR source was shielded by a polycarbonate lens to remove any UV that might have been produced.
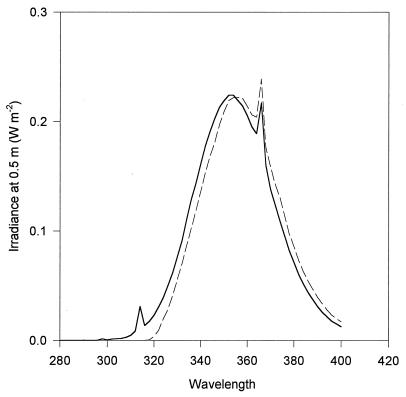
FIG. 1.
Irradiance of UV source at 0.5 m. The solid line indicates the maximum irradiance used in experiments in which organisms were exposed to UV-A+UV-B. The broken line indicates the maximum irradiance used in experiments in which organisms were exposed to UV-A alone. Irradiance is in watts per square meter per nanometer. Wavelength is in nanometers.
Gleason and Wellington (15) measured mean broadband UV irradiances at 10 m in the Caribbean of 15.70 (UV-A) and 0.32 (UV-B) W m−2. Thus, the broadband UV-A and UV-B irradiances used in these experiments approximate solar broadband UV. The maximum wavelength-specific irradiance to which organisms were exposed in experiments was between 0.22 and 0.24 W m−2 at 354 to 366 nm (Fig. 1); at these wavelengths, this irradiance occurs down to approximately 8 to 10 m in clear ocean water (1). Compared to sunlight, however, irradiance at longer wavelengths was depleted relative to irradiance at 354 nm (27).
Basic experimental protocol.
Twenty-four hours prior to an experiment, a portion of the nanoflagellate culture was filtered in series through a Whatman GF/C filter and a 1.0-μm-pore-size Nuclepore filter. This procedure removed all nanoflagellates but allowed heterotrophic bacteria to pass through the filters and repopulate the medium. The filtered medium was used for controls in which grazing on Synechococcus spp. was eliminated.
Experiments were initiated by introduction into sterile artificial seawater of either Synechococcus sp. strain WH8012 or WH7803 to a final concentration of 10 × 106 ml−1 or 5 × 106 ml−1, respectively. For experimental treatments, P. bandaiensis was introduced to a final concentration of approximately 25,000 cells ml−1. Controls containing Synechococcus organisms but no nanoflagellates were prepared for each UV irradiance. To controls, an aliquot of nanoflagellate-free filtrate was added, equal in volume to the additions containing nanoflagellates. Incubation was in UV-transparent polyethylene bags at 25°C. All bags were left open at the top and periodically gently mixed. For each treatment there were four replicates of 25 ml.
The bags were suspended 0.5 m from the UV source, and their contents were exposed to UV as explained above. At various times, samples were withdrawn for enumeration of Synechococcus cells, heterotrophic bacteria, and nanoflagellates. Samples preserved with unbuffered formalin (final concentration, 5%) were filtered onto 0.4-μm-pore-size polycarbonate filters for enumeration by epifluorescence microscopy. Synechococcus cells were visualized by autofluorescence (29). Nanoflagellates and heterotrophic bacteria were counted after being stained with acridine orange (23).
We also measured in vivo fluorescence of the Synechococcus photosynthetic pigments. Open-ocean isolates of Synechococcus spp., including the two strains used in this study, contain phycoerythrin as an accessory light-harvesting pigment (44). Phycoerythrins absorb green light and fluoresce yellow-orange (29). The fluorescence of unpreserved aliquots was measured in a 1-cm-wide cuvette by a Sequoia-Turner fluorometer using a 540-nm shortwave pass filter for excitation and a 585-nm longwave pass filter for emission. Both changes in the number of Synechococcus cells and the rate of change of population fluorescence were used for estimating grazing (see below).
Effect of UV-A and UV-B on nanoflagellate grazing.
Two experiments were performed to compare the effect of UV-A alone versus that of UV-A plus UV-B (UV-A+UV-B) on grazing of P. bandaiensis on Synechococcus spp. (Table 1, experiments 1 and 2). In both experiments, we used Synechococcus sp. strain WH8012. In the first experiment, UV-A+UV-B-treated organisms were exposed to 5.65 W of UV-A and 0.12 W of UV-B m−2. Organisms exposed to UV-A alone received an irradiance of 5.47 W m−2. In the second experiment, UV-A+UV-B-treated organisms were exposed to 9.36 W of UV-A and 0.19 W of UV-B m−2, and those exposed to UV-A alone received an irradiance of 9.48 W m−2. After 6 and either 9 or 10 h of irradiation, samples were collected and processed as described above.
TABLE 1.
Description of experiments examining effects of UV on nanoflagellate grazing
| Expt no. | Synechococcus straina | Nanoflagellatea | UV spectrum or spectra tested |
|---|---|---|---|
| 1 | WH8012 (10,000) | P. bandaiensis (25) | UV-A+UV-B; UV-A |
| 2 | WH8012 (10,000) | P. bandaiensis (25) | UV-A+UV-B; UV-A |
| 3 | WH7803 (5,000) | P. bandaiensis (25) | UV-A |
| 4 | WH8012 (10,000) | P. bandaiensis (25) | UV-A |
Numbers in parentheses indicate the densities of organisms (in cells × 1,000 per milliliter) at the start of the experiment.
Effect of a range of UV-A irradiances on nanoflagellate grazing and Synechococcus population production.
In two other experiments, we compared the effect of UV-A alone, at a range of irradiances, on grazing of P. bandaiensis on Synechococcus spp. and on primary production of the Synechococcus population (Table 1, experiments 3 and 4). In these experiments, the contents of all bags were shielded from UV-B by two sheets of Mylar 500D. Neutral-density screening and Plexiglas were used to produce UV-A irradiances of 0.00, 3.02, 5.47, and 9.48 W m−2. Samples were collected and processed as described above.
Synechococcus population production was determined by measuring the rate of synthesis of 14C-labeled biomass in controls. NaH14CO3 was added to an initial activity of 0.04 μCi ml−1. At 3-h intervals for 9 h, 1-ml aliquots were withdrawn, acidified with 100 μl of 1 N HCl, and shaken overnight to remove unincorporated radiolabel. Radioactivity in the samples was measured in a Beckman LS6500 scintillation counter. Rates are expressed as disintegrations per minute incorporated per milliliter per hour.
Calculation of Synechococcus grazing mortality and nanoflagellate-specific grazing rates.
Gross growth rates (b) of the Synechococcus population in each control were estimated by the formula b = (1/t1 − t0)ln(I1C/I0C), where I1C and I0C are the relative fluorescence intensities for samples without nanoflagellates at times t1 and t0, respectively. Net rates of growth (a) for each of the experimental treatments were estimated similarly by the formula a = (1/t1 − t0)ln(I1E/I0E), where I1E and I0E are the relative fluorescence intensities in samples containing nanoflagellates at times t1 and t0, respectively. Net and gross growth rates over more than one sampling interval (for example, from 0 to 9 h) were determined as the weighted average of growth rates calculated over individual sampling intervals (for example, from 0 to 6 and 6 to 9 h).
Assuming that the gross growth rates were equal in the control and experimental treatments, the rate of grazing mortality of Synechococcus spp., 1/time, is the difference between b and a (12); i.e., grazing mortality = b − a. Grazing mortality is a community-level measurement, reflecting both the abundance of nanoflagellate predators and the grazing rate of individual nanoflagellates. For calculation of nanoflagellate-specific grazing rates (nanoliters of prey cleared per nanoflagellate per hour), grazing mortality was divided by the average number of nanoflagellates per volume in the time interval (20).
In a recent article, Ochs (34) discusses the use of monitoring population fluorescence versus Synechococcus abundance for quantifying rates of nanoflagellate grazing. Briefly, as long as changes in population fluorescence at a particular UV irradiance and incubation period represent proportionately equivalent changes in cell number across the range of Synechococcus abundance in treatments with and without grazers, these methods will provide identical results. We found this condition to be satisfied for Synechococcus sp. strain WH8012 and nearly satisfied for Synechococcus sp. strain WH7803 (34). In the present study, we directly compared grazing rates measured by both methods.
Statistical analysis.
Differences in grazing mortality, nanoflagellate-specific grazing rates, nanoflagellate abundance, and primary production for the various treatments were examined by analysis of variance (ANOVA). Data that did not meet the criteria of a parametric test were analyzed by the Kruskal-Wallis one-way ANOVA on ranks. For isolation of treatment differences, the Student-Newman-Keuls test was used. All tests were two tailed with statistical significance set at P < 0.05.
RESULTS
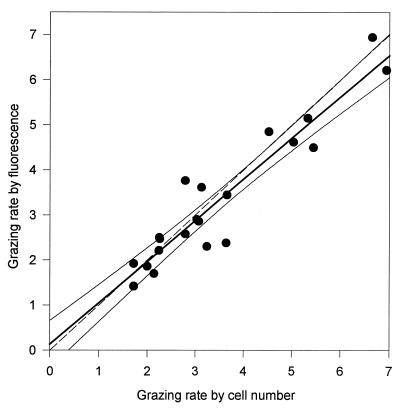
There was a close correspondence in the two methods of calculating grazing rates of P. bandaiensis (Fig. 2). As indicated by a comparison of the mean coefficient of variation for all estimates of nanoflagellate-specific grazing rates by fluorescence (0.13; standard deviation [SD] = 0.08, n = 22) with the mean coefficient of variation for estimates made by changes in cell number (0.30; SD = 0.18, n = 22), grazing measurements made by determining changes in fluorescence were more precise. For our discussion of results in individual experiments, therefore, we report only values of grazing mortality and nanoflagellate-specific grazing rates determined by fluorescence changes.
FIG. 2.
Relationship of specific grazing rates of P. bandaiensis as determined by changes in fluorescence and changes in Synechococcus sp. cell numbers: Y = 0.91X + 0.14; r2 = 0.91. All results for which both measurements were obtained were included; 95% confidence intervals are indicated. The broken line shows a 1:1 relationship. Units are nanoliters cleared per nanoflagellate per hour.
In experiment 1, there were no significant differences in grazing mortality between UV-A+UV-B-treated organisms and those exposed to UV-A alone, calculated over both 6 and 10 h of incubation (Table 2). Nanoflagellate-specific grazing rates with UV-A+UV-B and UV-A treatments also did not significantly differ. Thus, UV-B, at the relatively low irradiance used in experiment 1, appears not to have been a factor affecting nanoflagellate grazing. Synechococcus grazing mortality and nanoflagellate-specific grazing rate were highest for organisms shielded from UV-A+UV-B.
TABLE 2.
Effects of UV-A alone or UV-A+UV-B on grazing mortality (GM) of Synechococcus spp. and nanoflagellate-specific grazing rates (GR) of P. bandaiensis
| Expt no. | Irradiance (W m−2)
|
Grazing measurementa
|
||||
|---|---|---|---|---|---|---|
| UV-A | UV-B | 0–6 hb
|
0–9 (10) hb
|
|||
| GM | GR | GM | GR | |||
| 1 | 0.00 | 0.00 | 0.08 (0.02) a | 6.7 (1.6) a | 0.13 (0.05) a | 10.8 (2.7) a |
| 5.47 | 0.00 | 0.05 (0.01) b | 5.5 (1.0) a | 0.06 (0.00) b | 6.8 (0.7) b | |
| 5.65 | 0.12 | 0.05 (0.01) b | 5.8 (0.8) a | 0.06 (0.01) b | 6.7 (0.4) b | |
| 2 | 0.00 | 0.00 | 0.06 (0.00) a | 2.9 (0.4) a | 0.08 (0.00) a | 5.1 (0.5) a |
| 9.48 | 0.00 | 0.04 (0.01) b | 2.5 (0.3) a | 0.05 (0.01) b | 3.3 (0.5) b | |
| 9.36 | 0.19 | 0.03 (0.01) c | 1.7 (0.1) b | 0.03 (0.00) c | 2.6 (0.1) b | |
Units: GM, 1/hours; GR, nanoliters cleared per nanoflagellate per hour. Numbers in parentheses are SD. All grazing measurements were calculated by determining changes in in vivo population fluorescence. In each experiment, values followed by the same letter are not significantly different from each other (P > 0.05, ANOVA).
The duration of UV-A exposure over which the grazing rates were calculated. In experiment 1, the longer period was 10 h.
In experiment 2, the intensities of both UV-A and UV-B were higher than those used in experiment 1. Over 6 h, there were significant differences in grazing mortality and the nanoflagellate-specific grazing rate between organisms treated only with UV-A and those treated with UV-A+UV-B. Over a 9-h incubation, there was a significant difference in grazing mortality between the UV-A-treated organisms and those treated with UV-A+UV-B, but the difference between their nanoflagellate-specific grazing rates was not significant. As in experiment 1, the highest values for grazing mortality and nanoflagellate-specific grazing rate were measured in organisms shielded from all UV (Table 2).
In each of the two experiments in which only UV-A was used, there was an inverse relationship between UV-A irradiance and both grazing mortality and nanoflagellate-specific grazing rate (Table 3). In experiment 3, significant differences in the rate of Synechococcus sp. strain WH7803 grazing mortality with the various treatments were evident after 6 h. At the highest UV-A irradiance, grazing mortality over 6 h was suppressed by approximately one-third compared to mortality with treatment involving complete shielding from UV-A. Over the longer exposure period, grazing mortality was suppressed by up to 75% by UV-A. Nanoflagellate-specific grazing rates were not significantly different from each other after the 6-h incubation, although the pattern of mean rates is suggestive of a weak UV-A effect. Over a 9-h incubation period, nanoflagellate-specific grazing rates were significantly higher with lower UV-A irradiances. At the highest irradiance, there was a 56% suppression of nanoflagellate-specific grazing rates after 9 h compared to the rates with treatment involving complete shielding from UV-A.
TABLE 3.
Effects of UV-A exposure on grazing mortality (GM) and nanoflagellate grazing rates (GR) of P. bandaiensis on Synechococcus spp.
| Expt no. | UV-A irradiance (W m−2) | Grazing measurementa
|
|||
|---|---|---|---|---|---|
| 0–6 hb
|
0–9 hb
|
||||
| GM | GR | GM | GR | ||
| 3 | 0.00 | 0.03 (0.00) a | 2.4 (0.3) a | 0.04 (0.00) a | 3.2 (0.2) a |
| 3.02 | 0.03 (0.01) a,b | 2.2 (0.6) a | 0.03 (0.00) b | 2.3 (0.2) b | |
| 5.47 | 0.02 (0.00) b,c | 2.3 (0.2) a | 0.02 (0.00) c | 2.0 (0.3) b | |
| 9.48 | 0.02 (0.00) c | 1.9 (0.1) a | 0.01 (0.00) d | 1.4 (0.2) c | |
| 4 | 0.00 | 0.10 (0.00) a | 4.9 (0.2) a | 0.14 (0.01) a | 6.3 (0.4) a |
| 3.02 | 0.09 (0.00) b | 5.1 (0.3) a | 0.12 (0.01) b | 5.5 (0.4) b | |
| 5.47 | 0.07 (0.01) c | 4.5 (0.8) a | 0.10 (0.01) c | 4.7 (0.4) c | |
| 9.48 | 0.06 (0.02) c | 3.6 (1.2) a | 0.06 (0.01) d | 3.8 (0.8) c | |
Units: GM, 1/hours; GR, nanoliters cleared per nanoflagellate per hour. Numbers in parentheses are SD. All grazing measurements were calculated by determining changes in in vivo population fluorescence. In each experiment, values followed by the same letter are not significantly different from each other (P > 0.05, ANOVA).
The duration of UV-A exposure over which the grazing rates were calculated.
The results of experiment 4, in which the prey were Synechococcus sp. strain WH8012, were similar to results of experiment 3 (Table 3). At the highest UV-A irradiance, there was a 40% suppression of grazing mortality after 6 h and a 57% reduction after 9 h compared to the mortality of organisms shielded from UV-A. As in experiment 3, nanoflagellate-specific grazing rates did not differ significantly from each other until 9 h of UV-A exposure, at which point there were significant differences between treatments that corresponded to the gradient in UV-A irradiance. At an irradiance of 9.48 W m−2, there was a 40% reduction of grazing after 9 h compared to that with UV-shielded treatment.
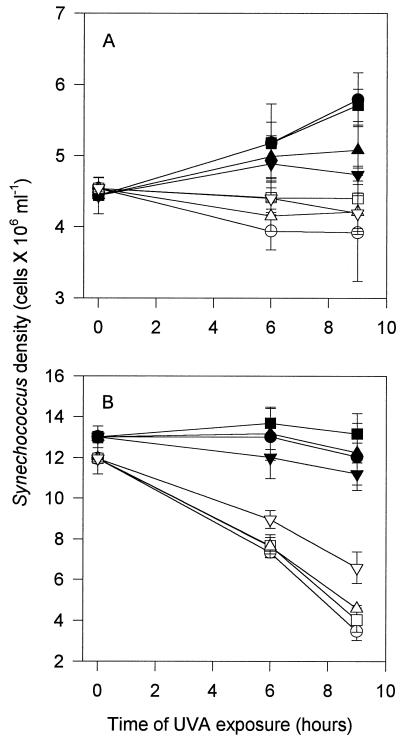
In the two experiments using UV-A alone, Synechococcus abundance in controls either remained stable or increased over the course of the incubations, and there was a decline in Synechococcus abundance when nanoflagellates were present (Fig. 3). Although the decline in abundance was slight in experiment 3, it was notable considering the increase of Synechococcus organisms in all four controls, especially those exposed to the lowest irradiances of UV-A. With the highest and lowest UV-A irradiances, the ratios of numbers of Synechococcus organisms to total numbers of heterotrophic bacteria at the beginning of the experiment and after 9 h of exposure were not significantly different (data not shown). Thus, for the various treatments, differences in the relative amounts of heterotrophic bacteria, an alternative prey, do not explain the differences in nanoflagellate grazing.
FIG. 3.
Synechococcus abundance in experiments 3 (A) and 4 (B) (see Table 1). Symbols for UV-A intensities: ▾, 9.48 W m−2; ▴, 5.47 W m−2; ▪, 3.02 W m−2; •, 0.0 W m−2. Open symbols indicate corresponding treatments in the presence of nanoflagellates.
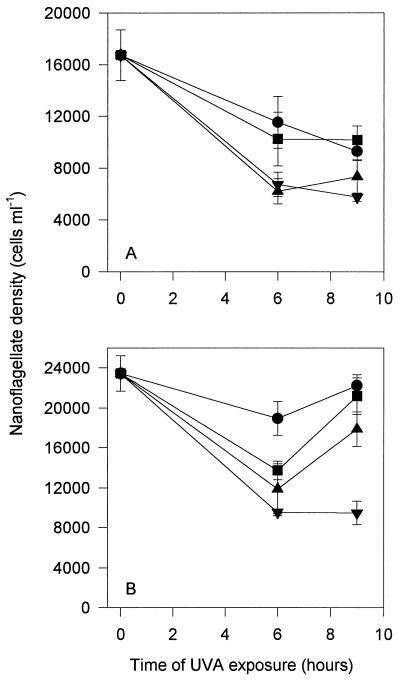
Nanoflagellate abundance declined with all treatments in both experiments using only UV-A (Fig. 4). In general, the decline was greatest with higher levels of UV-A. Between 6 and 9 h there was generally either no further decline or an increase in nanoflagellate numbers with all treatments.
FIG. 4.
Nanoflagellate abundance in experiments 3 (A) and 4 (B) (see Table 1). See the legend to Fig. 3 for definitions of symbols.
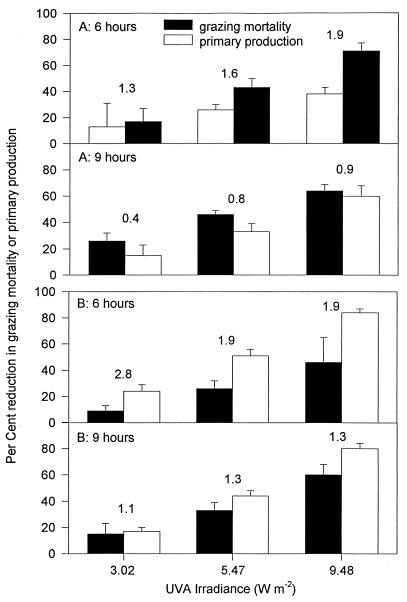
Primary productivity of Synechococcus spp. was increasingly suppressed as UV-A irradiance increased (Fig. 5). In both experiments, Synechococcus productivity was suppressed to a greater degree than was grazing mortality over 6 h. Unlike reductions in grazing mortality, which tended to become more severe with a longer incubation time, reductions of primary productivity were similar over both exposure periods. Consequently, as exposure time became longer, the degree to which grazing mortality was suppressed by UV-A tended to become more similar to the degree of suppression of primary productivity.
FIG. 5.
Percent reduction of grazing mortality or primary production, compared to treatments involving complete shielding from UV-A, in experiments 3 (A) and 4 (B). Results are shown over two time periods, 0 to 6 h and 0 to 9 h. The number over each pair of bars is the mean ratio of the reduction in productivity to the reduction in grazing mortality.
DISCUSSION
These experiments demonstrate that both grazing mortality of Synechococcus spp. and specific rates of grazing of P. bandaiensis on Synechococcus spp. are suppressed by exposure to UV for between 6 and 9 or 10 h at irradiances approximating surface water conditions of the oligotrophic ocean. Considering the well-known detrimental impact of UV-B absorption on biological molecules, especially nucleic acids, it is not surprising that UV-A+UV-B treatment had a somewhat greater impact on grazing by P. bandaiensis than UV-A alone. However, only at the higher irradiance of UV-B was there a significant suppression of grazing mortality or the nanoflagellate-specific rate of grazing compared to the effect of UV-A alone (Table 2).
UV-A alone suppressed the grazing mortality of Synechococcus spp. in all experiments. Differences in grazing mortality over the 6-h exposure periods are largely attributable to differential survival of nanoflagellates, as indicated by the nanoflagellate-specific grazing rates. Is it possible that in nonturbid seawater a diel effect of UV-A is to kill heterotrophic nanoflagellates? If this is so, the rate of nanoflagellate reproduction at night, or at times of the day (or on days) when UV-A irradiance is minimal, must be sufficient to replace individuals lost on sunny days. Available data on diel patterns in heterotrophic nanoflagellate abundance in the ocean do not permit resolution of this question. In measurements made in Woods Hole Harbor and in the oligotrophic Mediterranean Sea, Hagström et al. (19) observed decreases in nanoflagellate numbers of approximately 50% from midnight to midday. In other studies, there were equally large diel changes in nanoflagellate abundance, but in the opposite direction (13, 45). The maximum length of time in which samples were collected in these studies was 36 h, which makes it impossible to evaluate the consistency of the patterns observed. It is evident, however, assuming diel UV-induced mortality of nanoflagellates in the open ocean of up to 50%, that nanoflagellate growth rates are sufficient for recovery during the nightly UV-free period.
Differences in grazing mortalities with the various treatments were more pronounced after 9 h than after 6 h in both experiments 3 and 4, but there was little change or even an increase in numbers of nanoflagellates after between 6 and 9 h of UV-A exposure. Consequently, nanoflagellate-specific grazing rates, evaluated over 9 h, exhibited significant treatment effects, with grazing rates decreasing as UV-A exposure increased. These results suggest that after prolonged exposure to UV-A, the major factor contributing to differences in nanoflagellate-specific grazing rates was impairment of grazing by individual nanoflagellates. The mechanism of UV-A-induced impairment of nanoflagellate grazing is not clear. UV-A alone, as well as UV-B, interferes with the motility of phototropic flagellates of various kinds, at least in some cases by causing the loss or retraction of flagella (8, 18, 22, 42). We have observed that P. bandaiensis, following exposure to UV-A at the irradiances used in these experiments, tends to swim more slowly, and somewhat more erratically, than unexposed organisms, which is suggestive of flagellar damage (34). A freshwater nanoflagellate exhibited similar impairment of motility and suppression of grazing after exposure to artificial and natural UV-A (39). Impairment of flagellar movement may result in less-frequent encounters with potential prey, both by interfering with nanoflagellate motility and by disrupting flagellum-induced feeding currents (11). Alternatively, UV may suppress grazing rates by altering the palatability of prey (22); our visual observations, however, suggest that disruption of nanoflagellate flagella is more likely the cause of suppression of grazing.
Few studies have been conducted examining diel patterns in nanoflagellate grazing in the ocean, and these do not agree in their conclusions. Waterbury et al. (44) inferred from data on Synechococcus abundance collected over several diel cycles that grazing of nanoflagellates on Synechococcus spp., uncorrected for nanoflagellate abundance, occurred principally at night in surface water of the northern Sargasso Sea but continuously at a more turbid coastal water site. In turbid water, the effect of UV on plankton would be expected to be less than in a more clear environment. In two other studies, however, the diel pattern in grazing of marine protozoa was the opposite of that observed by Waterbury et al. (44). Rates of Synechococcus grazing mortality in the English Channel were consistently higher during the day than at night (46), and higher daytime grazing rates of nanoflagellates on bacteria were observed at several locations by Wikner et al. (45). None of these studies was designed specifically to test the effect of UV on grazing of nanoflagellates, a behavior which is probably influenced by multiple environmental factors, of which UV exposure may be of importance only at certain places and times.
Effects of UV on trophic-level interactions, as well as on the physiology or growth rates of individual species, may influence community structure and ecosystem-level productivity. Using predator-free enclosures, Bothwell et al. (2) discovered that growth rates of freshwater benthic diatoms were suppressed by exposure to ambient UV-B. In the presence of algivorous chironomid larvae, however, the net growth rate of the diatoms was higher than in controls in which the predators were present but UV-B was eliminated. Bothwell et al. (2) concluded that differential effects of UV-B on the diatoms compared to the chironomids suppressed predation to a greater degree than diatom growth was inhibited, with the net effect of UV-B exposure being to increase diatom abundance. In the present study, after 6 h of exposure, UV-A suppressed community-level primary production to a somewhat greater extent than the grazing mortality of Synechococcus was reduced. In terms of ecosystem-level productivity, therefore, the effects of this amount of UV-A exposure on suppression of grazing would partially, but not completely, cancel UV-A-induced reductions in Synechococcus productivity. The relative effects of UV-A on productivity and grazing mortality were more similar after 9 h than after 6 h, indicating that evaluations of the influence of UV-A on microbial-community structure must take into consideration both the UV-A irradiance and the duration of exposure.
Considering the simplicity of the system under study, it would be hazardous to noncritically extrapolate these results to a field situation. First, although the broadband UV-A and UV-B irradiances used in these experiments are realistic (15), an artificial source of UV can only approximate the spectral composition of solar UV. Second, the heterotrophic nanoflagellate used in this study appears to be sensitive to UV-A, but other species of nanoflagellates may not be as sensitive. For example, heterotrophic nanoflagellates in the family Bodonidae may be more sensitive to UV than are chrysomonads (39). Even among closely related chrysomonads there may be differences in UV sensitivity, as indicated by observations that grazing of Paraphysomonas imperforata was suppressed less by UV exposure than was grazing by the nanoflagellate used in these experiments (34). Finally, and perhaps most importantly, there is the question of whether the organisms used in this study were more sensitive to the effects of UV-A under the experimental conditions used than they would be in nature. For example, when exposed to UV, many prokaryotic and eukaryotic microorganisms will synthesize compounds that act as UV-absorbing sunscreens and that provide protection against damage to critical biomolecules (14, 25). Cultured organisms not previously exposed to UV may have reduced concentrations of these protective compounds; for these organisms, UV is likely to be particularly stressful or lethal (35, 36, 43). Considering these caveats, the effects of UV-A observed in these experiments perhaps represent an unusually severe or worst-case scenario.
There is a large and growing body of literature describing the effects of UV on microbial physiology, but there have been few studies examining effects on interactions between organisms. Until models of effects of UV-A and UV-B on ecological communities fully incorporate trophic-level interactions, they will be inadequate in helping us understand the role of natural UV irradiance in, or predict the effects of elevated UV on, microbial food webs or microbially mediated biogeochemical processes. Laboratory experiments are valuable as a preliminary means of exploring hypotheses regarding effects of UV on microbial food web interactions, but field studies will provide results that are more clearly relevant to the natural environment. These studies are strongly encouraged, especially in clear oligotrophic marine environments, where UV-A and UV-B may be significant factors influencing the structure and function of microbial food webs.
ACKNOWLEDGMENTS
Nanoflagellates and Synechococcus cultures were provided by D. Caron and J. Waterbury, respectively. We thank J. Beard of Schering-Plough HealthCare Products for excellent technical assistance and C. Webb for laboratory assistance. The manuscript was improved by the critical reading of K. Overstreet and M. Slattery.
Funding was provided by the College of Liberal Arts and Graduate School of the University of Mississippi and by a grant to L.P.E. from the Beta Beta Beta Research Scholarship Foundation Fund.
REFERENCES
- 1.Baker K S, Smith R C. Spectral irradiance penetration in natural water. In: Calkins J, editor. The role of solar ultraviolet radiation in marine ecosystems. New York, N.Y: Plenum Press; 1982. pp. 233–246. [Google Scholar]
- 2.Bothwell M L, Sherbot D M J, Pollock C M. Ecosystem response to solar ultraviolet-B radiation: influence of trophic-level interactions. Science. 1994;265:97–100. doi: 10.1126/science.265.5168.97. [DOI] [PubMed] [Google Scholar]
- 3.Campbell L, Carpenter E J. Estimating the grazing pressure of heterotrophic nanoplankton on Synechococcus spp. using the sea water dilution and selective inhibitor techniques. Mar Ecol Prog Ser. 1986;33:121–129. [Google Scholar]
- 4.Caron, D. A. Personal communication.
- 5.Caron D A, Lim E L, Miceli G, Waterbury J B, Valois F W. Grazing and utilization of chroococcoid cyanobacteria and heterotrophic bacteria by protozoa in laboratory cultures and a coastal plankton community. Mar Ecol Prog Ser. 1991;76:205–217. [Google Scholar]
- 6.Crutzen P J. Ultraviolet on the increase. Nature (London) 1992;356:104–105. [Google Scholar]
- 7.Cullen J C, Neale P J, Lesser M P. Biological weighing function for the inhibition of phytoplankton photosynthesis by ultraviolet radiation. Science. 1992;258:646–650. doi: 10.1126/science.258.5082.646. [DOI] [PubMed] [Google Scholar]
- 8.Davidson A T, Marchant H J. Comparative impact of in situ UV exposure on productivity, growth, survival of antarctic Phaeocystis and diatoms. Proc Natl Inst Polar Res Symp Polar Biol. 1994;7:53–69. [Google Scholar]
- 9.Döhler G, Hagmeier E, David C. Effects of solar and artificial UV irradiation on pigments and assimilation of 15N ammonium and 15N nitrate by macroalgae. J Photochem Photobiol B. 1995;30:179–187. [Google Scholar]
- 10.Donkor V A, Amewowor D H A K, Häder D-P. Effects of tropical solar radiation on the motility of filamentous cyanobacteria. FEMS Microbiol Ecol. 1993;12:143–148. [Google Scholar]
- 11.Fenchel T. The ecology of heterotrophic microflagellates. Adv Microb Ecol. 1986;9:57–97. [Google Scholar]
- 12.Frost B W. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr. 1972;17:805–815. [Google Scholar]
- 13.Fuhrman J A, Eppley R W, Hagström Å, Azam F. Diel variations in bacterioplankton, phytoplankton, and related parameters in the Southern California Bight. Mar Ecol Prog Ser. 1985;27:9–20. [Google Scholar]
- 14.Garcia-Pichel F, Wingard C E, Castenholz R W. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl Environ Microbiol. 1993;59:170–176. doi: 10.1128/aem.59.1.170-176.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleason D F, Wellington G M. Ultraviolet radiation and coral bleaching. Nature (London) 1993;365:836–838. [Google Scholar]
- 16.Glover H E, Prézelin B B, Campbell L, Wyman M. Pico- and ultraplankton Sargasso Sea communities: variability and comparative distributions of Synechococcus spp. and algae. Mar Ecol Prog Ser. 1988;49:127–139. [Google Scholar]
- 17.Green A E S, Cross K R, Smith L A. Improved analytic characterization of ultraviolet skylight. Photochem Photobiol. 1980;31:59–65. [Google Scholar]
- 18.Häder D. Effects of UV-B on motility and photobehavior in the green flagellate, Euglena gracilis. Arch Microbiol. 1985;141:159–163. [Google Scholar]
- 19.Hagström Å, Azam F, Andersson A, Wikner J, Rassoulzadegan F. Microbial loop in an oligotrophic pelagic marine ecosystem: possible role of cyanobacteria and nanoflagellates in the organic fluxes. Mar Ecol Prog Ser. 1988;49:171–178. [Google Scholar]
- 20.Heinbokel J F. Studies of the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar Biol. 1978;47:177–189. [Google Scholar]
- 21.Helbling E W, Villafañe V, Ferrario M, Holm-Hansen O. Impact of natural ultraviolet radiation on rates of photosynthesis and on specific marine phytoplankton species. Mar Ecol Prog Ser. 1992;80:89–100. [Google Scholar]
- 22.Hessen D O, Van Donk E, Andersen T. Growth responses, P-uptake, and loss of flagellae in Chlamydomonas reinhardtii exposed to UV-B. J Plankton Res. 1995;17:17–27. [Google Scholar]
- 23.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard K M, Joint I R. Physiological ecology of picoplankton in the North Sea. Mar Biol. 1989;102:275–281. [Google Scholar]
- 25.Karentz D, McEuen F S, Land M C, Dunlap W C. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Mar Biol. 1991;108:157–166. [Google Scholar]
- 26.Kim D S, Watanabe Y. Inhibition of growth and photosynthesis of freshwater phytoplankton by ultraviolet A (UVA) radiation and subsequent recovery from stress. J Plankton Res. 1994;16:1645–1654. [Google Scholar]
- 27.Kirk J T O. Light and photosynthesis in aquatic ecosystems. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 1994. [Google Scholar]
- 28.Li W K W, Subba Rao D V, Harrison W G, Smith J C, Cullen J J, Irwin B, Platt T. Autotrophic picoplankton in the tropical ocean. Science. 1983;219:292–295. doi: 10.1126/science.219.4582.292. [DOI] [PubMed] [Google Scholar]
- 29.MacIsaac E A, Stockner J G. Enumeration of phototrophic picoplankton by autofluorescence microscopy. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 187–197. [Google Scholar]
- 30.Magazzù G, Decembrino F. Primary production, biomass and abundance of phototrophic picoplankton in the Mediterranean Sea: a review. Aquat Microb Ecol. 1995;9:97–104. [Google Scholar]
- 31.Moeller R E. Contribution of ultraviolet radiation (UV-A, UV-B) to photoinhibition of epilimnetic phytoplankton in lakes of differing UV transparency. Arch Hydrobiol. 1994;43:157–170. [Google Scholar]
- 32.Morris D P, Zagarese H, Williamson C E, Balseiro E G, Hargreaves B R, Modenutti B, Moeller R, Queimalinos C. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol Oceanogr. 1995;40:1381–1391. [Google Scholar]
- 33.Morris I, Glover H. Physiology of photosynthesis by marine coccoid cyanobacteria—some ecological implications. Limnol Oceanogr. 1981;26:957–961. [Google Scholar]
- 34.Ochs C A. Effects of UV radiation on grazing by two marine heterotrophic nanoflagellates on autotrophic picoplankton. J Plankton Res. 1997;19:1517–1536. [Google Scholar]
- 35.Paerl H W. Cyanobacterial carotenoids: their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia. 1984;61:143–149. doi: 10.1007/BF00396752. [DOI] [PubMed] [Google Scholar]
- 36.Paerl H W, Bland P T, Bowles N D, Haibach M E. Adaptation to high-intensity, low-wavelength light among surface blooms of the cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol. 1985;49:1046–1052. doi: 10.1128/aem.49.5.1046-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt T, Subba Rao D V, Irwin B. Photosynthesis of picoplankton in the oligotrophic ocean. Nature (London) 1983;301:702–704. [Google Scholar]
- 38.Smith R C, Prézelin B B, Baker K S, Bidigare R R, Boucher N P, Coley T, Karentz D, MacIntyre S, Matlick H A, Menzies D, Ondrusek M, Wan Z, Waters K J. Ozone depletion: ultraviolet radiation and phytoplankton biology in Antarctic waters. Science. 1992;255:952–959. doi: 10.1126/science.1546292. [DOI] [PubMed] [Google Scholar]
- 39.Sommaruga R, Oberleiter A, Psenner R. Effect of UV radiation on the bacterivory of a heterotrophic nanoflagellate. Appl Environ Microbiol. 1996;62:4395–4400. doi: 10.1128/aem.62.12.4395-4400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockner J G, Anita N J. Algal picoplankton from marine and freshwater ecosystems: a multidisciplinary perspective. Can J Fish Aquat Sci. 1986;43:2472–2503. [Google Scholar]
- 41.Takahashi M, Bienfang P K. Size structure of phytoplankton biomass and photosynthesis in subtropical Hawaiian waters. Mar Biol. 1983;76:203–211. [Google Scholar]
- 42.Van Donk E, Hessen D O. Loss of flagella in the green alga Chlamydomonas reinhardtii due to in situ UV-exposure. Sci Mar. 1996;60:107–112. [Google Scholar]
- 43.Villafañe V E, Helbling E W, Holm-Hansen O, Chalker B E. Acclimatization of Antarctic natural phytoplankton assemblages when exposed to solar ultraviolet radiation. J Plankton Res. 1995;17:2295–2306. [Google Scholar]
- 44.Waterbury J B, Watson S W, Valois F W, Franks D G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci. 1986;214:71–120. [Google Scholar]
- 45.Wikner J, Rassoulzadegan F, Hagström Å. Periodic bacterivore activity balances bacterial growth in the marine environment. Limnol Oceanogr. 1990;35:313–324. [Google Scholar]
- 46.Xiu-ren N, Vaulot D. Simultaneous estimates of Synechococcus spp. growth and grazing mortality rates in the English Channel. Chin J Oceanol Limnol. 1996;14:8–16. [Google Scholar]