Abstract
The biodegradation by Rhizobium huakuii PMY1 of up to 10 mM phosphonomycin as a carbon, energy, and phosphorus source with accompanying Pi release is described. This biodegradation represents a further mechanism of resistance to this antibiotic and a novel, phosphate-deregulated route for organophosphonate metabolism by Rhizobium spp.
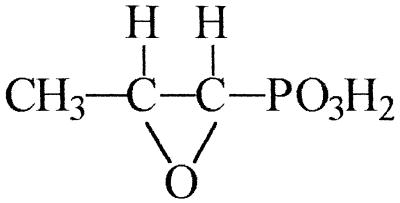
Phosphonomycin [fosfomycin; cis-(1R,2S)-1,2-epoxypropylphosphonic acid] is a broad-spectrum, cell wall-active, organophosphonate antibiotic, produced as a product of secondary metabolism by strains of Streptomyces fradiae, Streptomyces wedmorensis, Streptomyces viridochromogenes, and Pseudomonas syringae (7, 26, 27). Structurally, phosphonomycin is characterized by both an epoxide ring and a highly stable, covalent, carbon-phosphorus bond (Fig. 1) which is resistant to, for example, acid and base hydrolysis, thermolysis, and photolysis, and to phosphotransferase-catalyzed cleavage (12).
FIG. 1.
Structure of phosphonomycin (cis-1,2-epoxypropylphosphonic acid; fosfomycin).
The pathway leading to the formation of phosphonomycin has been extensively studied (1, 6, 24, 33); however, very little is known about its biodegradation. Previous studies have shown that environmental microorganisms undergoing phosphate starvation are capable of the utilization of phosphonomycin as the sole source of phosphorus. Cleavage of the constituent carbon-to-phosphorus bond is presumed to be catalyzed by a C-P lyase enzyme complex(es) which, being under the direct control of the PHO regulon, is inducible only under conditions of phosphate limitation (9, 22, 23, 31). We now report the isolation of a bacterium capable of utilizing phosphonomycin independently of the phosphate status of the cell, as either a carbon source or as a carbon and phosphorus source, with essentially quantitative extracellular release of organophosphonate-derived orthophosphate. Degradation of phosphonomycin by cleavage of the carbon-phosphorus bond may also provide a novel mechanism of resistance to the antibiotic.
Enrichment was done with a basal mineral salts medium (pH 6.8) which contained the following (per liter): KCl, 0.2 g; MgSO4 · 7H2O, 0.2 g; CaCl2 · 2H2O, 1.0 mg; NH4Cl, 1.0 g; ferric ammonium citrate, 1.0 mg; phosphate-free yeast extract (32), 0.05 g; BME essential amino acids solution, 20 ml/liter (Sigma); and 1 ml each of trace element solution (11) and vitamin solution (17). Filter-sterilized (0.22-μm pore size) phosphonomycin (5 mM) was routinely added as a carbon and phosphorus source.
Enrichment cultures (25 ml in 250-ml Erlenmeyer flasks) were inoculated with a 0.5% (vol/vol) combined inoculum from an activated sludge plant (Dunmurry, Northern Ireland), a laundry effluent disposal lagoon (Summit Lake, Wis.) and a sheep dip disposal site (County Antrim, Northern Ireland); each site was known to have a previous history of exposure to organophosphonate xenobiotics, although significant exposure to phosphonomycin is unlikely. Cultures were incubated at 30°C on an orbital shaker at 100 rpm. Growth was measured by the increase in optical density at 650 nm with a Pye-Unicam PU 8200 UV/Vis spectrophotometer (Pye-Unicam Ltd., Cambridge, United Kingdom), while phosphate release into the culture supernatant was monitored by the method of Fiske and SubbaRow (4).
After 11 serial transfers, a bacterial isolate, designated PMY1, capable of growth on phosphonomycin (5 mM) as a carbon, energy, and phosphorus source was purified on medium solidified by the addition of 1.2% purified agar (Oxoid) by the plate screening method of McGrath and Quinn (16).
Isolate PMY1 was found to be gram negative, nonmotile, and oxidase and catalase positive and grew poorly on all conventional rich laboratory media tested. Partial 16S rRNA sequencing (ca. 450 nucleotides), performed by Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany), revealed 100% 16S rRNA gene similarity to Rhizobium huakuii and indicated a close relationship to other Rhizobium spp.
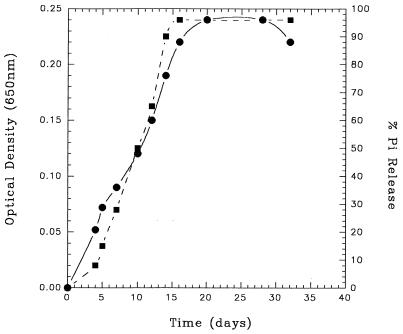
Growth of R. huakuii PMY1 on 5 mM phosphonomycin as a carbon, energy, and phosphorus source is shown in Fig. 2. Phosphonomycin biodegradation was observed by virtue of its total disappearance from growth medium as detected by the gas chromatography-mass spectrometry method of Longo et al. (15). This strain was unable to utilize hydroxymethylphosphonate, phosphonobutyrate, phosphonoformate, 2-phosphonopropionate, 3-phosphonopropionate, phosphonomethylglycine (glyphosate), methylphosphonate, trimethylphosphonate, ethylphosphonate, dimethylmethylphosphonate, 2-amino-3-phos- nopropionate, 1-aminoethylphosphonate, 2-aminoethylphosphonate, phenylphosphonate, or phosphonoacetate as carbon, energy, and phosphorus sources.
FIG. 2.
Growth of isolate PMY1 on phosphonomycin (5 mM) as the sole carbon, energy, and phosphorus source. Cultures were incubated at 30°C on an orbital shaker at 100 rpm. Growth was measured by the increase in optical density at 650 nm, while phosphate release was determined by the method of Fiske and SubbaRow (4). Symbols: •, growth (optical density at 650 nm); ▪, percentage of phosphate released from substrate.
The metabolism of 5 mM phosphonomycin was accompanied by the concomitant release of up to 96% inorganic phosphate (Fig. 2). No spontaneous orthophosphate release was observed either in uninoculated control experiments or from cultures incubated in the absence of substrate.
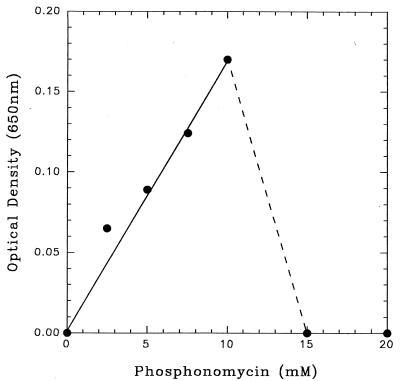
R. huakuii PMY1 was grown on a range of concentrations (0 to 20 mM) of phosphonomycin as a carbon, energy, and phosphorus source (Fig. 3). Final cell yields were directly proportional to phosphonomycin concentrations within the range of 0 to 10 mM and were accompanied by the extracellular release of >95% phosphonate-phosphorus as Pi. Negligible growth rates and cell yields were observed at phosphonomycin concentrations of 15 and 20 mM (Fig. 3).
FIG. 3.
Stationary-phase cell yields of isolate PMY1 as a function of phosphonomycin concentration (0 to 20 mM).
Cell extracts of R. huakuii PMY1, grown on phosphonomycin (5 mM) as a carbon, energy, and phosphorus source and prepared by sonication as described by McGrath et al. (17), contained no in vitro C—P bond-cleaving activity either on phosphonomycin or any of the other organophosphonate substrates tested. The addition of either Mg2+ (1 mM), Fe3+ (1 mM), Mn2+ (1 mM), Co2+ (1 mM), Zn2+ (1 mM), or NAD+ (3 mM) or exhaustive dialysis against 50 mM Tris-HCl buffer (pH 6.8) produced no subsequent detectable in vitro C—P bond-cleaving activity. No other previously described C—P bond-cleaving enzyme, neither phosphonoacetate hydrolase nor phosphonatase (assayed by the methods of McGrath et al. [17] and La Nauze et al. [13], respectively) could be detected in cell extracts of the isolate.
This report represents not only the first demonstration of the microbial biodegradation of phosphonomycin but also the first description of the metabolism of an organophosphonate as a source of carbon by any member of the Rhizobiaceae family. Other members of the Rhizobiaceae have been shown capable of the utilization of organophosphonates, including the herbicide glyphosate, as a source of phosphorus; metabolism occurs only under conditions of strict phosphate limitation with no detectable in vitro C—P bond-cleaving activity (14). Indeed, the utilization of compounds containing a direct carbon-to-phosphorus bond as a source of phosphorus is widespread. The utilization of organophosphonates as a source of carbon is more limited; this may be explained by the proposed regulation of both phosphonate uptake and catabolism as part of the PHO regulon, with degradation being repressed and/or inhibited by the excess phosphate released during mineralization of the organophosphonate carbon skeleton. Thus, the utilization of phosphonomycin by R. huakuii PMY1 as a carbon source is surprising and would suggest the existence and involvement of an enzyme system not under classical PHO regulon control. Only two other organisms have previously been reported to utilize organophosphonates as the sole carbon source; Pseudomonas fluorescens 23F mineralized phosphonoacetic acid via acetic acid and orthophosphate (18–20) while Pseudomonas putida utilized phosphonoacetaldehyde via C—P bond cleavage to yield acetaldehyde (2). R. huakuii PMY1 represents only the third reported exception to the stringent control of microbial organophosphonate uptake and metabolism by inorganic phosphate.
Rhizobia are commonplace in the biosphere and may contribute to the rapid degradation of organophosphonate herbicides and pesticides in the soil (14). Whether this report, detailing phosphate-deregulated organophosphonate metabolism, is of ecological significance to the environmental fate of organophosphonate residues, such as glyphosate in the rhizosphere, remains to be seen.
Our failure to detect any C—P bond-cleaving activity in cell extracts of R. huakuii PMY1 leaves the route of phosphonomycin metabolism uncertain. Of the documented C—P bond-cleaving enzymes, only phosphonatase (13) and phosphonoacetate hydrolase (17) (each specific for their respective substrates phosphonoacetaldehyde and phosphonoacetate) have been detected in vitro, while cell-free C-P lyase activity has remained elusive despite numerous attempts (3, 5, 9, 10, 14, 21, 25, 31). Neither phosphonatase nor phosphonoacetate hydrolase was demonstrable in cell extracts of R. huakuii PMY1, suggesting the likely presence of a previously undescribed C—P bond-cleaving enzyme (although a specific phosphate-deregulated C-P lyase cannot be ruled out; the existence of different classes of C-P lyase, each with a defined organophosphonate substrate range has previously been postulated [9]). Further enzyme characterization may become possible only after elucidation of the phosphonomycin degradative pathway through the synthesis of potential pathway intermediates. In this way, the actual substrate for the enzyme involved in C—P bond cleavage may eventually be identified, thus allowing for further enzymatic characterization in vitro.
The degradation of phosphonomycin may also afford R. huakuii PMY1 a novel resistance mechanism to the antibiotic, through the cleavage of the constituent carbon-phosphorus bond. This isolate is resistant to antibiotic concentrations many times greater than the inhibitory concentration ranges described for susceptible bacteria (1 to 200 μmol/liter [8]). Three other methods of bacterial phosphonomycin resistance have been reported: (i) impermeability owing to chromosome mutations affecting phosphonomycin uptake (8, 29); (ii) loss of target enzyme affinity and thus sensitivity to phosphonomycin (29, 30); (iii) modification of the molecule through the production of a 16-kDa polypeptide (glutathione S-transferase), which catalyzes the formation of a phosphonomycin adduct with glutathione, thus inactivating the antibiotic (28). Cleavage of the C—P bond may therefore be regarded as a fourth mechanism of phosphonomycin detoxification, confirming the postulated existence of antibiotic resistance mechanisms based on C—P bond cleavage (23, 31).
Acknowledgments
This work was supported in part by the Science and Engineering Research Council, UK (grant GR/H 29568), the UK Clean Technology Unit, the Queen’s University Environmental Science and Technology Research Centre (QUESTOR) and by a Distinction Award to J. W. McGrath from the Department of Education for Northern Ireland Postgraduate Training Scheme. F. Hammerschmidt is supported by the Fonds zur Förderung der wissenschaftlichen Forschung (Vienna; project number P11929-CHE).
REFERENCES
- 1.Aisaka K, Ohshiro T, Uwajima T. Optimum culture conditions for the epoxidation of cis-propenylphosphonate to fosfomycin by Cellvibrio gilvus. Appl Microbiol Biotechnol. 1992;36:431–435. doi: 10.1007/BF00170177. [DOI] [PubMed] [Google Scholar]
- 2.Cook A M, Daughton C G, Alexander M. Phosphonate utilization by bacteria. J Bacteriol. 1978;133:85–90. doi: 10.1128/jb.133.1.85-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daughton C G, Cook A M, Alexander M. Bacterial conversion of alkylphosphonates to natural products via carbon-phosphorus bond cleavage. J Agric Food Chem. 1979;27:1375–1382. [Google Scholar]
- 4.Fiske C H, SubbaRow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 5.Frost J W, Loo M L, Cordeiro M L, Li D. Radical-based dephosphorylation and organophosphonate biodegradation. J Am Chem Soc. 1987;109:2166–2171. [Google Scholar]
- 6.Hammerschmidt F, Kählig H. Incorporation of D-[1-2H1]glucose into 2-aminoethylphosphonic acid in Tetrahymena thermophila and into fosfomycin in Streptomyces fradiae—the stereochemical course of a phosphoenolpyruvate mutase-catalysed reaction. Liebigs Ann Chem. 1992;11:1201–1203. [Google Scholar]
- 7.Hendlin D, Stapley E O, Jackson M, Wallick H, Miller A K, Wolf F J, Miller T W, Chaiet L, Kanan F M, Foltz E L, Woodruff H B, Mata J, Hernandez S, Mochales S. Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 8.Kahan F M, Kahan J S, Cassidy P J, Kroop H. The mechanism of action of fosfomycin (phosphonomycin) Ann N Y Acad Sci. 1974;235:364–385. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 9.Kertesz M, Elgorriaga A, Amrhein N. Evidence for two distinct phosphonate degrading enzymes (C-P lyases) in Arthrobacter sp. GLP-1. Biodegradation. 1991;2:53–59. doi: 10.1007/BF00122425. [DOI] [PubMed] [Google Scholar]
- 10.Kishore G M, Jacob G S. Degradation of glyphosate by Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem. 1987;262:12164–12168. [PubMed] [Google Scholar]
- 11.Krieg N R. Enrichment and isolation. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 112–142. [Google Scholar]
- 12.Lacoste A M, Dumora C, Cassaigne A. Cleavage of the carbon to phosphorus bond of organophosphonates by bacterial systems. Biochem Adv (Life Sci) 1989;8:97–111. [Google Scholar]
- 13.La Nauze J M, Rosenberg H, Shaw D C. The enzymatic cleavage of the carbon-phosphorus bond: purification and properties of phosphonatase. Biochim Biophys Acta. 1970;212:332–350. doi: 10.1016/0005-2744(70)90214-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu C-M, McLean P A, Sookdeo C C, Cannon F C. Degradation of the herbicide glyphosate by members of the family Rhizobiaceae. Appl Environ Microbiol. 1991;57:1799–1804. doi: 10.1128/aem.57.6.1799-1804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo A, Di Toro M, Pagani E, Carenzi A. Simple selected ion monitoring method for determination of fosfomycin in blood and urine. J Chromatogr. 1981;224:257–264. [Google Scholar]
- 16.McGrath J W, Quinn J P. A plate assay for the detection of organophosphonate mineralization by environmental bacteria and its modification as an activity stain for identification of the carbon-phosphorus bond cleaving enzyme phosphonoacetate hydrolase. Biotechnol Tech. 1995;9:497–502. [Google Scholar]
- 17.McGrath J W, Wisdom G B, McMullan G, Larkin M J, Quinn J P. The purification and properties of phosphonoacetate hydrolase, a novel carbon-phosphorus bond-cleaving enzyme from Pseudomonas fluorescens 23F. Eur J Biochem. 1995;234:225–230. doi: 10.1111/j.1432-1033.1995.225_c.x. [DOI] [PubMed] [Google Scholar]
- 18.McMullan G, Harrington F, Quinn J P. Metabolism of phosphonoacetate as the sole carbon and phosphorus source by an environmental bacterial isolate. Appl Environ Microbiol. 1992;58:1364–1366. doi: 10.1128/aem.58.4.1364-1366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMullan G, Quinn J P. Detection of a novel carbon phosphorus bond cleavage activity in cell free extracts of an environmental Pseudomonas fluorescens isolate. Biochem Biophys Res Commun. 1992;184:1022–1027. doi: 10.1016/0006-291x(92)90693-f. [DOI] [PubMed] [Google Scholar]
- 20.McMullan G, Quinn J P. In vitro characterization of a phosphate starvation-independent carbon-phosphorus bond cleavage activity in Pseudomonas fluorescens 23F. J Bacteriol. 1994;176:320–324. doi: 10.1128/jb.176.2.320-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pipke R, Amrhein N. Carbon-phosphorus lyase activity in permeabilized cells of Arthrobacter sp. GLP-1. FEMS Microbiol Lett. 1988;236:135–138. [Google Scholar]
- 22.Quinn J P, Peden J M M, Dick R E. Carbon-phosphorus bond cleavage by Gram positive soil bacteria. Appl Microbiol Biotechnol. 1989;31:283–287. [Google Scholar]
- 23.Quinn J P. Carbon-phosphorus lyase activity—a novel mechanism of bacterial resistance to the phosphonate antibiotics? Lett Appl Microbiol. 1989;8:113–116. [Google Scholar]
- 24.Rogers T O, Birnbaum J. Biosynthesis of fosfomycin by Streptomyces fradiae. Antimicrob Agents Chemother. 1974;5:121–132. doi: 10.1128/aac.5.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shames S L, Wackett L P, LaBarge S, Kuckowski R L, Walsh C T. Fragmentative and stereochemical isomerization probes for homolytic carbon to phosphorus bond scission catalysed by bacterial carbon-phosphorus lyase. Bioorg Chem. 1987;15:366–373. [Google Scholar]
- 26.Shoji J, Kato T, Hinoo H, Hattori T, Hirooka K, Matsumoto K, Tanimoto T, Kondo E. Production of fosfomycin (phosphonomycin) by Pseudomonas syringae. J Antibiot. 1986;39:1011–1012. doi: 10.7164/antibiotics.39.1011. [DOI] [PubMed] [Google Scholar]
- 27.Stapley E O, Hendlin D, Mata J M, Jackson M, Wallick H, Hernández S, Mochales S, Currie S A, Miller R M. Phosphonomycin. 1. Discovery and in vitro characterisation. Antimicrob Agents Chemother. 1969;9:284–290. [PubMed] [Google Scholar]
- 28.Suárez J E, Arca P, Villar C J, Hardisson C. Evolutionary origin, genetics, and biochemistry of clinical fosfomycin resistance. In: Hershberger C L, Queener S W, Hegeman G, editors. Genetics and molecular biology of industrial microorganisms. Washington, D.C: American Society for Microbiology; 1990. pp. 93–98. [Google Scholar]
- 29.Tsuruoka T, Yamada Y. Characterisation of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B “in vitro.”. J Antibiot. 1975;28:906–911. doi: 10.7164/antibiotics.28.906. [DOI] [PubMed] [Google Scholar]
- 30.Venkateswaran T S, Wu H C. Isolation and characterization of a fosfomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972;110:935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wackett L P, Shames S L, Venditti C P, Walsh C T. Bacterial carbon-phosphorus lyase: products, rates, and regulation of phosphonic and phosphinic acid metabolism. J Bacteriol. 1987;169:710–717. doi: 10.1128/jb.169.2.710-717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weimberg R, Orton W L. Repressible acid phosphomonoesterase and constitutive pyrophosphatase of Saccharomyces mellis. J Bacteriol. 1963;86:805–813. doi: 10.1128/jb.86.4.805-813.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White R F, Birnbaum J, Meyer R T, Broeke J T, Chemerde J M, Demain A L. Microbial epoxidation of cis-propenylphosphonic acid to (−)-cis-1,2-epoxypropylphosphonic acid. Appl Microbiol. 1971;22:55–60. doi: 10.1128/am.22.1.55-60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]