Abstract
Phages infecting Vibrio vulnificus were abundant (>104 phages g of oyster tissue−1) throughout the year in oysters (Crassostrea virginica) collected from estuaries adjacent to the Gulf of Mexico (Apalachicola Bay, Fla.; Mobile Bay, Ala.; and Black Bay, La.). Estimates of abundance ranged from 101 to 105 phages g of oyster tissue−1 and were dependent on the bacterial strain used to assay the sample. V. vulnificus was near or below detection limits (<0.3 cell g−1) from January through March and was most abundant (103 to 104 cells g−1) during the summer and fall, when phage abundances also tended to be greatest. The phages isolated were specific to strains of V. vulnificus, except for one isolate that caused lysis in a few strains of V. parahaemolyticus. Based on morphological evidence obtained by transmission electron microscopy, the isolates belonged to the Podoviridae, Styloviridae, and Myoviridae, three families of double-stranded DNA phages. One newly described morphotype belonging to the Podoviridae appears to be ubiquitous in Gulf Coast oysters. Isolates of this morphotype have an elongated capsid (mean, 258 nm; standard deviation, 4 nm; n = 35), with some isolates having a relatively broad host range among strains of V. vulnificus. Results from this study indicate that a morphologically diverse group of phages which infect V. vulnificus is abundant and widely distributed in oysters from estuaries bordering the northeastern Gulf of Mexico.
Vibrio vulnificus is an estuarine bacterium (10, 22, 24, 28, 29, 34, 37) that is capable of causing primary septicemia following its ingestion and secondary septicemia through skin lesions in individuals with underlying chronic diseases (7, 25). In both cases, the onset of illness is usually rapid and potentially fatal. Most food-borne illness due to V. vulnificus is linked to consumption of raw oysters (19). Typically, Gulf Coast oysters harbor about 103 to 104 V. vulnificus cells g−1 during the warmer months of April through October and usually fewer than 10 cells g−1 during other months (13, 36). Both physical and biological factors probably regulate the abundance of V. vulnificus in nature. For example, the survival of V. vulnificus in seawater has been shown to be temperature and salinity dependent (21), while within oysters, V. vulnificus is readily phagocytized by oyster hemocytes (16). Phages are another factor that may affect populations of V. vulnificus within oysters and estuarine waters. Phages are extremely abundant in marine systems, with concentrations in excess of 107 phages ml−1 routinely measured in coastal waters of the Gulf of Mexico (8, 20, 32). Moreover, phages infecting Vibrio spp. can be readily isolated from seawater. Moebus and Nattkemper (27) found that 362 of 366 phage-sensitive bacteria isolated from the Atlantic belonged to the family Vibrionaceae and that 280 of them could be assigned to Vibrio spp. Phages which infect Vibrio parahaemolyticus have been isolated from a variety of estuarine samples as well, with greater abundances associated with higher water temperatures and higher concentrations of mesophilic vibrios (5, 6). A variety of Vibrio phages are also prevalent in the Gulf of Mexico (23); recently, phages causing lysis of V. vulnificus were recovered in approximately 5% of estuarine water samples collected from Louisiana (30).
Given that phages infecting V. vulnificus can be isolated in estuarine waters of the northern Gulf of Mexico, we wished to determine whether they were also present in oysters from this region. In this study, we used a quantitative assay for phages infecting V. vulnificus to determine the seasonal distribution and abundance of these phages in oysters collected from Louisiana, Alabama, and Florida. In addition, we collected representative isolates of these phages and characterized them by transmission electron microscopy and through host range studies.
Source of bacterial host strains.
Strains of V. vulnificus were provided by the following individuals: strains A-9 (moderately virulent environmental isolate) and J-7 (a virulent environmental isolate), Jerry Stelma, Environmental Protection Agency, Cincinnati, Ohio (31); strain VBNO (isolated from a Blue Crab, Callinectes sapidus), Ron Sizemore, University of North Carolina—Wilmington; strain 304C (isolated from an oyster, Crassostrea virginica), David Cook, Gulf Coast Seafood Laboratory, U.S. Food and Drug Administration, Dauphin Island, Ala.; strain MO6-24 (human primary septicemia blood isolate), Glenn Morris, Center for Vaccine Development, University of Maryland, Baltimore, Md. Virulence was determined by the method of Stelma et al. (31); isolate A-9 was lethal to mice that were simultaneously iron overloaded and immunosuppressed, and isolate J-7 was lethal to mice that were iron overloaded.
Sample collection and preparation.
Oysters were collected weekly from October through December 1994 and March through September 1995 from Apalachicola Bay, Fla., Mobile Bay, Ala., and Black Bay, La., with tongs or a dredge. These areas were sampled monthly in January and February 1995. The oysters were held at 5 to 10°C during shipment for 24 to 30 h prior to analysis. Duplicate oyster samples (12 each) were scrubbed, shucked, mixed with an equal (1:1) weight of Butterfield’s phosphate-buffered saline and blended (4).
V. vulnificus enumeration.
The abundance of V. vulnificus was determined by most-probable-number analysis with enzyme immunoassay identification by the FDA Bacteriological Analytical Manual method (12). Tenfold dilutions of oyster homogenate were inoculated into alkaline peptone water (three tubes per dilution) and incubated at 35°C for 12 to 16 h. Portions from tubes with turbid growth were streaked to modified cellobiose-polymyxin-colistin agar, and plates were incubated at 39°C for 18 to 24 h for colony isolation. Cellobiose-fermenting colonies (yellow), typical of V. vulnificus, were picked with sterile toothpicks and transferred for confirmation as V. vulnificus by an enzyme immunoassay. The V. vulnificus species-specific monoclonal antibody used for the enzyme immunoassay was prepared in-house as previously described (35).
Phage enumeration and isolation.
Phages were enumerated directly in the supernatant of oyster homogenates as previously described (11). All media and diluents were prepared in seawater (35 ppt collected 50 km offshore from Alabama), which was filtered through 0.2-μm-pore-size cellulose-acetate bottletop filters (Corning Glass Works, Corning, N.Y.) and diluted with deionized seawater to 20 ppt. Casamino Acids peptone marine (CPM) broth (5.0 g of Casamino Acids [Difco], 5.0 g of Bacto Peptone [Difco], and 1.0 liter of seawater, autoclaved for 15 min at 121°C) was used as a growth medium. Serial 10-fold dilutions of oyster supernatant were prepared in sterile seawater. Aliquots (0.1 ml) of each dilution were adsorbed to 0.2 ml of log-phase host cultures for 15 min, and virulent phages were enumerated by using the soft-agar overlay technique (2). The plating medium and soft-agar overlay were prepared with CPM medium supplemented with 1.5 and 0.7% Bacto Agar (Difco), respectively. The plates were incubated at 26°C, and plaques were counted at 24 and 48 h.
Purification and preparation of phage stock.
Plaque assays were performed by using either enrichment or direct enumeration procedures; a Pasteur pipette was used to pull plugs of agar containing plaques from plates containing fewer than 250 PFU. The plugs were suspended in sterile seawater, and dilutions of this suspension were replated to purify the phage. This procedure was repeated. High-titer stocks of phages (108 to 1010 phages ml−1) were prepared from the highest dilution of a phage suspension which gave confluent lysis on a soft-agar overlay plate. The phages were extracted by covering the agar surface with 10 ml of sterile seawater and storing the plate at 3°C for 1 to 2 h. The seawater-phage suspension was centrifuged as described above and filtered through a 0.2-μm-pore-size filter.
Bacterial susceptibility.
Five strains of V. vulnificus and 18 other isolates of mesophilic Vibrio spp. were grown to log phase and plated on CPM medium by the soft-agar overlay technique. After 1 h, 4 μl from a phage stock was spotted onto the plates, and the plates were incubated overnight at 26°C. Bacterial strains were considered susceptible to phages that produced either clear or turbid plaques.
Phage morphology.
The morphology of the phage isolates was determined by transmission electron microscopy (33). Phage lysates were filtered through 0.2-μm-pore-size filters and, if necessary, concentrated by ultracentrifugation (146,000 × g) at 20°C in an AH-629 swinging-bucket rotor (Sorvall) for 2.5 h. The phages were transferred to 400-mesh carbon-coated copper grids by floating the grids on drops of filtered lysate for ca. 30 min. The grids were stained with 1% uranyl acetate and photographed at 80 kV with a Philips EM 301 transmission electron microscope. The sizes of the six different phage morphotypes were estimated from photographic images of negatively stained phage particles. A diffraction grating replica calibration standard (2,160 lines/mm) with latex beads (0.216 mm in diameter) was used to calibrate the magnification of the electron microscope.
Changes in the abundances of phages and V. vulnificus.
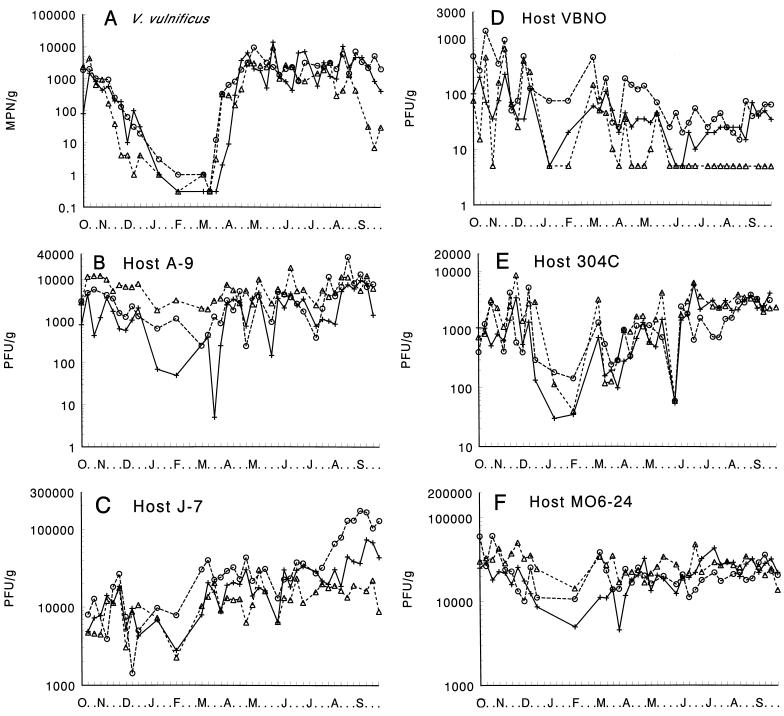
Phages infecting a clinical strain (strain MO6-24) and a virulent environmental isolate (strain J-7) of V. vulnificus were abundant throughout the year in oysters from Apalachicola Bay, Fla., Mobile Bay, Ala., and Black Bay, La., three distinct estuaries along the northern coast of the Gulf of Mexico (Fig. 1C and F). Abundances ranged from 103 to more than 105 phages g of oyster tissue−1 for strain J-7, with the greatest concentrations occurring in the summer and fall in oysters from Black Bay (Fig. 1C). The abundance of phages infecting strain MO6-24 tended to be less variable and ranged from ca. 104 to 105 phages g−1. Phages infecting strain A-9 (Fig. 1A), a moderately virulent environmental isolate, and strain 304C (Fig. 1E), an oyster isolate of unknown virulence, were not as abundant and ranged in titer from 102 to 104 phages g−1, except for a few occasions when the abundance was <102 phages g−1. The phages infecting strain VBNO of V. vulnificus, which was isolated from a blue crab and is of unknown virulence, were typically the least abundant and varied between being undetectable (<5 phages g−1) to ca. 103 phages g−1 (Fig. 1D). Relative to the abundance of infectious phages, V. vulnificus was much more variable, ranging from near or below detection limits (<0.3 cell g−1) from January through March to ca. 103 to 104 cells g−1 during April through October (Fig. 1A). The densities of V. vulnificus and its phages were remarkably similar among the Gulf Coast estuaries (Fig. 1).
FIG. 1.
Seasonal changes in the abundance of V. vulnificus (A) and of phages that infect strains of V. vulnificus (B to F) in oysters from Florida (▵), Alabama (+), and Louisiana (○).
Morphology of phages and host range studies.
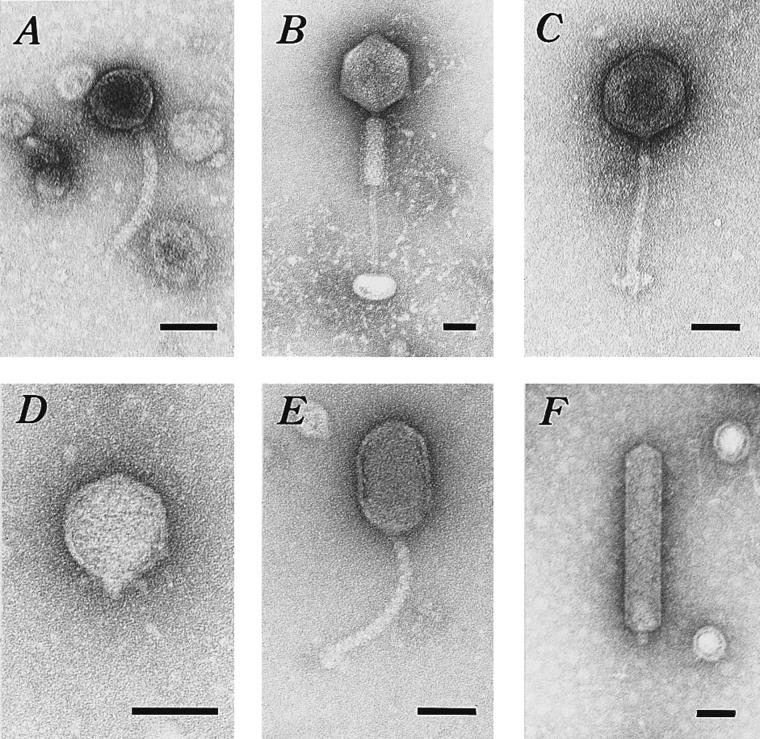
We examined 43 different phage isolates with the electron microscope and found six distinct phage morphologies belonging to the bacteriophage families Myoviridae, Styloviridae, and Podoviridae (Fig. 2). The first morphotype was a stylophage with a collar-like structure between the head and tail and a thin threadlike extension from the end of the noncontractile tail (Bradley group B-1; Fig. 2A). The mean head diameter was 65 nm (standard deviation [SD] = 4 nm; n = 10), and the mean total length of the phage was 219 nm (SD = 14 nm; n = 10). The second morphotype was a myophage with a long contractile tail that ended in a plate-like structure (Bradley group A-1; Fig. 2B). The mean head diameter was 109 nm (SD = 12 nm; n = 11), and the mean total length of the phage was 366 nm (SD = 45 nm; n = 11). The third morphotype was a stylophage with appendages at the end of the thin, flexible tail (Bradley group B-1; Fig. 2C). The mean head diameter was 90 nm (SD = 4 nm; n = 6), and the mean total length of the phage was 262 nm (SD = 9 nm; n = 6). The fourth morphotype was a podophage with short extensions at the neck (Bradley group C-1; Fig. 2D). The mean head diameter was 72 nm (SD = 5 nm; n = 20). The fifth morphotype was a stylophage with elongated head (Bradley group B-2; Fig. 2E). The mean head length was 109 nm (SD = 10 nm; n = 10), and the mean head width was 64 nm (SD = 5 nm; n = 10). The mean total length of this phage was 274 nm (SD = 8 nm; n = 10). The sixth morphotype was a podophage with an extensively elongated head (Bradley group C-3; Fig. 2F). The mean head length was 258 nm (SD = 4 nm; n = 35), and the mean head width was 47 nm (SD = 3 nm; n = 35). The mean total length of this phage was 270 nm (SD = 5 nm; n = 35).
FIG. 2.
Transmission electron micrographs of representative V. vulnificus phages. (A) Stylophage with collar. A thin threadlike structure extends from the end of the tail; isolated from location for isolate 70A-4. (B) Myophage with contracted tail sheath revealing tail core and plate-like structure at the end of the tail; isolated from location for isolate 1-11. (C) Stylophage; isolated from location for isolate 11-8. (D) Podophage; isolated from location for 1-9. (E) Stylophage with elongated capsid; isolated from location for isolate 353B. (F) Podophage with very elongated capsid; isolated from location for isolate 71A-6. Bars, 50 nm.
Host range studies with the five host strains of V. vulnificus revealed 16 distinct morphotype and host range patterns among the 25 phage strains tested (Table 1). Podoviridae strains in Bradley groups C-1 and C-3 caused lysis in all five host strains. The Bradley C-3 morphotype has not previously been reported for phages infecting Vibrio spp., yet it was one of the most common morphotypes observed in this investigation. Nineteen mesophilic bacterial isolates, primarily Vibrio spp., were tested for susceptibility to 25 representative V. vulnificus phage isolates. A single phage isolate lysed several atypical (cellobiose-fermentering) V. parahaemolyticus strains. Other bacterial species including V. cholerae (seven strains), V. alginolyticus (three strains), V. parahaemolyticus (two strains), V. fluvialis (one strain), V. mimicus (one strain), Aeromonas hydrophila (one strain), and Pseudomonas putricida (one strain) were resistant to all 25 of the phage isolates.
TABLE 1.
V. vulnificus phage morphology and host specificity
| Original host strain | Phage morphol- ogya | Strain | Susceptibility of strain:
|
||||
|---|---|---|---|---|---|---|---|
| A-9 | J-7 | VBNO | 304C | MO6-24 | |||
| A-9 | A | 72A-1; 110A-2 | + | − | − | − | − |
| A-9 | F | 70A-2 | + | + | + | + | + |
| A-9 | F | 110A-1 | + | − | − | − | + |
| J-7 | A | 70A-4; 108A-3 | − | + | − | − | − |
| J-7 | C | 70A-3 | − | + | − | − | − |
| VBNO | A | 109B-1 | − | + | + | − | − |
| VBNO | D | 72A-4 | − | − | + | − | − |
| 304C | D | 1-9 | − | − | − | + | + |
| 304C | D | 70A-8; 110A-4 | − | − | − | + | − |
| 304C | F | 72A-5 | − | − | − | + | − |
| 304C | F | 109A-6 | + | + | + | + | + |
| 304C | F | 110A-5 | − | − | − | + | + |
| M06-24 | B | 1-11 | − | − | − | + | + |
| M06-24 | C | 70A-10 | − | − | − | − | + |
| M06-24 | D | 72A-10 | + | + | + | + | + |
| M06-24 | E | 353B | + | − | − | − | + |
| M06-24 | F | 7-8; 71A-6 | + | + | + | + | + |
| 72A-8; 108A-10 | |||||||
| 109A-8; 110A-7 | |||||||
Phage morphologies A to F correspond to those described in Fig. 2A to F, respectively.
Phage abundance and distribution.
This study demonstrates that a diverse group of phages infecting V. vulnificus was abundant in oysters from estuaries in the northern Gulf of Mexico. Pelon et al. (30) reported a low incidence of phages infecting V. vulnificus in estuarine water samples from Louisiana; quantitative procedures for enumeration were not used. The total concentration of phages infecting V. vulnificus cannot be determined from our data. Many of the phages that we isolated caused lysis in only one of the five stains assayed, indicating that only a subset was detected. This finding suggests that more phages would be found if a larger number of host strains were used to assay the phages. It is also possible that the efficiencies of the plaque assays were lower than 100%. Nonetheless, given the abundance and diversity of phages that we detected in oysters from the Gulf of Mexico, it seems evident that phages probably play an important role in the ecology of cooccurring V. vulnificus.
Phages that cause lysis of V. parahaemolyticus have been isolated from seawater samples (18) and have also been found in molluscan shellfish from Washington and Oregon (5, 6). The abundance of these phages in shellfish increased with increasing water temperatures and with the abundance of mesophilic vibrios but not with increases in the abundance of V. parahaemolyticus. Baross et al. (5) hypothesized that other mesophilic vibrios served as hosts for phages that infected V. parahaemolyticus. We also observed that the abundance of phages infecting some strains of V. vulnificus remained high even when V. vulnificus was present in small or undetectable numbers. We examined 19 mesophilic isolates for susceptibility to 25 phages that caused lysis of V. vulnificus in order to test the hypothesis that other Vibrio spp. might serve as alternate hosts. Of these phages, only a single phage isolate was able to cause lysis of a bacterial strain other than V. vulnificus, namely, several atypical strains (cellobiose fermenters) of V. parahaemolyticus. Although the possibility remains that the production of phages which infect V. vulnificus was supported by other species of bacteria, we were not able to provide convincing evidence of this in our experiments.
Phage morphology and host range.
The phages found in our study were morphologically diverse, and several were distinct from those previously isolated from Louisiana waters (30). A number of the phages we isolated belonged to the Podoviridae C Bradley group, although they have not been reported in past surveys of Vibrio phages (1). However, in an extensive study of phages isolated from the North Atlantic which infected 366 strains of bacteria belonging primarily to the Vibrionaceae, Moebus and coworkers (14, 26, 27) isolated a number of phages belonging to the Podoviridae. The group of short-tailed phages that have an exceptionally long capsid (Fig. 2F) appear to belong to a previously undescribed morphotype within the Podoviridae. Unlike most of the phage isolates, which had relatively narrow host ranges and caused lysis only of the strain on which they were originally isolated, some of the phages belonging to the newly described group lysed all five of the host strains as well as all clinical isolates of V. vulnificus tested thus far (data not shown).
That many of the phages have a relatively narrow host range suggests that they may be useful in developing a phage-typing system for V. vulnificus, such as has been used for epidemiological studies of related pathogens including V. cholerae (3, 9, 15). Indeed, susceptibility patterns of V. vulnificus strains to the phage isolates listed in Table 1 clearly distinguished among the five host strains of V. vulnificus. Moreover, encapsulated (associated with virulence) strains of V. vulnificus appear to be more susceptible to infection by phages than unencapsulated (not associated with virulence) strains do (30). Phage typing may also be useful in distinguishing strain virulence, a capability not currently available (17).
Acknowledgments
We thank Florida State Department of Natural Resources, Alabama State Health Department, and Louisiana State Department of Health and Hospitals for assistance with sample collection and shipment.
REFERENCES
- 1.Ackermann H W, Kasatiya S S, Kawata T, Koga T, Lee J V, Mbiguino A, Newman F S, Vieu J F, Zachary A. Classification of Vibrio bacteriophages. Intervirology. 1984;22:61–71. doi: 10.1159/000149535. [DOI] [PubMed] [Google Scholar]
- 2.Adams M H. Bacteriophages. New York, N.Y: Interscience; 1959. [Google Scholar]
- 3.Almeida R J, Cameron D N, Cook W L, Wachsmuth I K. Vibriophage VcA-3 as an epidemic strain marker for the U.S. Gulf Coast Vibrio cholerae O1 clone. J Clin Microbiol. 1992;30:300–304. doi: 10.1128/jcm.30.2.300-304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Public Health Association. Recommended procedures for the examination of seawater and shellfish. 4th ed. Washington, D.C: American Public Health Association; 1970. [Google Scholar]
- 5.Baross J A, Liston J, Morita R Y. Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl Environ Microbiol. 1978;36:492–499. doi: 10.1128/aem.36.3.492-499.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baross J A, Liston J, Morita R Y. Ecological relationship between Vibrio parahaemolyticus and agar-digesting vibrios as evidenced by bacteriophage susceptibility patterns. Appl Environ Microbiol. 1978;36:500–505. doi: 10.1128/aem.36.3.500-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake P A, Merson A M, Weaver R E, Hollis D G, Heublein P C. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 8.Boehme J, Frischer M E, Jiang S C, Kellogg C A, Pichard S, Rose J B, Steinway C, Paul J H. Phages, bacterioplankton, and phytoplankton in the southeastern Gulf of Mexico: distribution and contribution to oceanic DNA pools. Mar Ecol Prog Ser. 1993;97:1–10. [Google Scholar]
- 9.Chattopadhyay D J, Sarkar B L, Ansari M Q, Chakrabarti B K, Roy M K, Ghosh A N, Pal S C. New phage typing scheme for Vibrio cholerae O1 biotype El Tor strains. J Clin Microbiol. 1993;31:1579–1585. doi: 10.1128/jcm.31.6.1579-1585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePaola A, Capers G M, Alexander D. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast Appl Environ Microbiol. 1994;60:984–988. doi: 10.1128/aem.60.3.984-988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola A, McLeroy S, McManus G. Distribution of Vibrio vulnificus phage in oyster tissues and other estuarine habitats. Appl Environ Microbiol. 1997;63:2464–2467. doi: 10.1128/aem.63.6.2464-2467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot E L, Kaysner C A, Tamplin M L. FDA bacteriological analytical manual. 7th ed. Arlington, Va: Association of Official Analytical Chemists; 1992. V. cholerae, V. vulnificus, and other Vibrio spp; pp. 111–140. [Google Scholar]
- 13.Food and Drug Administration. Hazard analysis of Vibrio vulnificus in oysters. Dauphin Island, Ala: Food and Drug Administration; 1996. [Google Scholar]
- 14.Frank H, Moebus K. An electron microscopic study of bacteriophages from marine waters. Helgol Meeresunters. 1987;41:385–414. [Google Scholar]
- 15.Goldberg S, Murphy J R. Molecular epidemiological studies of United States Gulf Coast Vibrio cholerae strains: integration site of mutator vibriophage VcA-3. Infect Immun. 1983;42:224–230. doi: 10.1128/iai.42.1.224-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris-Young L, Tamplin M L, Fisher W S, Mason J W. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl Environ Microbiol. 1993;59:1012–1017. doi: 10.1128/aem.59.4.1012-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayat U, Reddy G P, Bush C A, Johnson J A, Wright A C, Morris J G., Jr Capsular types of Vibrio vulnificus: an analysis of strains from clinical and environmental sources. J Infect Dis. 1993;168:758–762. doi: 10.1093/infdis/168.3.758. [DOI] [PubMed] [Google Scholar]
- 18.Hidaka T, Tokushige A. Isolation and characterization of Vibrio parahaemolyticus bacteriophages in sea water. Mem Fac Fish Kagoshima Univ. 1978;27:79–90. [Google Scholar]
- 19.Hlady G. Risk assessment and current data. In: Watkins W, McCarthy S, editors. Proceedings of the 1994 Vibrio vulnificus Workshop. Washington, D.C: Food and Drug Administration; 1994. pp. 27–37. [Google Scholar]
- 20.Jiang S C, Paul J H. Seasonal and diel abundance of phages and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar Ecol Prog Ser. 1994;104:163–172. [Google Scholar]
- 21.Kaspar C W, Tamplin M L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol. 1993;59:2425–2429. doi: 10.1128/aem.59.8.2425-2429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaysner C A, Abeyta C, Jr, Wekell M M, DePaola A, Jr, Stott R F, Leitch J M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987;53:1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellogg C A, Rose J B, Jiang S C, Thurmond JM, Paul J H. Genetic diversity of related vibriophages isolated from marine environments around Florida and Hawaii, USA. Mar Ecol Prog Ser. 1995;120:89–98. [Google Scholar]
- 24.Kelly M T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982;44:820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy L M, Gnn R A. Syndromes of Vibrio vulnificus infections, clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 26.Moebus K, Nattkemper H. Bacteriophage sensitivity patterns among bacteria isolated from marine waters. Helgol Meeresunters. 1981;34:375–385. [Google Scholar]
- 27.Moebus K, Nattkemper H. Taxonomic investigations of bacteriophage sensitive bacteria isolated from marine waters. Helgo Meeresunters. 1983;36:357–373. [Google Scholar]
- 28.Oliver J D, Warner R A, Cleland D R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983;45:985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill K R, Jones S H, Grimes D J. Seasonal incidence of Vibrio vulnificus in the Great Bay Estuary of New Hampshire and Maine. Appl Environ Microbiol. 1992;58:3257–3262. doi: 10.1128/aem.58.10.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelon W, Siebeling R J, Simonson J, Luftig R B. Isolation of bacteriophage infectious for Vibrio vulnificus. Curr Microbiol. 1995;30:331–336. doi: 10.1007/BF00369859. [DOI] [PubMed] [Google Scholar]
- 31.Stelma G N, Jr, Reyes A L, Peeler J T, Johnson C H, Spaulding P L. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992;58:2776–2782. doi: 10.1128/aem.58.9.2776-2782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suttle C A, Chan A M, Cottrell M T. Infection of phytoplankton by phages and reduction of primary productivity. Nature. 1990;347:467–469. [Google Scholar]
- 33.Suttle C A, Chan A M. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Mar Ecol Prog Ser. 1993;92:99–109. [Google Scholar]
- 34.Tamplin M, Rodrick G E, Blake N J, Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982;44:1466–1477. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamplin M L. The seasonal occurrence of Vibrio vulnificus in shellfish, seawater, and sediment of United States coastal waters and the influence of environmental factors on survival and virulence. 1994. Final report to the Salstonstall-Kennedy Program. [Google Scholar]
- 37.Wright A C, Hill R T, Johnson J A, Roghman M, Colwell R R, Morris J G. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol. 1996;62:717–724. doi: 10.1128/aem.62.2.717-724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]