Abstract
In this study, we showed that the cell wall anchor of protein A from Staphylococcus aureus is functional in the food-grade organism Lactococcus lactis. A fusion protein composed of the lactococcal Usp45 secretion signal peptide, streptavidin monomer, and the S. aureus protein A anchor became covalently attached to the peptidoglycan when expressed in L. lactis. The streptavidin moiety of the fusion protein was functionally exposed at the cellular surface. L. lactis cells expressing the anchored fusion polypeptide could be specifically immobilized on a biotinylated alkaline phosphatase-coated polystyrene support.
Cell wall sorting of surface proteins in gram-positive bacteria has been attributed to the presence of a particular carboxy-terminal protein domain composed of an LPXTG motif, a hydrophobic core of approximately 30 amino acid residues, and a positively charged (Arg- or Lys-rich) tail (3, 18, 19). The mechanism and the chemical nature of the coupling have been described previously (13, 17) and involve a proteolytic cleavage of the polypeptide after the threonine residue in the LPXTG motif followed by covalent linking to the pentaglycine peptide in the peptidoglycan layer of Staphylococcus aureus. The LPXTG motif and overall structure of the anchor have been identified in several surface-associated proteins in gram-positive organisms (4, 11, 12, 18, 20, 22, 25). Also, the PrtP anchor has been used for the attachment of heterologous proteins to the cell wall (27). Wells et al. (27) described a potential use of surface anchoring by demonstrating the enhancement of the immune response to a heterologous antigen in vaccination vectors. Other possible applications reside in the immobilization of enzymes at the bacterial surface, the development of enzyme-coated microspheres, and the immobilization of productory microorganisms at ligand-coated surfaces.
An enzymatic activity termed “sortase” has been postulated to be involved in coupling and cell wall localization (17). It is now generally accepted that the mechanism of sorting in gram-positive organisms is highly conserved and may have evolved through gene copying and lateral transfer of genetic information (4). It has also been shown that surface-localized protein domains can be grafted on the S. aureus protein A (SPA) anchor and be efficiently sorted to the S. aureus cell wall (18). The SPA anchor sequence has been used successfully in the attachment of chimeric proteins, consisting in part of a single-chain antibody fragment and the anchor, to the cell wall of Staphylococcus xylosus and Staphylococcus carnosus (5). Here we show that this anchor structure retains its sorting capacity for recombinant molecules when used in a different genus of gram-positive bacteria. We made use of the SPA anchor to achieve functional exposition of streptavidin (SA) at the surface of the food-grade organism Lactococcus lactis. We chose to use SA as a tool for the detection and demonstration of surface anchoring. However, the described constructs could be used to immobilize L. lactis at solid surfaces for production purposes.
Expression vectors for surface display.
By PCR amplification (15), we cloned the SPA anchor from S. aureus Cowan I (NCTC 8530) from genomic DNA isolated by the method of Marmur (10). PCR amplification was performed with Vent DNA polymerase (New England Biolabs, Beverly, Mass.) and the oligonucleotides 5′-GCTCAGGATCCAAAAGAGGAAGACAACAACAAGCC-3′ and 5′-CCGCGTCTAGATATCTATCGTTGTGTATTGTTTGTTTTTATAGTTCGCG-3′. The oligonucleotides were designed to introduce a BamHI and an XbaI restriction site 5′ and 3′ from the SPA anchor, respectively. The sequence of the subcloned 621-bp PCR fragment was determined by the dye terminator method and was found to be identical to the SPA anchor sequence reported earlier (21).
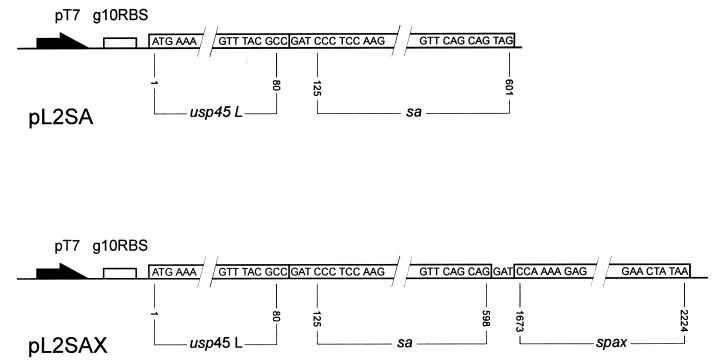
Several intermediate plasmid constructs were used to arrive at the structures of plasmids pL2SA and pL2SAX, depicted in Fig. 1. Primary transformants were obtained by the CaCl2 method in Escherichia coli MC1022 [araD139 Δ(ara leu)7697 Δ(lacZ)M15 galU galK rpsL] (2). For reasons which are unclear, plasmids carrying the pSH71 replicon (8) suffered rearrangements when transformed into the more commonly used isogenic strain MC1061 (same genotype as above except for Δ(lac)X74 and hsdR2) (2). Plasmid pL2SA contains the coding sequence for mature SA fused precisely to the sequence encoding the last amino acid of the Usp45 signal peptide (26), as present in pLET2N (24). In plasmid pL2SAX, the termination codon was changed to a sense codon by site-directed mutagenesis. The resulting BamHI site was joined to the BamHI site of the SPA fragment present in a pLET2N-type vector. The sequences at the several junction points are shown in Fig. 1.
FIG. 1.
Schematic overview of the described expression units for SA (pL2SA) and the SA-SPA anchor (pL2SAX) fusion protein. The genes are preceded by the phage T7 gene 10 promoter (pT7) and ribosome binding site (g10RBS). Sequence numbering: usp45, as in reference 26; streptavidin (sa), as in reference 1; protein A anchor (spax), as in reference 21
The expression plasmids pL2SA and pL2SAX were introduced by electroporation (28) into the expression strain MG1820(pILPOL) (29). Conditions for growth and induction were as described previously (24). Essentially, cells were pregrown at 30°C in GM17S, which is M17 (Difco) containing (per liter) 5 g of glucose, 5 mg of chloramphenicol, and 5 mg of erythromycin. At an optical density at 600 nm of 0.5, the cells were collected by centrifugation and resuspended in LM9S, which contains (per liter) 6 g of Na2HPO4, 3 g of KH2PO4, 1 g of NH4Cl, 0.5 g of NaCl, 2 mmol of MgSO4, 25 mmol of NaHCO3, 25 mmol of Na2CO3, 0.1 mmol of CaCl2, 5 g of lactose, 5 g of Casitone (Difco), 5 mg of chloramphenicol, and 5 mg of erythromycin. The cells were harvested in mid-log phase at an optical density at 600 nm of 1.5. The plasmid pILPOL carries a lac expression cassette driving the T7 RNA polymerase which is repressed in the absence of lactose by the LacR protein. In this system, the expression plasmids pL2SA and pL2SAX directed the inducible synthesis of polypeptides, reactive with rabbit antiserum to SA (R-α-SA), with apparent molecular masses of 17 and 32 kDa, respectively, which is in good agreement with the calculated values (Fig. 2).
FIG. 2.
Expression and fractionation of recombinant SA proteins synthesized from pL2SA and pL2SAX in L. lactis MG1820(pILPOL). Proteins were separated by SDS–15% PAGE and revealed by immunoblotting with rabbit anti-SA antiserum. Molecular mass markers were low-range prestained markers (Bio-Rad, Hercules, Calif.). Fractions represent proteins present in the growth medium, proteins released by lysostaphin treatment, and proteins present in the residue after that treatment. Proteins released by boiling of intact cells in Laemmli cracking buffer are presented in the lanes labeled “cracking released.” Arrowhead indicate discrete protein bands of relevant sizes corresponding to SA and SAX derivatives.
Cellular location of the expressed polypeptides.
The cells from 10-ml induced cultures were collected by low-speed centrifugation. The supernatant was further centrifuged at 100,000 × g for 1 h. This supernatant was extracted with equilibrated phenol. The proteins were precipitated from the phenol phase at −20°C by the addition of 2.5 volumes of ethanol. The precipitate was resuspended in sample buffer and prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The collected cells were washed three times with a Tris-buffered saline solution (20 mM Tris-HCl, pH 7.5; 150 mM NaCl) (TBS) and resuspended in 250 μl of a 10% sucrose solution in 20 mM Tris-HCl, pH 7.5. After the addition of 0.6 mg of lysostaphin (Sigma, St. Louis, Mo.), the cell suspension was incubated at 37°C for 1 h and centrifuged at low speed. The supernatant was called the lysostaphin-released protein fraction. The remaining pellet was boiled in SDS-PAGE sample buffer. This fraction was called the residue. Quantities equivalent to 1 ml of culture were separated by SDS-PAGE, and the protein bands were revealed by standard immunoblotting (Western blotting) procedures (Fig. 2).
SA was efficiently secreted and accumulated in the growth medium of MG1820(pILPOL, pL2SA). In contrast, no proteins reacting with R-α-SA could be observed in the growth medium of MG1820(pILPOL, pL2SAX). This finding indicates that the SAX (SA-SPA anchor) fusion protein was anchored in the cell wall of L. lactis. To further strengthen this observation, washed cells were treated with lysostaphin. This enzyme has been shown to release proteins which are linked to the pentaglycine peptide in the staphylococcal peptidoglycan (18). We found that this hydrolase could also act on the L. lactis peptidoglycan, although its activity was strongly dependent on pretreatment of the cells with 150 mM NaCl. Induced MG1820(pILPOL, pL2SA) released only a slight amount of SA after this treatment. This material is likely to represent either a fraction in transit or molecules nonspecifically trapped in the cell wall. MG1820(pILPOL, pL2SAX) cells, on the other hand, released a major part of their R-α-SA-reactive protein. Residue fractions from MG1820(pILPOL, pL2SA) showed no traces of R-α-SA-reactive polypeptides. A major band, equal in size to the lysostaphin-released protein, was detected in the residue of the induced MG1820(pILPOL, pL2SAX) culture. This probably represented proteins which had not diffused out of the cell wall during lysostaphin digestion. After being boiled in SDS-PAGE sample buffer, induced MG1820(pILPOL, pL2SAX) cells released an R-α-SA-reactive polypeptide with an apparent molecular mass slightly larger than that of the protein found in the fractions discussed above. We expect these to be non-carboxy-terminally truncated, and therefore not covalently attached, SAX molecules which are physiologically relevant intermediates in sorting (14). The bulk of the R-α-SA-reactive proteins, i.e., the sum of the lysostaphin-released and residue fractions, was not released by boiling in SDS, indicating a very firm attachment of the SAX fusion protein to the cell wall of L. lactis. The coupling of the SPA anchor to the S. aureus peptidoglycan occurs at the pentaglycine moiety after the threonine in an LPETG motif present in the anchor. This coupling has been proposed to be performed by a sortase (17). Anchored molecules can be released by the action of lysostaphin, a hydrolase which cleaves in the pentaglycine (18). In the L. lactis peptidoglycan, the l-Ala–d-Glu(NH2)–l-Lys–d-Ala peptides, bound at the N-acetylmuramic acid residues in the glycan strands, are connected from the l-Lys of one chain to the d-Ala of another by a d-Asp interpeptide bridge (16). This d-Ala thus forms the structural analog of the pentaglycine interpeptide bridge in the S. aureus peptidoglycan. We showed that the SAX molecules were released by treatment with lysostaphin. Other workers have reported that this lysostaphin preparation contains many impurities (9) and that, among other activities, an N-acetylmuramyl-l-alanine amidase activity forms part of the crude starting material (7). We believe that the SAX molecules are connected covalently to the peptidoglycan. Treatment with an N-acetylmuramyl-l-alanine amidase activity would then yield a discrete SAX band of the size seen in Fig. 2. In particular, our finding supports the high degree of functional homology between the L. lactis and S. aureus sortases. Up to now, the high degree of similarity between the sorting mechanisms of different gram-positive bacteria has been postulated by the demonstration of the presence of the conserved anchor structure in different cell wall-bound proteins, by the coupling of foreign proteins to homologous anchors (18), and by the use of heterologous anchors (6, 18). Here we have provided evidence that a heterologous anchor can be sorted successfully in L. lactis, in agreement with earlier observations (14).
Surface localization of the SAX fusion protein.
The presence of SA at the outside surface of MG1820(pILPOL, pL2SAX) was assessed as described for surface-localized fusion proteins in E. coli (23). The results are shown in Fig. 3. Control setups, involving the incubation of induced cultures of MG1820(pILPOL, pL2SA) and MG1820(pILPOL, pL2SAX) in TBS containing 1% bovine serum albumin (BSA) followed by three washing cycles, filtration, and reaction with horseradish peroxidase (POD) substrate, showed that the cells were essentially free of nonspecific background activity in this assay. On the other hand, when induced MG1820(pILPOL, pL2SA) and MG1820(pILPOL, pL2SAX) cultures were challenged with biotinylated POD (B-POD), a strong signal was observed in the SAX-expressing cells whereas no signal could be detected for cells expressing secreted SA. To verify that the observed signals were due to the actual binding of the biotin moiety of B-POD to the immobilized SA and not, e.g., to the nonspecific binding of the B-POD complex to the anchor, or to any other altered structure in the cell wall, caused by the expression of SAX, we incubated induced cultures with a B-POD solution which had previously reacted with a 30-μg ml−1 solution of SA in TBS. The cultures were further processed in a manner identical to that described above. No signal was detected in this assay.
FIG. 3.
Biotin binding capability of induced L. lactis MG1820(pILPOL) cells carrying the SA expression plasmids indicated. The lanes marked “negative” represent cells which were incubated in TBS–1% BSA. The lanes marked “B-POD” represent cells which were incubated with B-POD. The lanes marked “B-POD/SA” represent cells incubated with B-POD, which had been blocked with an excess of SA. After incubation, cells were washed, filtered, and revealed with POD substrate.
Binding of SAX-expressing cells to biotinylated surfaces.
In this assay, biotinylated alkaline phosphatase was immobilized on a polystyrene surface (custom-made Maxisorp 35-mm-diameter petri dishes; Nunc, Roskilde, Denmark). The surface was further blocked with a 1% solution of BSA in TBS. Induced cells of strain MG1820(pILPOL, pL2SA) or MG1820(pILPOL, pL2SAX) were allowed to interact on these surfaces for 1 h. After washing, the number of bound cells present in randomly picked areas was counted under the light microscope. The results are presented in Fig. 4. SAX-expressing cells, but not SA-expressing cells, were found to bind with high efficiency to the biotinylated surfaces. The binding could be completely prevented by preincubation of the plates with a 30-μg ml−1 solution of SA (Fig. 4). The density of binding was calculated to be approximately 2 × 105 per mm2. The cells were harvested in mid-log phase, and approximately 109 were applied per plate. From the observations recorded, we concluded that about 108 cells were bound. Clearly, this method could be used in a panning selection strategy depending on surface-located receptor-like structures.
FIG. 4.
Immobilization of SAX-expressing L. lactis cells on a biotinylated alkaline phosphatase (B-AP)-coated polystyrene surface. The frame labeled “negative controls” is representative of all negative controls, including administration of SAX-expressing L. lactis to non-B-AP-coated plates, or B-AP-coated plates which were previously blocked with an excess of soluble SA, and administration of prewashed L. lactis cells expressing the secreted SA to all types of pretreated plates used. The preparations were observed through a Zeiss Microsystems Axiovert 100 confocal laser scanning microscope equipped with a 100× objective.
Our observations show that a heterologous anchor sequence can function in a highly efficient manner to expose functional units at the surface of the food-grade microorganism L. lactis. The biotechnological applications of this system in antigen presentation, immobilization of enzymatic groups at the surface, and immobilization of production strains are currently under investigation.
Acknowledgments
This work was supported by grants from the Flemish IWT-COT, the FGWO, and the National Lottery.
REFERENCES
- 1.Argarana C E, Kuntz I D, Birken S, Axel R, Cantor C R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986;14:1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 3.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 4.Goward C R, Scawen M D, Murphy J P, Atkinson T. Molecular evolution of bacterial cell-surface proteins. Trends Biochem Sci. 1993;18:136–140. doi: 10.1016/0968-0004(93)90021-e. [DOI] [PubMed] [Google Scholar]
- 5.Gunneriusson E, Samuelson P, Uhlén M, Nygren P-Å, Ståhl S. Surface display of a functional single-chain Fv antibody on staphylococci. J Bacteriol. 1996;178:1341–1346. doi: 10.1128/jb.178.5.1341-1346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iversen O-J, Grov A. Studies on lysostaphin. Separation and characterisation of three enzymes. Eur J Biochem. 1973;38:293–300. doi: 10.1111/j.1432-1033.1973.tb03061.x. [DOI] [PubMed] [Google Scholar]
- 8.Kok J, van der Vossen J M B M, Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984;48:726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malatesta M L, Heath H E, LeBlanc P A, Sloan G L. EGTA inhibition of DNase activity in commercial lysostaphin preparations. BioTechniques. 1992;12:70–72. [PubMed] [Google Scholar]
- 10.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 11.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 12.McNab R, Jenkinson H F, Loach D M, Tannock G W. Cell surface associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol Microbiol. 1994;14:743–754. doi: 10.1111/j.1365-2958.1994.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 13.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 14.Piard J-C, Hautefort I, Fischetti V A, Ehrlich S D, Fons M, Gruss A. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J Bacteriol. 1997;179:3068–3072. doi: 10.1128/jb.179.9.3068-3072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiki R K, Scharf S J, Faloona F A, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 16.Schleifer K H, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneewind O, Fowler A, Faull K E. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–105. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 18.Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 20.Sela S, Aviv A, Burstien I, Tovi A, Caparon M G, Hanski E. Protein F: an adhesion of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993;10:1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 21.Shuttleworth H L, Duggleby C J, Jones S A, Atkinson T, Minton N P. Nucleotide sequence analysis of the gene for protein A from Staphylococcus aureus Cowan 1 (NCTC 8530) and its enhanced expression in Escherichia coli. Gene. 1987;58:283–295. doi: 10.1016/0378-1119(87)90383-0. [DOI] [PubMed] [Google Scholar]
- 22.Signäs C, Raucci G, Jönsson K, Lindgren P-E, Anatharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steidler L, Remaut E, Fiers W. LamB as a carrier molecule for the functional exposition of IgG-binding domains of the Staphylococcus aureus protein A at the surface of Escherichia coli K12. Mol Gen Genet. 1993;236:187–192. doi: 10.1007/BF00277111. [DOI] [PubMed] [Google Scholar]
- 24.Steidler L, Wells J M, Raeymaeckers A, Vandekerckhove J, Fiers W, Remaut E. Secretion of biologically active murine interleukin-2 by Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1995;61:1627–1629. doi: 10.1128/aem.61.4.1627-1629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talay S R, Valentin-Weigand P, Timmis K N, Chatwal G S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesion of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 26.van Asseldonck M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 27.Wells J M, Norton P M, Le Page R W F. Progress in the development of mucosal vaccines based on Lactococcus lactis. Int Dairy J. 1995;5:1071–1079. [Google Scholar]
- 28.Wells J M, Wilson P W, Le Page R W F. Improved cloning vectors and transformation procedure for Lactococcus lactis. J Appl Bacteriol. 1993;74:629–636. doi: 10.1111/j.1365-2672.1993.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 29.Wells J M, Wilson P W, Norton P M, Gasson M J, Le Page R W F. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–1162. doi: 10.1111/j.1365-2958.1993.tb01660.x. [DOI] [PubMed] [Google Scholar]