Abstract
A strain of the aerobic anoxygenic photosynthetic bacteria was isolated from a deep-ocean hydrothermal vent plume environment. The in vivo absorption spectra of cells indicate the presence of bacteriochlorophyll a incorporated into light-harvesting complex I and a reaction center. The general morphological and physiological characteristics of this new isolate are described.
Deep-ocean hydrothermal volcanic vents, including so-called black smokers, were first discovered near the Galapagos Island (2) and later were found at Atlantic, Indian, and other Pacific ocean sites. The initial reports of these vents were followed by discoveries of unusual microbial and invertebrate populations associated with vent environments (5, 13–15, 18, 30). High-temperature vents have been proposed as models of ecosystems developed on the early Archean Earth (11).
The documentation of geothermal light at otherwise dark deep-sea ecosystems led to the suggestion that geothermally driven photosynthesis could exist on the seafloor (17, 28, 29). Nisbet et al. proposed that light emitted at deep-sea vents may have provided the selective force for the evolution of photosynthesis, through initial development of phototaxis toward geothermal light followed by the evolution of rudimentary photosynthesis (19). Sulfide flanges on the Endeavour Segment of the Juan de Fuca Ridge were recognized as a habitat that fulfills the requirements of photosynthetic bacteria because of geothermally illuminated stratified microenvironments of mineral precipitation that promote microbial growth (8, 17). If photosynthetic bacteria were to be found at deep-sea hydrothermal vents, their attributes could provide insight into the evolution and diversity of photosynthesis. The idea that photosynthesis may have originated at deep-sea hydrothermal vents and then dispersed to favorable refuges in shallow-water habitats raises the possibility that if phototrophs exist at vents they may exhibit physiological properties and have genetic compositions that could enhance our understanding of the evolution of primitive phototrophs.
We used water samples obtained in August, 1996, from vent plumes on the Juan de Fuca Ridge (northeastern Pacific Ocean; ca. 47°57′N, 129°05′W; 2,000 m beneath the ocean surface) to isolate bacteria containing photosynthetic pigments such as bacteriochlorophylls (Bchls) and carotenoids. Descriptions of the samples, which were obtained from the vicinity of nonbuoyant regions of plumes emitted from hydrothermal vents, are given in Table 1. After their recovery on board ship, samples were immediately transferred into sterile screw-cap bottles and placed in dark storage at 4°C. In the laboratory, decimal dilutions of the samples were prepared in sterile media. Thereafter, 0.1 ml of each dilution was spread in duplicate on agar plates of a medium previously used for the isolation of marine aerobic photosynthetic bacteria (36), containing the following (in grams per liter): acetate, 1; yeast extract, 1; Bacto Peptone, 1; agar, 20. The medium also contained 1 ml of a trace element solution (4) per liter and 20 μg of vitamin B12 per liter and was obtained at pH 7.8 to 8.0. Plates were incubated aerobically at room temperature in the dark for 5 days and, after the identification of colonies, were incubated at 30°C for 5 days. The number and pigmentation of the colonies obtained are shown in Table 1.
TABLE 1.
Abundances of various aerobic bacteria cultivated from samples obtained from the vicinity of vent plumes at the Juan de Fuca Ridge
| Samplea | Abundance (cells/ml) of:
|
Location of sample site (lat, long, depth [m])b | Additional information | ||
|---|---|---|---|---|---|
| Noncolored cells | Pigmented cells (color) | Bchl a-containing cells (color) | |||
| 1 | 100 | 20 (yellow) | 0 | 47°57′N, 129°05′W, 2,170 | Main Endeavour Field; 25 m below bottom of nonbuoyant region of vent plume |
| 2 | 250 | 50 (yellow) | 20 (yellow) | 47°57′N, 129°05′W, 1,978 | Main Endeavour Field; 50 m below top of nonbuoyant region of vent plum |
| 3 | 300 | 20 (pink); 80 (yellow) | 40 (yellow) | 47°57′N, 129°05′W, 1,762 | Main Endeavour Field; 170 m above top of nonbuoyant region of vent plume |
| 4 | 200 | 40 (yellow) | 0 | 47°56′N, 129°07′W, 1,864 | Mothra Field (3); 65 m above top of nonbuoyant region of vent plume |
| 5 | 170 | 0 | 0 | 47°56′N, 129°06′W, 2,154 | Near the Mothra Field; 20 m above bottom of light-scattering region |
Samples were obtained from Niskin bottles in vertical sampling casts of sensors of conductivity, temperature, light scattering, and depth at the designated locations. For samples 1 to 3, the nonbuoyant regions of the vent plume extended from 1,925 to 2,150 m of depth. For sample 4, the region of the plume extended from 1,930 to 2,150 m. For sample 5, a distinct plume was not detected, although moderate light scattering was found from 1,950 to 2,175 m of depth.
lat, latitude; long, longitude.
All samples analyzed gave rise to many noncolored colonies (representative of 100 to 300 cells/ml), about 30% of which demonstrated exoagarase activity resulting in agar hydrolysis. Cells from pigmented colonies were suspended in a liquid medium identical in composition to the plates, and pure cultures were obtained by repeated plating. Purified cells from pigmented colonies were used for absorption spectrum analysis in the region of 350 to 1,100 nm to evaluate the presence of Bchls and carotenoids. Most of the pigmented isolates contained only carotenoids, but a Bchl was detected in about 30% of the pigmented strains, all of which were yellow in color (Table 1).
The capability for anaerobic photosynthetic growth (with tungsten filament lamp illumination levels of 10, 20, and 100 microeinsteins/m2/s) of aerobically isolated Bchl-containing strains was tested in completely filled screw-cap test tubes and in agar (1%) deeps by using media for purple sulfur (H2S or Na2S2O3 and CO2 with or without acetate) or nonsulfur (acetate, malate, or succinate) bacteria (12). None of the strains tested grew anaerobically in the light in these media.
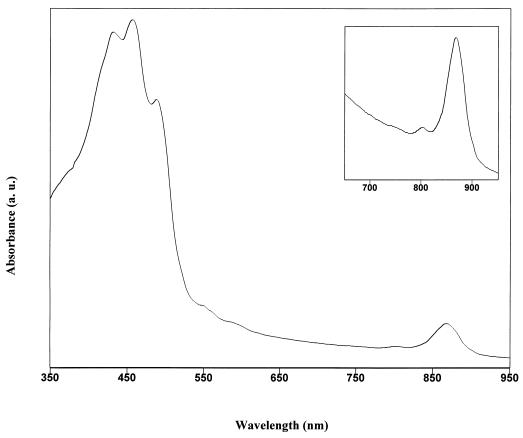
The in vivo absorption spectra of the yellow-pigmented isolates (Fig. 1) gave a major peak at 867 nm, indicating the presence of Bchl a incorporated into light-harvesting complex I (LHI), and the small peak at 800 nm indicates the presence of the photosynthetic reaction center (RC) (1). Although the presence of LHI and the RC is established by these in vivo spectra, the total number of photosynthetic units per cell is small compared to the numbers of units determined from the spectra of (facultatively) anaerobic purple bacteria. Cells contained 0.4 to 0.6 nmol of Bchl a per mg of protein. The yellow color of cells and the three peaks at 433, 457, and 487 nm indicate the presence of carotenoids, apparently of the carotene type (Fig. 1) (32). The ratio of the absorbance at the LHI Bchl a absorption peak to that at the main carotenoid absorption peak is about 1:8 (ratio of absorbance at 867 nm to absorbance at 457 nm).
FIG. 1.
In vivo absorption spectrum of representative yellow-pigmented strain JF-1 intact cells showing carotenoid and Bchl peaks. Inset: enlarged 650-to-950-nm region of the spectrum demonstrating the presence of the photosynthetic RC (small peak at 800 nm) and LHI (major peak at 867 nm).
The presence of Bchl a and carotenoids, the inability to grow anaerobically in the light, the small number of photosynthetic units, and the abundance of carotenoids indicate that the yellow-pigmented bacterium is a member of the aerobic anoxygenic photosynthetic bacteria (25).
The aerobic anoxygenic photosynthetic bacteria are phylogenetically diverse within the α subclass of the Proteobacteria and comprise a variety of species with different morphologies and carotenoid pigments, at present classified into the genera Erythrobacter, Erythromicrobium, Roseobacter, Roseococcus, Porphyrobacter, and Acidiphilium (6, 21, 23, 31, 35). These bacteria have been found at several geographic sites in different ecological niches, and Erythromicrobium and Roseococcus species are associated with hydrothermal spring communities (6, 22, 24, 31, 33, 36).
The bacteria we describe here are the first species containing photosynthetic pigments that have been recovered from deep-ocean hydrothermal-vent plume waters. The absence of pigmented strains containing Bchl a at Mothra Field and in sample 1 (Table 1) indicates that sites 2 and 3 of the Main Endeavour Field are ecological niches of these bacteria. Furthermore, the absence of strain JF-1 from marine surface samples that were collected throughout the world and that contained a variety of aerobic photosynthetic bacteria (21–25, 36) indicates that JF-1 is not a common inhabitant of the marine environment and thus is unlikely to have sedimented from the surface.
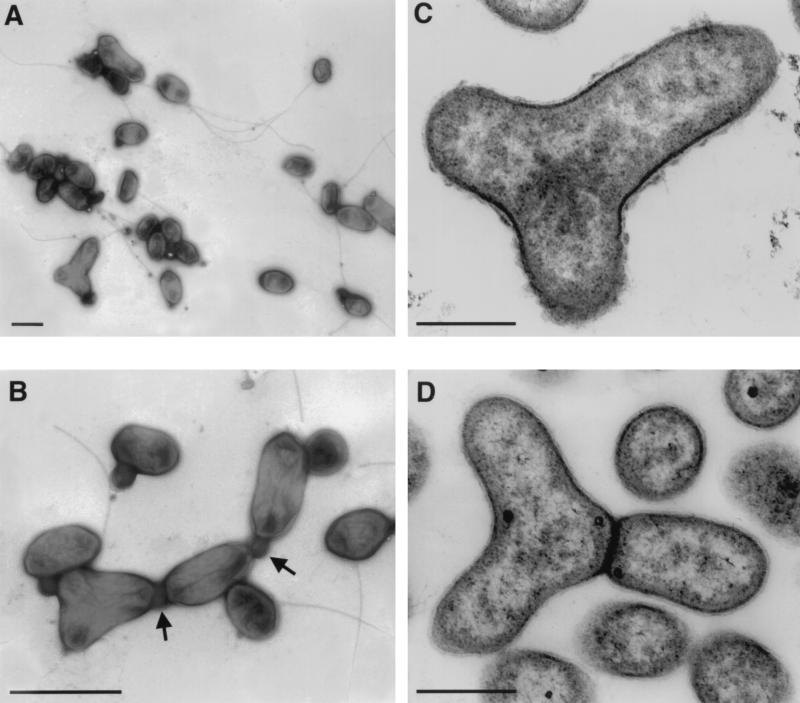
The representative strain, JF-1, is citron-yellow colored and unusually polymorphic (Fig. 2). Depending on the age of cultures in liquid medium, cells are coccoid to ovoid rods, often forming so-called Y cells, which is rare for bacteria. Coccoid cells from young cultures are motile by one polar or subpolar flagellum. This microorganism is unusually variable in its means of multiplication. Budding, ternary fission, binary division, and symmetric and asymmetric constrictions of cell division were found. Cells often remained attached after division, apparently by means of a connective material of unknown nature, and surrounded by a membrane (Fig. 2). Therefore, individual cells would remain in contact after division within a free-floating population.
FIG. 2.
Electron micrographs of negatively stained (A and B) and thin-sectioned (C and D) cells of strain JF-1. (A) Cell groups demonstrating polymorphism and the presence of flagella. (B) Cells of different morphology are connected by an unknown material (indicated by arrows). (C) A Y cell preceding division to form three daughter cells. The nucleoid is seen as light zones of the section, distributed in three directions. (D) A later stage of Y-cell division. One daughter cell is separated by the cell wall from two as yet unseparated nascent cells. Bars: A, 1 μm; B, 400 nm; C and D, 200 nm.
The Gram stain determination (9) and the appearance of cells in electron microscopic thin sections (16) showed that strain JF-1 has a gram-negative cell wall. The cytoplasmic membrane was visible, but no obvious intracytoplasmic membranes (ICM) were detected (Fig. 2). The absence of an extensive ICM system is a common trait of aerobic anoxygenic photosynthetic bacteria, in contrast to (facultatively) anaerobic anoxygenic purple photosynthetic bacteria (10, 35). Thus, the photosynthetic apparatus of JF-1 is probably located in the cytoplasmic membrane.
Because of the high degree of phenotypic similarity, six individually purified strains are assumed to be representatives of the same species, although at present no rigorous conclusions can be drawn with respect to their taxonomic status and phylogenetic relationships. However, the sequences of two segments (about nucleotides 110 to 570 and 970 to 1390) of the 16S rDNA place JF-1 within the α subclass of the Proteobacteria (26) (data not shown). Some determinative characteristics of strain JF-1 in comparison to other aerobic photosynthetic bacteria that contain yellow carotenoids are presented in Table 2.
TABLE 2.
Some determinative characteristics of strain JF-1 and other aerobic anoxygenic photosynthetic bacteria that contain yellow carotenoidsa
| Strain or genus | Environment | Cell shape and size (μm) | Color | In vivo peaks (nm)
|
Sensitivityb to:
|
DNA G+C content (mol %) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carotenoid | Bchl | Peni- cillin | Tetra- cycline | Strepto- mycin | Chloram- phenicol | |||||
| JF-1 | Deep-sea hydrothermal vent plume water | Pleomorphic; 0.4–0.5 by 1.0–1.2 | Citron-yellow | 433, 457, 487 | 800, 867 | − | + | − | + | 67.5 |
| Erythrobacter | Marine surface | Rods; 0.4–0.5 by 1.0–5.0 | Orange | 470 | 800, 869 | + | + | − | + | 60–64 |
| Sandaracinobacter | Freshwater | Thin, long rods; 0.3–0.5 by 1.5–2.5 | Yellow-orange | 424, 450, 474 | 800, 867 | − | − | + | + | 68.5 |
| Erythromonas | Freshwater | Ovoid; 0.8–1.0 by 1.3–2.6 | Orange-brown | 430, 458, 485 | 800, 867 | − | + | − | + | 65.4 |
| Erythromicrobium | Freshwater | Branched rods; 0.7–1.0 by 1.6–2.5 | Red-orange | 466, 478 | 798, 832, 868 | − | − | + | + | 64.2 |
| Porphyrobacter | Freshwater | Pleomorphic; 0.4–0.8 by 1.1–2.0 | Orange-red | 464, 491 | 799, 869 | + | ND | − | + | 65–66 |
Aerobic anoxygenic photosynthetic bacteria are thought of as atypical photosynthetic microorganisms in contrast to purple sulfur or nonsulfur bacteria, which can utilize light as the sole source of energy for anaerobic growth. Nevertheless, photosynthetic activity in these bacteria was shown by (i) the reversible photooxidation of cytochromes and the reversible photobleaching of Bchl in the RC; (ii) photoinhibition of respiration; and (iii) a light-stimulated increase in ATP pools and an increase in growth rate and biomass production (7, 20, 27, 34). The contribution of light to energy metabolism in aerobic photosynthetic bacteria seems to be small and was best evinced during an alternating light-and-dark regimen of cultivation, and so light seems to be used as a supplementary source of energy during intermittent illumination of these bacteria (37).
Our discovery of aerobic anoxygenic photosynthetic bacteria in a deep-ocean hydrothermal vent environment raises broad possibilities for future investigations. Although we cannot say whether JF-1 evolved in proximity to a hydrothermal vent or came to colonize this environment later, the abundance (approaching 10% of all cells cultivated on the media used; see Table 1) of this bacterium in plume samples indicates that the ability to produce Bchl may be of selective advantage. It will be interesting to see if it is possible to differentiate between the evolution of photosynthesis at deep hydrothermal vents and that in sun-irradiated environments. Perhaps, isolate JF-1 exhibits some photosynthetic properties of evolutionarily early photosynthetic bacteria and the respiratory dependence of this species developed during subsequent evolution.
Acknowledgments
We thank K. Juniper (Université du Québec á Montreal) for ship time to obtain samples, S. Delcardayre (University of British Columbia) for sample collection and preliminary 16S rDNA analysis, and D. Kelley (University of Washington) for assistance in sample characterization.
REFERENCES
- 1.Aagaard J, Sistrom W R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972;15:209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- 2.Corlis J B, Dymond J, Gordon L, Edmond J, von Herzen R D, Green J, Williams D, Crane K, van Adel J H. Submarine thermal springs on the Galapagos Rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 3.Delaney J R, Lilley M D, McDurf R E, Kelley D S, Wilcock W S, Robigou V. Cellular hydrothermal circulation in a submarine system. EOS. 1996;77:756. . (American Geophysical Union Abstracts.) [Google Scholar]
- 4.Drews G. Mikrobiologisches Praktikum. Berlin, Germany: Springer Verlag; 1983. [Google Scholar]
- 5.Felbeck H. Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachiptila (Vestimentifera) Science. 1981;213:336–338. doi: 10.1126/science.213.4505.336. [DOI] [PubMed] [Google Scholar]
- 6.Fuerst J A, Hawkins J A, Holmes A, Sly L I, Moore C J, Stackebrandt E. Porphyrobacter neustonensis gen. nov., sp. nov., an aerobic bacteriochlorophyll-synthesizing budding bacterium from freshwater. Int J Syst Bacteriol. 1993;43:125–134. doi: 10.1099/00207713-43-1-125. [DOI] [PubMed] [Google Scholar]
- 7.Garcia D, Richaud P, Breton J, Vermeglio A. Structure and function of the tetraheme cytochrome associated to the reaction centers of Roseobacter denitrificans. Biochimie. 1994;76:666–673. doi: 10.1016/0300-9084(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 8.Goldfarb M S, Delaney J R. Layering in hydrothermal flanges, Endeavour Segment, Juan de Fuca Ridge. EOS. 1989;70:1163. [Google Scholar]
- 9.Gregersen T. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur J Appl Microbiol Biotechnol. 1978;5:123–127. [Google Scholar]
- 10.Harashima K, Nakagava M, Murata N. Photochemical activity of bacteriochlorophyll in aerobically grown cells of heterotrophs, Erythrobacter species (OCh114) and Erythrobacter longus (OCh101) Plant Cell Physiol. 1982;23:185–193. [Google Scholar]
- 11.Holm N G. Why are hydrothermal systems proposed as plausible environments for the origin of life? In: Holm N G, editor. Marine hydrothermal systems and the origin of life. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 5–14. [Google Scholar]
- 12.Imhoff J F. Anoxygenic phototrophic bacteria. In: Austin B, editor. Methods in aquatic bacteriology. New York, N.Y: John Wiley & Sons Ltd., Inc.; 1988. pp. 207–240. [Google Scholar]
- 13.Jannasch H W, Wirsen C O. Chemosynthetic primary production at East Pacific sea floor spreading centers. Bioscience. 1979;29:592–598. [Google Scholar]
- 14.Jannasch H W, Wirsen C O. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981;41:528–538. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karl D M, Wirsen C O, Jannasch H W. Deep-sea primary production at the Galapagos hydrothermal vents. Science. 1980;207:1345–1347. [Google Scholar]
- 16.Kellenberger E, Ryter A, Sechaud J. Electron microscope study of DNA-containing plasms. J Biophys Biochem Cytol. 1958;4:671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LITE Workshop Participants. RIDGE program report. Woods Hole, Mass: NSF and NASA; 1994. Light in thermal environments; p. 44. [Google Scholar]
- 18.Lonsdale P. Clustering of suspension-feeding macrobentos near hydrothermal vents at oceanic spreading centers. Deep Sea Res. 1977;24:857–863. [Google Scholar]
- 19.Nisbet E G, Cann J R, van Dover C L. Origins of photosynthesis. Nature (London) 1995;373:479–480. [Google Scholar]
- 20.Okamura K, Mitsuori F, Ito O, Takamiya K, Nishimura M. Photophosphorylation and oxidative phosphorylation in intact cells and chromatophores of an aerobic photosynthetic bacterium, Erythrobacter sp. strain OCh114. J Bacteriol. 1986;168:1142–1146. doi: 10.1128/jb.168.3.1142-1146.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiba T. Roseobacter litoralis gen. nov., sp. nov. and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. System Appl Microbiol. 1991;14:140–145. [Google Scholar]
- 22.Shiba T, Shioi Y, Takamiya K I, Sutton D C, Wilkinson C R. Distribution and physiology of aerobic bacteria containing bacteriochlorophyll a on the east and west coasts of Australia. Appl Environ Microbiol. 1991;57:295–300. doi: 10.1128/aem.57.1.295-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiba T, Simidu U. Erythrobacter longus gen. nov., sp. nov., an aerobic bacterium which contains bacteriochlorophyll a. Int J Syst Bacteriol. 1982;32:211–217. [Google Scholar]
- 24.Shiba T, Simidu U, Taga N. Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl Environ Microbiol. 1979;38:43–45. doi: 10.1128/aem.38.1.43-45.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada K. Aerobic anoxygenic phototrophs. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 105–122. [Google Scholar]
- 26.Stackebrandt E, Rainey F A, Ward-Rainey N. Anoxygenic phototrophy across the phylogenetic spectrum: current understanding and future perspectives. Arch Microbiol. 1996;166:211–223. doi: 10.1007/s002030050377. [DOI] [PubMed] [Google Scholar]
- 27.Takamiya K, Okamura K. Photochemical activities and photosynthetic ATP formation in membrane preparation from a facultative methylotroph, Protaminobacter ruber strain NR-1. Arch Microbiol. 1984;140:21–26. [Google Scholar]
- 28.Van Dover C L, Cann J R, Cavanaugh C, Chamberlain S, Delaney J R, Janecky D, Imhoff J, Tyson J A the LITE Workshop Participants. Light at deep sea hydrothermal vents. EOS. 1994;75:44–45. [Google Scholar]
- 29.Van Dover C L, Reynolds G T, Chave A D, Tyson J A. Light at deep sea hydrothermal vents. Geophys Res Lett. 1996;23:2049–2052. [Google Scholar]
- 30.Van Dover C L, Szuts E, Chamberlain S C, Cann J R. A novel eye in “eyeless” shrimp from hydrothermal vents of the Mid-Atlantic Ridge. Nature (London) 1989;337:458–460. doi: 10.1038/337458a0. [DOI] [PubMed] [Google Scholar]
- 31.Wakao N, Shiba T, Hiraishi A, Ito M, Sakurai Y. Distribution of bacteriochlorophyll a in species of the genus Acidiphilium. Curr Microbiol. 1993;27:277–279. [Google Scholar]
- 32.Yurkov V, Gad’on N, Drews G. The major part of polar carotenoids of the aerobic bacteria Roseococcus thiosulfatophilus, RB3 and Erythromicrobium ramosum, E5 is not bound to the bacteriochlorophyll a complexes of the photosynthetic apparatus. Arch Microbiol. 1993;160:372–376. [Google Scholar]
- 33.Yurkov V, Gorlenko V M. Ecophysiological peculiarities of phototrophic microbial communities of Bolsherechensky thermal springs. Microbiology (New York) 1992;61:115–122. [Google Scholar]
- 34.Yurkov V, Schoepp B, Vermeglio A. Electron transfer carriers in obligately aerobic photosynthetic bacteria from genera Roseococcus and Erythromicrobium. In: Mathis P, editor. Photosynthesis: from light to biosphere. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 543–546. [Google Scholar]
- 35.Yurkov V, Stackebrandt E, Holmes A, Fuerst J A, Hugenholtz P, Golecki J, Gad’on N, Gorlenko V M, Kompantseva E I, Drews G. Phylogenetic positions of novel aerobic, bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int J Syst Bacteriol. 1994;44:427–434. doi: 10.1099/00207713-44-3-427. [DOI] [PubMed] [Google Scholar]
- 36.Yurkov V, van Gemerden H. Abundance and salt tolerance of obligately aerobic, phototrophic bacteria in a microbial mat. Neth J Sea Res. 1993;31:57–62. [Google Scholar]
- 37.Yurkov V, van Gemerden H. Impact of light/dark regime on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch Microbiol. 1993;159:84–89. [Google Scholar]