Abstract
Batch sorption experiments were carried out with the bacteriophages MS2 and φX174. Two types of reactor vessels, polypropylene and glass, were used. Consistently lower concentrations of MS2 were found in the liquid phase in the absence of soil (control blanks) than in the presence of soil after mixing. High levels of MS2 inactivation (∼99.9%) were observed in control tubes made of polypropylene (PP), with comparatively little loss of virus seen in PP tubes when soil was present. Minimal inactivation of MS2 was observed when the air-water interface was completely eliminated from PP control blanks during mixing. All batch experiments performed with reactor tubes made of glass demonstrated no substantial inactivation of MS2. In similar experiments, bacteriophage φX174 did not undergo inactivation in either PP or glass control blanks, implying that this virus is not affected by the same factors which led to inactivation of MS2 in the PP control tubes. When possible, phage adsorption to soil was calculated by the Freundlich isotherm. Our data suggest that forces associated with the air-water-solid interface (where the solid is a hydrophobic surface) are responsible for inactivation of MS2 in the PP control tubes. The influence of air-water interfacial forces should be carefully considered when batch sorption experiments are conducted with certain viruses.
Application of wastewater effluents to land for treatment purposes must take into consideration the potential for groundwater pollution due to virus transport through the subsurface. A number of studies have clearly documented the ability of viruses to migrate significant distances through soil, resulting in groundwater contamination (16, 21, 41). One of the major factors limiting virus transport is sorption to soil particles (14, 46). The factors which influence virus sorption to soil have been extensively studied (7, 15, 17, 24, 28, 29, 42) but, to date, have not been fully characterized. Furthermore, the majority of available data are of a qualitative, rather than a quantitative, nature.
Traditionally, virus sorption has been analyzed by the batch equilibrium method, which quantifies the partitioning of virus between solid and liquid phases at equilibrium (7, 28). The method consists of mixing a suspension of virus solution and soil in a batch reactor vessel, or tube. The soil-virus suspension is mixed or shaken to allow complete and thorough contact between soil particles and the aqueous phase. After equilibrium partitioning is reached, virus concentrations in the aqueous phase are measured and the amount of virus sorbed to the soil is calculated based on mass balance considerations. A control sample (virus and water, no soil) is used to determine whether virus loss may occur due to factors other than sorption to soil during the shaking process (e.g., inactivation or sorption to the reactor vessel). Several investigators using the batch equilibrium method for studying bacteriophage sorption to soil have reported unexplained results associated with anomalous control samples (6, 18, 30). In these studies, virus concentrations in control tubes (virus and water) were consistently lower than in experimental tubes (virus, soil, and water) after shaking or mixing. Furthermore, the reactor vessels used were all made of polypropylene (PP). One possible explanation of this anomalous behavior is the inactivation of viruses due to the presence of an air-water interface (AWI) in the batch reactor vessel. To facilitate mixing, batch tubes are only partially filled with soil and virus solution, thereby leaving an AWI present within the tube, as was the case in the three studies mentioned above (6, 18, 30).
Previous work has shown that shaking viral suspensions or exposing viruses to AWIs may lead to inactivation of virus particles (2, 5, 8, 9, 26, 37–40). These studies suggest that viruses in solution approach the AWI via convection and diffusion and that they are sorbed and subsequently inactivated at the AWI by forces deforming the virus particle. By shaking a virus suspension, the AWI is continuously being regenerated, providing a renewed location for virus inactivation. Virus capsids have localized polar and nonpolar regions (27), making them similar in nature to amphipathic molecules, which are known to accumulate at the surfaces of aqueous systems (35). At the AWI, hydrophobic regions of the virus will partition out of solution and into the gas phase via the reconfiguration of capsid proteins. This reconfiguration, or denaturation, may result in the loss of virus infectivity. To date, it has not been proposed that loss of bacteriophage in certain batch adsorption studies (6, 18, 30) was the result of virus inactivation associated with the presence of an AWI.
The purpose of this study was to examine the role of the AWI in virus sorption experiments. The batch equilibrium method was used (i) to quantify sorption of MS2 and φX174 to different soils and more importantly (ii) to determine the mode of virus inactivation as a function of the interfacial forces present in the dynamic reactor vessel.
MATERIALS AND METHODS
Bacteriophage stocks and enumeration.
Two bacteriophages, MS2 and φX174, were used as model viruses in this investigation. Bacteriophage MS2, obtained from the American Type Culture Collection (ATCC 15597B1), is an icosahedral, single-stranded RNA phage with a diameter of approximately 26 nm (34) and an isoelectric point of 3.9 (47). Bacteriophage φX174 (ATCC 13706B1) is an icosahedral, single-stranded DNA phage with a diameter of approximately 23 nm and an isoelectric point of 6.6 (1).
MS2 and φX174 were grown on lawns of host Escherichia coli (ATCC 15597 and ATCC 13706, respectively) by the agar-overlay method (3). Bacteriophages were harvested and suspended in phosphate-buffered saline composed of the following: NaCl (0.1 mol liter−1), KCl (0.003 mol liter−1), and Na2HPO4 (0.02 mol liter−1). pH was adjusted to 7.45 with HCl. Harvested virus was centrifuged for 15 min at approximately 12,000 × g in a model SS-34 rotor (7 ± 1°C) followed by filtration through a succession of 0.45-μm-pore-size membrane filters (Gelman Sciences, Ann Arbor, Mich.). The concentrations of prepared stocks typically ranged from 1010 to 1012 PFU per ml. Before use, a small fraction of the prefiltered virus was passed through a series of 0.2- and 0.05-μm-pore-size polycarbonate membrane filters (COSTAR Corp., Pleasanton, Calif.) to remove viral aggregates.
Enumeration of MS2 and φX174 was performed according to the PFU method (3) with the aforementioned bacterial hosts. One milliliter of sample and 1 ml of log-phase E. coli were combined in a tube of molten Trypticase soy agar (Difco Laboratories, Detroit, Mich.) and poured onto Trypticase soy agar plates to be incubated overnight at 37°C. Replicate plating was performed on each sample, with countable numbers of plaques ranging from 25 to 250 per plate.
Experimental soils.
The soil materials used were Ottawa sand (OS), Tujunga loamy sand (TLS), and Greenfield sandy loam (GSL). OS (Fisher Scientific, Los Angeles, Calif.) is a uniform quartz sand with a mean grain diameter of 0.6 to 0.8 mm, no clay fraction, and an organic carbon content of 0 mg g−1 (28). The sand was washed with detergent and rinsed thoroughly with distilled water prior to oven drying at 105°C for 24 h. TLS (6.7 mg of organic carbon g−1, 4.5% clay) (10) and GSL (9.21 mg of organic matter g−1, 9.5% clay) (13) were taken from two University of California field sites located in southern California; samples were obtained from the top 30 cm of the soil surface. Both soils were air dried for 24 h, passed through a 2-mm-pore-size sieve, and oven dried at 105°C for 24 h prior to use.
Batch sorption study.
Batch sorption experiments were performed in 15-ml PP centrifuge tubes (Fisher Scientific) and in Pyrex glass screw-cap tubes (16 by 125 mm; Fisher Scientific). Glass tubes were washed with detergent, soaked in 6 N HCl, rinsed thoroughly in deionized water, autoclave sterilized, and oven dried at 105°C overnight. A minimum of six different virus stock concentrations (∼102 to 107 PFU ml−1) were used to establish the isotherm curves. Physiological conditions (i.e., presence of phosphate-buffered saline) were used in an effort to promote virus stability and thereby eliminate potential confounding factors which could contribute to inactivation. Experimental tubes received 10 ml of virus stock solution and 10 g of sand or soil; control tubes received only virus solution (10 ml). A 1:1 ratio of soil to virus solution was used in each batch experiment. Soil-virus suspensions were mixed for 3 h at 7 ± 1°C by rotating the tubes end over end (∼20 rpm) on a tube rotator (Fisher Scientific) so that the soil remained in a dynamic state. The 3-h reaction time was chosen based on initial studies (performed with glass tubes) which demonstrated that equilibrium sorption was reached within the first 1.5 h of mixing (data not shown). The suspension was then transferred to Teflon tubes (Nalgene, Rochester, N.Y.) and centrifuged for 10 min at approximately 12,000 × g in a model SS-34 rotor at 7 ± 1°C. Control tubes were treated in the same manner as experimental tubes. All experiments were performed in triplicate. Virus sorption was determined with the following formula:
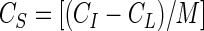 |
1 |
where CI, CL, and CS are, respectively, the concentrations of virus in the control liquid phase (PFU per milliliter), in the experimental liquid phase (PFU per milliliter), and adsorbed to the soil (PFU per gram) and M is the total mass of soil per unit volume of virus suspension (grams per milliliter) used in each batch experiment. In the instances where MS2 concentrations were lower in control tubes than in experimental tubes, CS values could not be determined because of the negative result of CI − CL.
Determination of the air-water-solid effect on the fate of MS2.
Only the behavior of MS2 was examined in this study, since φX174 did not undergo inactivation during batch adsorption experiments in PP control blanks. Four types of reactor vessels were selected for this study: 15-ml PP centrifuge tubes, 50-ml PP centrifuge tubes (Fisher Scientific), 250-ml PP bottles (Fisher Scientific), and Pyrex glass screw-cap tubes (16 by 125 mm). The volume of MS2 solution added to each tube was varied while the starting concentration of MS2 (C0) was held constant at ∼105 PFU ml−1. Tube types and the volumes of solution added were as follows: 15-ml PP tubes with 4, 5, 7, 9, 10, 11, 13, 15, and 15.7 ml of solution; 50-ml PP tubes with 10, 25, and 50 ml of solution; 250-ml PP bottles with 10, 50, 100, and 250 ml of solution; and glass tubes with 5, 10, and 15 ml of solution. It should be noted that the measured total capacities (no gas phase present) for the 15-ml PP, 50-ml PP, 250-ml PP, and glass tubes were, respectively, 15.7, 55.5, 304, and 17.4 ml. No soil was added so that comparisons could be made between any changes in MS2 concentration and the total volume of phage suspension added to each tube. The experimental procedure was identical to that for the control blanks described in the batch sorption study, with two exceptions: (i) centrifugation was not performed and (ii) a series of tubes was held static to act as nonshaken controls, with solution volumes being the same as those in the experimental tubes. The 15-ml PP tubes receiving 15.7 ml of MS2 solution were filled to capacity so that no gas phase (no AWI) was present within the tube. This was accomplished by aseptically immersing the tube and cap into a 2-liter beaker of virus suspension and attaching the cap so that no air bubbles remained. To maintain the solution in a dynamic state during mixing, 15 Teflon beads (average diameter, ∼6 mm; Norton Performance Plastics, Akron, Ohio) were added to each tube. Preliminary studies revealed that MS2 did not sorb to the Teflon beads (data not shown).
After the tubes were mixed for 3 h and the solutions were assayed for MS2, a select number of samples were treated by adding various eluants directly to the MS2 suspension and continuing the mixing process. The following eluants were added (values given reflect the final eluant concentration in the batch tubes): 2.5% (wt/vol) beef extract (Beef Extract V; Becton Dickinson and Co., Cockeysville, Md.), 2.0% (vol/vol) Tween 80 (Aldrich Chemical Co., Milwaukee, Wis.), 0.6% (wt/vol) gelatin (Sigma Chemical Co., St. Louis, Mo.), and 0.15 mol glycine (Sigma Chemical Co.) liter−1. Beef extract, Tween 80, and glycine were prepared in 0.05 mol of Na2HPO4 liter−1 and adjusted to a pH of 9.5 (glycine was adjusted to a pH of 10.0) with NaOH or HCl as needed. The gelatin was prepared in 0.02 mol of Na2HPO4 liter−1 and adjusted to a pH of 7.5 with HCl. Upon addition of eluant to the vessel, each sample was remixed for an additional 0.5 h and reassayed to determine if MS2 had been desorbed from the vessel walls. All experiments were performed at 7 ± 1°C to limit virus inactivation due to temperature effects.
RESULTS
Batch sorption study.
Table 1 presents the MS2 sorption data from batch experiments performed in PP tubes. Solution-phase MS2 concentrations in the control blanks (CI) were on average 2.34 log10 units lower than in the tubes containing soil. Consequently, calculation of sorbed-phase MS2 concentrations (CS) by the traditional mass balance method (equation 1) was not possible because of the resultant negative value for the term CI − CL. The input concentrations may be used as control values; however, if this is done, there is no way to distinguish between the effects of adsorption to soil and other factors which might influence virus fate during the experiment (e.g., natural inactivation, container wall effects, and influence of the AWI). When the same experiments were performed with glass tubes, no substantial loss of MS2 was observed in the control blanks, thereby permitting the quantification of sorbed-phase MS2 (CS). OS did not adsorb MS2; values of CI and CL were not significantly different at a significance level of 0.1% (Student’s t test) at any of the concentrations tested.
TABLE 1.
MS2 batch adsorption data from experiments performed with PP tubesa
| Input concn (PFU ml−1) | Control blank concn (CI) (PFU ml−1) | Solution-phase concn (CL) (PFU ml−1) in:
|
||
|---|---|---|---|---|
| OS | TLS | GSL | ||
| 3.24 × 102 | (1.00 ± 1.26) × 100 | (2.63 ± 0.42) × 102 | (2.17 ± 0.28) × 102 | (1.30 ± 0.28) × 102 |
| 2.40 × 103 | (3.32 ± 1.29) × 100 | (2.45 ± 0.50) × 103 | (1.68 ± 0.31) × 103 | (1.32 ± 0.34) × 103 |
| 2.57 × 104 | (2.24 ± 0.42) × 102 | (2.56 ± 0.21) × 104 | (1.81 ± 0.41) × 104 | (1.56 ± 0.18) × 104 |
| 4.17 × 105 | (8.32 ± 0.61) × 102 | (3.77 ± 0.36) × 105 | (3.29 ± 0.22) × 105 | (2.69 ± 0.37) × 105 |
| 3.98 × 106 | (1.58 ± 0.37) × 104 | (2.21 ± 0.41) × 106 | (2.94 ± 0.25) × 106 | (2.53 ± 0.33) × 106 |
| 4.16 × 107 | (1.82 ± 0.35) × 105 | (4.08 ± 0.26) × 107 | (3.38 ± 0.48) × 107 | (2.80 ± 0.28) × 107 |
Sorbed-phase concentrations (CS) were not calculated because CL is greater than CI. The concentrations are means ± standard deviations.
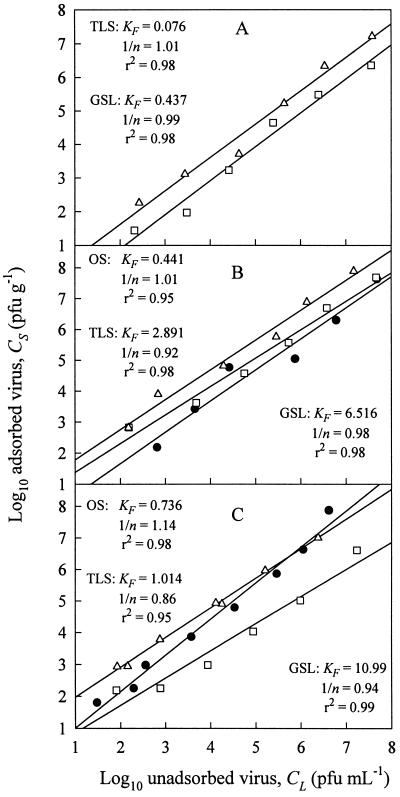
Adsorption of MS2 to TLS and GSL was quantified by the Freundlich isotherm, CS = KFCL1/n, where CS is the quantity of virus sorbed to the soil (PFU per gram), CL is the concentration of virus remaining in the liquid phase (PFU per milliliter), and KF (Freundlich constant) and 1/n are constants. The parameters KF and 1/n were estimated by linear regression of the log10-transformed data (i.e., log10 CS versus log10 CL). Figure 1A presents the isotherms and corresponding Freundlich constants for MS2 adsorption to TLS and GSL from experiments performed with glass tubes. Data from the MS2 PP batch studies are not presented as isotherms because of the negative CS term resulting from CL being greater than CI (Table 1).
FIG. 1.
MS2 and φX174 Freundlich adsorption isotherm plots for OS (circles), TLS (squares), and GSL (triangles). (A) MS2 adsorption data from experiments performed with glass tubes. No significant adsorption of MS2 to OS was observed (see the text). (B) φX174 adsorption data from experiments performed with glass tubes. (C) φX174 adsorption data from experiments performed with polypropylene tubes. All values of KF are in milliliters per gram.
Unlike MS2, φX174 did not undergo substantial inactivation in the PP control blanks during mixing. φX174 CI values averaged 93% of input (data not shown) compared to MS2 CI values, which averaged just 0.4% of input (Table 1). CI values from batch experiments performed with glass tubes were similar to those seen in the PP studies (94 versus 93% of input). Figure 1B and C show the φX174 adsorption data from batch experiments performed with PP and glass tubes, respectively. Adsorption of φX174 was greatest to GSL, followed by that to TLS and OS. Except in experiments with TLS, φX174 KF values were greater when the batch experiments were done with PP tubes. Adsorption of φX174 to all three porous media was much greater than that of MS2 (Fig. 1A).
Determination of the air-water-solid effect on the fate of MS2.
Batch experiments performed with the three different PP containers showed an increase in the rate of MS2 loss (over 3 h of mixing) as the starting volume of virus solution was decreased (Table 2). When the 15-ml PP tubes were completely filled with solution and there was no AWI present (i.e., 15.7-ml solution volume), the MS2 inactivation rate was very low (Table 2). The presence of a small amount of gas phase increased the inactivation rate by more than 1 order of magnitude (0.056 versus 0.599 h−1). Although a considerable amount of AWI was present in the 50-ml PP (50-ml solution volume) and the 250-ml PP (100- and 250-ml solution volumes) containers during mixing, MS2 inactivation rates were much lower than those observed in the 15-ml PP tubes. In the glass tube experiments, no pronounced MS2 inactivation was observed, even when the initial volume of solution was varied. None of the static controls (nonrotated) exhibited substantial decreases in MS2 concentration (data not shown). This was true for each of the four container types and the different volumes tested. Final concentrations of MS2 in the PP static controls typically ranged from 74.2 to 99.7% of input values.
TABLE 2.
Data from batch experiments (no soil) examining the air-water-solid effect on the fate of MS2a
| Tube type | MS2 solution vol (ml) | Mean C/C0 ± SDb | Decay rate (α) (h−1)c |
|---|---|---|---|
| PP, 15 ml | 4 | (3.09 ± 0.52) × 10−4 | 1.170 |
| 5 | (5.81 ± 0.27) × 10−4 | 1.078 | |
| 7 | (1.25 ± 0.27) × 10−3 | 0.968 | |
| 9 | (2.44 ± 0.23) × 10−3 | 0.871 | |
| 10 | (2.54 ± 0.38) × 10−3 | 0.866 | |
| 11 | (3.50 ± 0.23) × 10−3 | 0.818 | |
| 13 | (5.98 ± 1.06) × 10−3 | 0.741 | |
| 15 | (1.59 ± 0.29) × 10−2 | 0.599 | |
| 15.7 | (6.81 ± 3.01) × 10−1 | 0.056 | |
| PP, 50 ml | 10 | (7.79 ± 0.86) × 10−4 | 1.037 |
| 25 | (1.76 ± 0.77) × 10−2 | 0.584 | |
| 50 | (1.93 ± 0.63) × 10−1 | 0.238 | |
| PP, 250 ml | 10 | (6.67 ± 1.29) × 10−4 | 1.058 |
| 50 | (8.86 ± 1.16) × 10−2 | 0.351 | |
| 100 | (2.93 ± 0.77) × 10−1 | 0.178 | |
| 250 | (8.96 ± 1.04) × 10−1 | 0.016 | |
| Glass | 5 | (1.04 ± 0.16) × 100 | 0.000 |
| 10 | (8.12 ± 0.63) × 10−1 | 0.030 | |
| 15 | (9.60 ± 0.87) × 10−1 | 0.006 |
All batch experiments were conducted for 3 h at 7 ± 1°C.
C0 = 1.57 × 105 PFU ml−1.
Assuming first-order decay.
Of the four eluting agents tested on the mixed samples, none produced a significant increase in the concentration of MS2 (data not shown). The largest detectable increase in the titer of MS2 (43%) occurred after the addition of 0.6% gelatin to the 250-ml PP bottles (10-ml solution volume). This corresponded to an increase in C/C0 from 6.69 × 10−4 to 1.17 × 10−3. In no case did C/C0 values approach C0 after addition of eluant.
DISCUSSION
Loss of MS2 was observed in PP vessels lacking soil (Tables 1 and 2) but not in glass vessels (Table 2). Loss due to adsorption by the PP container walls is not supported by our elution experiments. Speculation that MS2 inactivation might be specific to PP vessels was eliminated when similar studies with polystyrene, Teflon, and polyethylene tubes revealed similar rates of inactivation (data not shown). The possibility of losses being due to temperature and turbulence effects was also eliminated based on data from the controls.
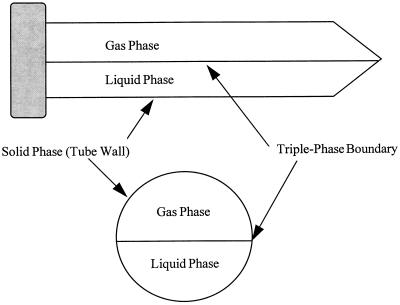
Previous studies have shown that viruses and proteins can lose viability upon exposure to AWIs (2, 12, 19, 39, 40). However, our data suggest that factors other than the AWI alone are responsible for viral inactivation. The lack of MS2 inactivation in the glass tubes with AWI present indicates that the forces responsible for virus loss are present in the PP system but not in the glass system or that if they are present in the glass system, they do not influence virus fate in the same manner. This possibility implies that the site of phage inactivation is not simply the AWI itself, since the AWI is the same in both systems (glass and PP), but rather is the interface at which the liquid, solid, and gas phases meet (the triple-phase-boundary [TPB]). As Fig. 2 depicts, the TPB is the line, or perimeter, at which the AWI meets the container wall.
FIG. 2.
Schematic of a PP tube (partially filled with phage suspension) depicting the air, water, and solid phases, as well as the TPB.
Although the mechanism of inactivation is not fully understood, we propose the following. Viruses in solution reach the AWI, where they adsorb, via convection and diffusion. This adsorption is dominated by electrostatic, hydrophobic, hydration, and capillary forces; solution ionic strength; pH; and various other factors (25, 33, 36, 43–45). As a virus adsorbs to the AWI, hydrophobic domains on the protein capsid partition out of the solution and into the more nonpolar gas phase. We suggest that such exposed domains on the virus capsid are susceptible to forces at the TPB which are not present at the AWI itself.
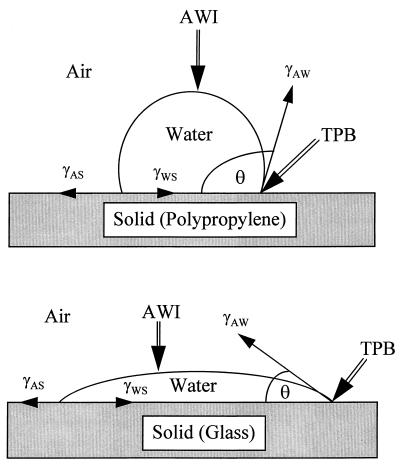
Unlike the AWI, the balance of forces at the TPB will be influenced by the surface characteristics of the solid (tube). Glass is hydrophilic, with a contact angle against water of <45°, while PP is a hydrophobic organic polymer with a contact angle of 108° (4). Figure 3 demonstrates the interaction of a water droplet with glass and PP surfaces at equilibrium. The forces acting on the water droplet will balance at equilibrium according to Young’s equation, γSA = γSW + γAW · cosθ, where γSA, γSW, and γAW are, respectively, the solid-air, solid-water, and air-water surface tensions and cosθ is the cosine of the angle of contact between liquid and solid. From Fig. 3 it is clear that the forces influencing an exposed virus particle at the TPB will be much different from those at the bulk AWI. Since the air-water surface tension (γAW) will be the same for both systems, the other forces (i.e., γSA and γSW) will be dictated by the type of tube used. Furthermore, it has been demonstrated that the orientations of water molecules at large hydrophobic surfaces are considerably different from those in bulk water (22).
FIG. 3.
Water droplets resting on PP and glass surfaces. Shown are the equilibrium forces involved (i.e., γAW, γWS, and γAS), the AWI, and the TPB.
We propose that virus particles partitioned at the TPB experience destructive forces as a result of the reconfiguration of water molecules near the hydrophobic PP surface. Virus proteins projecting into the gas phase may also interact with the PP surface at the TPB. As the AWI is sheared away from the PP wall during mixing, partitioned virus particles may experience shear stress. Such forces may cause structural changes to a virus capsid, resulting in loss of infectivity.
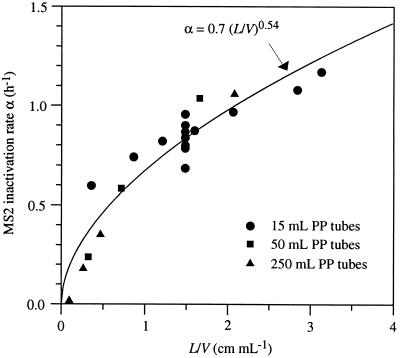
The role of the air-water interface in MS2 inactivation is clearly demonstrated in Table 2. The presence of a small area of AWI caused a considerable increase in the viral inactivation rate in the 15-ml tubes. Moreover, the experimental data relate the magnitude of MS2 inactivation to the relative amount of TPB within the system. Assuming that viruses are completely inactivated at the TPB line, and that mass transfer at the interface is a first-order process, the following relationship (11) applies: dC/dt = (Ak/V) · C, where C is the virus concentration in solution [ML−3], t is time, k is the mass transfer coefficient [LT−1], V is the volume of solution [L3], and A is the interface through which the mass transfer occurs [L2]. For the TPB, this interface (A) is equal to the length of the TPB (L) times an infinitesimal thickness (Δz). The effective virus inactivation rate (α) is then given as α = Ak/V = (L/V)Δzk. Thus, the inactivation rate is proportional to the ratio of TPB length to solution volume (L/V). Figure 4 shows experimentally determined inactivation rates plotted versus the ratio L/V. The ratio L/V was estimated by averaging the minimum and maximum TPB perimeters occurring during one revolution of the sample tube (Fig. 2). Figure 4 clearly indicates that MS2 decay in the PP tubes increases with increasing L/V and is independent of the starting concentration of virus. The nonlinearity of the relationship is due to more rapid mixing at lower solution volumes (larger void volumes).
FIG. 4.
Relationship between MS2 inactivation rate (α) and the ratio of TPB to virus solution volume (L/V). Experiments were performed with 15-, 50-, and 250-ml PP tubes. All vessels were mixed for 3 h at 7 ± 1°C. The data were fit to the exponential equation α = a(L/V)b, where a and b are, respectively, 0.7 and 0.54. To determine whether the relationship held for various concentrations as well as for a constant concentration of MS2, control blank data from Table 1 were used in conjunction with data from Table 2.
The protective effect of the TLS and GSL (Table 1) is likely due to the accumulation of clay-sized particles at the AWI (23, 43). Clay adsorption to the AWI may prohibit viruses from reaching it or alter the forces at the TPB so that MS2 inactivation is prevented. It is not clear why MS2 inactivation was negligible in the presence of OS, as the particles are too large to accumulate at the AWI. It is possible that traces of detergent remained on the sand after washing, thereby preventing MS2 inactivation (36).
φX174 is resistant to the interfacial forces which appear to cause inactivation of MS2. The reasons for this observation are as yet unclear; however, Trouwborst and coworkers (40) reported a wide range of effects from exposure to large AWIs on four bacteriophage and two animal viruses. These data, in conjunction with ours, suggest that interfacial inactivation is dependent upon the type of virus under investigation. Furthermore, a batch study investigating the adsorption of φX174 to five soils (7) did not report any bacteriophage loss in PP control tubes, supporting the evidence presented here.
Although the batch equilibrium method has sometimes been shown to be inadequate for predicting virus sorption during subsurface transport (20, 31, 36), it remains the primary method for obtaining adsorption coefficient values. In virus adsorption studies it is important to generate accurate data from both control and experimental samples. We have shown that reliable data from control samples may be difficult to acquire, particularly when working with MS2 bacteriophage in PP vessels. The loss of MS2 seen in our batch adsorption experiments with PP tubes has been documented elsewhere (6, 18, 30) and may have gone unreported in many other instances. The results presented here suggest that there are three factors which should be more closely considered before batch adsorption experiments are conducted with certain viruses. These factors include (i) the type of reactor vessel used, (ii) the type of virus under investigation, and (iii) the presence of an AWI within the batch system. Our findings may also have application to reports of greater viral inactivation during transport through unsaturated soils (31, 32), where the AWI is a significant component of the system, as opposed to saturated soils, where AWIs are generally not present.
ACKNOWLEDGMENT
This research was supported by a grant from the Kearney Foundation of Soil Science.
REFERENCES
- 1.Ackerman H-W, DuBow M S. Viruses of prokaryotes. Vol. 2. Boca Raton, Fla: CRC Press; 1987. [Google Scholar]
- 2.Adams M H. Surface inactivation of bacterial viruses and of proteins. J Gen Physiol. 1948;31:417–432. doi: 10.1085/jgp.31.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959. [Google Scholar]
- 4.Adamson A W. Physical chemistry of surfaces. 4th ed. New York, N.Y: Wiley Interscience; 1982. [Google Scholar]
- 5.Bald J G, Samuel G. Some factors affecting the inactivation rate of the virus of tomato spotted wilt. Ann Appl Biol. 1934;21:179–190. [Google Scholar]
- 6.Bradford A W. Transport of MS-2 virus through saturated soil columns. M.S. thesis. Tucson: University of Arizona; 1987. [Google Scholar]
- 7.Burge W D, Enkiri N K. Virus adsorption by five soils. J Environ Qual. 1978;7:73–76. [Google Scholar]
- 8.Campbell-Renton M L. Radiation of bacteriophage with ultraviolet light. J Pathol Bacteriol. 1937;45:237–251. [Google Scholar]
- 9.Campbell-Renton M L. Experiments on shaking bacteriophage. J Pathol Bacteriol. 1942;54:235–245. [Google Scholar]
- 10.Clendening L D. A field mass balance study of pesticide volatilization, leaching and persistence. Ph.D. thesis. Riverside: University of California; 1988. [Google Scholar]
- 11.Cussler E L. Diffusion, mass transfer in fluid systems. New York, N.Y: Cambridge University Press; 1984. [Google Scholar]
- 12.Donaldson T L, Boonstra E F, Hammond J M. Kinetics of protein denaturation at gas-liquid interfaces. J Colloid Interface Sci. 1980;74:441–450. [Google Scholar]
- 13.Gan J, Yates S R, Anderson M A, Spencer W F, Ernst F F, Yates M V. Effect of soil properties on degradation and sorption of methyl bromide in soil. Chemosphere. 1994;29:2685–2700. [Google Scholar]
- 14.Gerba C P, Yates M V, Yates S R. Quantitation of factors controlling viral and bacterial transport in the subsurface. In: Hurst C J, editor. Modeling the environmental fate of microorganisms. Washington, D.C: American Society for Microbiology; 1991. pp. 77–88. [Google Scholar]
- 15.Goyal S M, Gerba C P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979;38:241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal S M, Keswick B H, Gerba C P. Viruses in groundwater beneath sewage irrigated cropland. Water Res. 1984;18:299–302. [Google Scholar]
- 17.Grant S B, List E J, Lidstrom M E. Kinetic analysis of virus adsorption and inactivation in batch experiments. Water Resour Res. 1993;29:2067–2085. [Google Scholar]
- 18.Grondin G H. Transport of MS-2 and f2 bacteriophage through saturated Tanque Verde Wash soil. M.A. thesis. Tucson: University of Arizona; 1987. [Google Scholar]
- 19.James L K, Augenstein L G. Adsorption of enzymes at interfaces: film formation and the effect on activity. Adv Enzymol. 1966;28:1–40. doi: 10.1002/9780470122730.ch1. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Yates M V, Thompson S S, Jury W A. Sorption of viruses during flow through saturated sand columns. Environ Sci Technol. 1997;31:548–555. [Google Scholar]
- 21.Keswick B H, Gerba C P. Viruses in groundwater. Environ Sci Technol. 1980;14:1290–1297. [Google Scholar]
- 22.Lee C Y, McCammon J A, Rossky P J. The structure of liquid water at an extended hydrophobic surface. J Chem Phys. 1984;80:4448–4455. [Google Scholar]
- 23.Leja J. Surface chemistry of froth flotation. New York, N.Y: Plenum Press; 1982. [Google Scholar]
- 24.Loveland J P, Ryan J N, Amy G L, Harvey R W. The reversibility of virus attachment to mineral surfaces. Colloids Surf A. 1996;107:205–221. [Google Scholar]
- 25.MacRitchie F. Air/water interface studies of proteins. Anal Chim Acta. 1991;249:241–245. [Google Scholar]
- 26.McLimans W F. The inactivation of equine encephalitis virus by mechanical agitation. J Immunol. 1947;56:385–391. [PubMed] [Google Scholar]
- 27.Mix T W. The physical chemistry of membrane-virus interactions. Dev Ind Microbiol. 1974;15:136–142. [Google Scholar]
- 28.Moore R S, Taylor D H, Sturman L S, Reddy M M, Fuhs G W. Poliovirus adsorption by 34 minerals and soils. Appl Environ Microbiol. 1981;42:963–975. doi: 10.1128/aem.42.6.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore R S, Taylor D H, Reddy M M, Sturman L S. Adsorption of reovirus by minerals and soils. Appl Environ Microbiol. 1982;44:852–859. doi: 10.1128/aem.44.4.852-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poletika N N, Jury W A, Yates M V. Transport of bromide, simazine, and MS-2 coliphage in a lysimeter containing undisturbed, unsaturated soil. Water Resour Res. 1995;31:801–810. [Google Scholar]
- 31.Powelson D K, Gerba C P. Virus removal from sewage effluents during saturated and unsaturated flow through soil columns. Water Res. 1994;28:2175–2181. [Google Scholar]
- 32.Powelson D K, Gerba C P. Fate and transport of microorganisms in the vadose zone. In: Wilson L G, Everett L G, Cullen S J, editors. Handbook of vadose zone characterization and monitoring. Boca Raton, Fla: CRC Press, Inc.; 1995. pp. 123–125. [Google Scholar]
- 33.Song K B, Damodaran S. Influence of electrostatic forces on the adsorption of succinylated β-lactoglobulin at the air-water interface. Langmuir. 1991;7:2737–2742. [Google Scholar]
- 34.Strauss J H, Jr, Sinsheimer R L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- 35.Stumm W, Morgan J J. Aquatic chemistry. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 36.Thompson S S. An integrative study on the transport and fate of wastewater microorganisms in the subsurface and the effects of air-water-solid interfacial forces on the fate of bacterial viruses in dynamic aqueous batch systems. Ph.D. thesis. Riverside: University of California; 1997. [Google Scholar]
- 37.Trouwborst T, de Jong J C, Winkler K C. Mechanism of inactivation in aerosols of bacteriophage T1. J Gen Virol. 1972;15:235–242. doi: 10.1099/0022-1317-15-3-235. [DOI] [PubMed] [Google Scholar]
- 38.Trouwborst T, Winkler K C. Protection against aerosol-inactivation of bacteriophage T1 by peptides and amino acids. J Gen Virol. 1972;17:1–11. doi: 10.1099/0022-1317-17-1-1. [DOI] [PubMed] [Google Scholar]
- 39.Trouwborst T, de Jong J C. Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Appl Microbiol. 1973;26:252–257. doi: 10.1128/am.26.3.252-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trouwborst T, Kuyper S, de Jong J C, Plantinga A D. Inactivation of some bacterial and animal viruses by exposure to liquid-air interfaces. J Gen Virol. 1974;24:155–165. doi: 10.1099/0022-1317-24-1-155. [DOI] [PubMed] [Google Scholar]
- 41.Vaughn J M, Landry E F, Baranosky L J, Beckwith C A, Dahl M C, Delihas N C. Survey of human virus occurrence in wastewater-recharged groundwater on Long Island. Appl Environ Microbiol. 1978;36:47–51. doi: 10.1128/aem.36.1.47-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilker V L, Fong J C, Seyyed-Hoseyni M. Poliovirus adsorption to narrow particle size fractions of sand and montmorillonite clay. J Colloid Interface Sci. 1983;92:422–435. [Google Scholar]
- 43.Wan J, Wilson J L. Colloid transport and the gas-water interface in porous media. In: Sabatini D A, Knox R C, editors. Transport and remediation of subsurface contaminants. Washington, D.C: American Chemical Society; 1992. pp. 55–70. [Google Scholar]
- 44.Wan J, Wilson J L. Visualization of the role of the gas-water interface on the fate and transport of colloids in porous media. Water Resour Res. 1994;30:11–23. [Google Scholar]
- 45.Williams D F, Berg J C. The aggregation of colloidal particles at the air-water interface. J Colloid Interface Sci. 1992;152:218–229. [Google Scholar]
- 46.Yates M V, Yates S R. Modeling microbial fate in the subsurface environment. Crit Rev Environ Control. 1988;17:307–344. [Google Scholar]
- 47.Zerda K S. Adsorption of viruses to charge-modified silica. Ph.D. thesis. Houston, Tex: Baylor College of Medicine; 1982. [Google Scholar]