Abstract
Degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of bacteria in soil was investigated by applying different inoculation protocols. The soil was inoculated with Sphingomonas paucimobilis BA 2 and strain BP 9, which are able to degrade anthracene and pyrene, respectively. CFU of soil bacteria and of the introduced bacteria were monitored in native and sterilized soil at different pHs. Introduction with mineral medium inhibited PAH degradation by the autochthonous microflora and by the strains tested. After introduction with water (without increase of the pore water salinity), no inhibition of the autochthonous microflora was observed and both strains exhibited PAH degradation.
Polycyclic aromatic hydrocarbons (PAHs) are pollutants which are widely distributed in the ecosphere. These compounds enter the environment either adsorbed onto particles by emissions from combustion processes or from spilling of mineral or tar oils. Pollution of soil by tar oil from coal liquefaction and gasification facilities is the source of considerable contamination by PAHs (5). Those sites are subject to remediation activities, since PAHs are a serious risk to human health as a result of their carcinogenic potential (30). For bioremediation of contaminated environments, seeding by introduction of microorganisms has been considered a valuable tool for increasing the rate and extent of biodegradation of pollutants (2). Increased biodegradation of xenobiotic pollutants by such treatments has been demonstrated in several studies (3, 4, 10, 12, 21). However, degradation by introduced bacteria could not be established in other soils, and the reasons for the failures have not been further investigated (9, 11, 19). The advantage of seeding is not generally accepted, since inoculation experiments have shown ambiguous results in comparison to degradation by indigenous microorganisms (2, 7, 18). We have isolated several strains of bacteria from a site contaminated with tar oil. The bacteria are able to grow on PAHs and were found in the soil at levels of 102 to 105 CFU per g of soil (15). However, no PAH degradation was observed after introduction of these strains into artificially contaminated soil. The goal of the present study was to investigate and overcome this inhibition of the introduced bacteria. The activity and survival of the PAH-degrading bacteria were examined by applying different inoculation protocols in native and sterilized soils with different pHs.
Experimental methods.
The experiments were carried out with soil from an Ah horizon of a Luvisol (German systematic) from a noncontaminated rural area (16). All concentrations are dry weights of soil. The experiments were conducted with 100 g of soil in 1.5-liter jars at 25°C, as described previously (17). The soil was sterilized three times by autoclaving of 500 g for 30 min followed by incubation for 24 h at 37°C. The pH of the soil was determined to be 5.2. The pH was shifted to 7.0 by adding 80 mg of CaO to 100 g of soil. After neutralization, the soil was distributed into vessels and contaminated with PAHs as described previously (17). The first set of experiments (see Table 1) was conducted with 100 mg (each) of phenanthrene, anthracene, fluoranthene, and pyrene per kg. All other experiments were conducted with 25 mg of anthracene or pyrene per kg. The suspended bacteria were added to the soil after contamination. The water content of the soil was finally adjusted to 60% of the water-holding capacity (WHC). Sampling, isolation, identification, culture conditions, and physiological characteristics of the PAH-degrading bacteria used have already been described (15). The phenanthrene- and anthracene-degrading strain BA 2 was identified as Sphingomonas paucimobilis, and the phenanthrene-, anthracene-, fluoranthene-, and pyrene-degrading strain BP 9 was classified as a Gordona-like species by the Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany (15).
TABLE 1.
Degradation of PAHs in contaminated soils and after inoculation with PAH-degrading strain BP 9
| Soil and inoculation | PAH concn (mg/kg)a at day:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Phenanthrene
|
Anthracene
|
Fluoranthene
|
Pyrene
|
|||||
| 0 | 98 | 0 | 98 | 0 | 98 | 0 | 98 | |
| Native | 107 ± 5.3 | 1.5 ± 0.02 | 111 ± 4.7 | 0.07 ± 0.01 | 107 ± 5.5 | 89 ± 1.8 | 103 ± 5.3 | 85 ± 2.0 |
| Sterilized | 99 ± 4.6 | 82 ± 1.4 | 108 ± 4.7 | 94 ± 1.4 | 103 ± 1.8 | 88 ± 1.8 | 99 ± 0.9 | 84 ± 1.7 |
| Native + BP 9 | 90 ± 3.7 | 78 ± 1.1 | 95 ± 2.0 | 72 ± 1.0 | 89 ± 3.0 | 84 ± 1.3 | 89 ± 2.8 | 79 ± 1.1 |
| Sterilized + BP 9 | 85 ± 1.9 | 58 ± 1.0 | 103 ± 2.2 | 74 ± 0.5 | 85 ± 1.8 | 62 ± 1.1 | 82 ± 1.9 | 60 ± 0.9 |
Values are means ± standard deviations.
The cultures for the inocula were incubated in 250-ml serum bottles on a shaker (150 rpm) at 25°C. The mineral medium contained, per liter, 2.13 g of Na2HPO4, 1.3 g of KH2PO4, 0.5 g of NH4Cl, 0.2 g of MgSO4 · 7H2O, 1 ml of trace element solution, and 3 ml of vitamin solution (pH 6.9). Strain BA 2 was cultivated in the presence of 50 mg of anthracene per liter, and strain BP 9 was cultivated with 200 mg of pyrene per liter. The cultures were harvested in log phase by centrifugation, and the pellets were washed twice with decreasing concentrations of the mineral medium for introduction protocols B and C. Protocol A (introduction with mineral medium) was performed by resuspending the pellet in 5 ml of mineral medium. Protocol B (introduction with deionized water) was carried out by resuspending the pellet in 5 ml of deionized water. This solution was mixed with 100 g of contaminated soil and the water content was adjusted to 60% of the WHC. In protocol C (slurry phase introduction), the pellet was resuspended in 50 ml of water. The water was mixed with 100 g of soil, and the slurry was dried to 60% of the WHC at 20°C within 24 h. The bacteria were introduced to a final concentration of 2 × 108 cells/g of soil. Microorganisms were extracted from the soil by mixing 1 g of soil with 10 ml of sterile Na2P2O7 solution (2.8 g/liter) and 3 g of glass beads (diameter, 5 mm) in 50-ml tubes for 2 h in a horizontal position on a shaker (350 rpm). The soil particles were allowed to sediment for 30 min. The supernatant was diluted and plated on solid media. Total CFU were determined after 8 days by counting the colonies on mineral medium without additional carbon sources. The total CFU of the native Ah soil amounted to 107/g. PAH-degrading bacteria were determined by clear-zone-forming colonies on mineral media coated with a crystal layer of the respective PAH (15). In addition, due to their characteristic visible shape and color, colonies of strain BP 9 were counted separately from other colonies on the media. Colonies of strain BA 2 could be detected only by anthracene degradation. No PAH-degrading organisms were detected in the native Ah soil. PAH concentrations in the soil were analyzed by ultrasonic extraction with ethylacetate. This was followed by alkaline hydrolysis to extract PAH residues not extractable by organic solvents (8). PAHs were quantified by high-performance liquid chromatography as described previously (17). The presented data are summarized from both extraction procedures and are means of triplicate analyses.
Results and discussion.
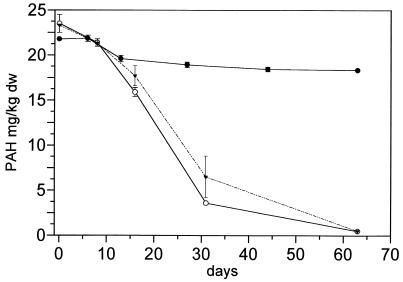
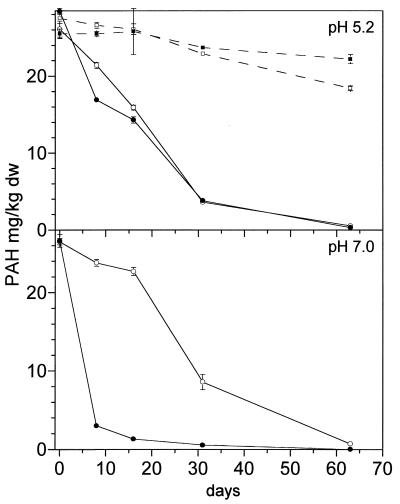
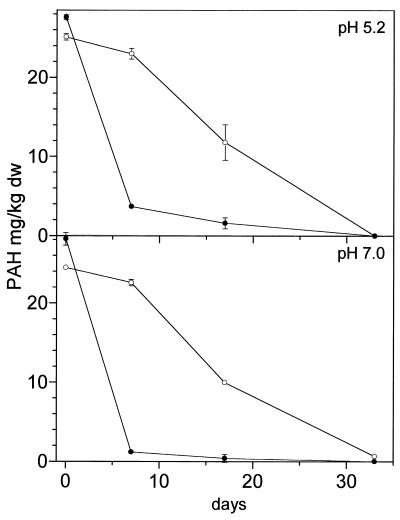
Native Ah soil showed degradation of anthracene and phenanthrene, whereas fluoranthene and pyrene were not degraded (Table 1). Due to sequestering into soil micropores, a slight abiotic loss was observed in sterilized Ah soil. To improve the PAH degradation, the soil was inoculated with PAH-degrading bacteria. The Gordona-like strain BP 9, which is able to degrade the respective PAHs (see “Experimental methods”), was introduced by protocol A with mineral medium. However, no decrease in the PAHs was observed in the seeded culture (Table 1). Moreover, degradation of phenanthrene and anthracene by the autochthonous soil microflora was completely inhibited. Only slight degradation was observed in sterilized soil. However, 108 CFU/g of viable pyrene-degrading colonies of BP 9 were detected after 5 days, and 106 CFU/g were still present after 35 days in the native soil. The decline in CFU of strain BP 9 was more rapid in sterilized soil (only 3 × 104 CFU/g after 5 days), indicating better survival in native soil. Similar results were obtained with the anthracene-degrading strain BA 2 (Fig. 1). After introduction by protocol A with mineral medium, no anthracene degradation was observed and the degradation by the autochthonous microflora was also inhibited. Anthracene-degrading colonies at >102 CFU/g were still detected after 30 days. Thus, the introduced bacteria were able to survive and to compete with the indigenous microflora. Introduction of strain BA 2 by protocol C in slurry phase with water did not inhibit degradation by the native soil (Fig. 1). However, degradation was not improved by strain BA 2. The results suggest that the inhibition of PAH degradation in the native soil was caused by the salt content of the mineral medium used in protocol A. Therefore, introduction protocol B with water was performed without increasing the salt content of the soil. Both strains were tested with protocol B. Anthracene degradation by the autochthonous microflora was not inhibited. However, no improved anthracene degradation by strain BA 2 was observed in native or sterilized soil (pH 5.2) (Fig. 2), whereas a sixfold increase in pyrene degradation was caused by BP 9 (Fig. 3). The lack of degrading activity of strain BA 2 in the native soil suggested additional inhibition by the pH level. This hypothesis was tested by neutralizing the soil pH with CaO. Cultures at pH 7 showed a 10-fold-increased degradation rate in the soil after introduction by protocol B (Fig. 2). Pyrene degradation by strain BP 9 was not significantly affected by the soil pH, and similar rates were observed in sterilized soil cultures (data not shown).
FIG. 1.
Degradation of anthracene in contaminated soil after inoculation with S. paucimobilis BA 2 by different procedures. Symbols: ○, native soil (control); •, introduction protocol A (addition of strain BA 2 with mineral medium); ▾, introduction protocol C (addition of strain BA 2 by slurry application). dw, dry weight.
FIG. 2.
Degradation of anthracene by S. paucimobilis BA 2 in contaminated soils with different pHs. Symbols: ○, native soil (control); •, introduction protocol B (addition of strain BA 2 with water); □, sterilized native soil; ▪, introduction protocol B with sterilized soil. dw, dry weight.
FIG. 3.
Degradation of pyrene by strain BP 9 in contaminated soils with different pHs. Symbols: ○, native soil (control); •, introduction protocol B (addition of strain BP 9 with water). dw, dry weight.
The results demonstrate that introduction by protocol B with water is the appropriate method to overcome the inhibition observed by use of protocol A with mineral medium. We present, to our knowledge, the first evidence that the salinity of the introduction medium may repress the PAH degradation activity of the introduced bacteria and of the autochthonous soil microflora. This confirms the essential impact of the inoculation protocol on the activity of bacteria in soil. Introduction of bacteria into soil has usually been conducted with buffers or media (26) without focused attention on introduction media and details of the protocol. A Mycobacterium sp. introduced into contaminated soils simply as free cells in Tris buffer increased the mineralization of pyrene from 1 to 55% (12). PAH degradation in other soils was only slightly enhanced by inoculation (10). Previous studies have presented liquid inoculation methods, such as simple spraying or mixing procedures, or semisolid inoculation methods, such as immobilization techniques (26). Immobilization is considered to promote better survival and activity of the introduced organisms (3, 6, 21, 29). However, an immobilized phenanthrene-degrading Pseudomonas sp. did not enhance degradation in soil slurries. Additional substrates (28) or inorganic nutrients (11) had a greater impact on degradation and survival. Our results imply that inoculation by media may have caused several failures in the introduction of degradation activities and may have supported the higher effectiveness of immobilized cells. The possible reasons for such failures have not been extensively discussed (9). However, changes in environmental conditions of the soil by the inoculation procedure have been considered, and no attempts to overcome these limitations have been reported.
The focus of previously published inoculation experiments has been the inoculum size (1, 23). The data can be summarized by the fact that increasing the inoculum size increases degradation activity (13). Inoculation levels of 107 to 108 cells/g are considered sufficient to establish degradation activity (13, 14); therefore, 2 × 108 CFU/g were applied in this work. After addition of strain BP 9, total CFU and pyrene-degrading CFU were doubled in native soil (pH 5.2) during initial degradation until day 7. Neutralization of the soil led to 10-fold-increased CFU after 7 days. CFU began to decrease after 7 days, and decline factors of ≈100 CFU within 30 days have also been observed by other authors (22, 27). The decline in pyrene-degrading CFU of strain BP 9 was significantly more rapid than that in total CFU (≈500 at pH 7 and ≈1,000 at pH 5.2). Considerable amounts of the BP 9 colonies lost the ability to degrade pyrene after extraction from soil. This phenomenon, and whether it is an effect of soil cultures or is inherent to the organisms, should be investigated further.
Some authors (6) have argued that pure cultures of bacteria introduced into soil often do not persist due to limited competitiveness, since degradation was observed only in sterilized soil (9, 19). Those considerations imply that survival leads automatically to activity. However, we have shown that bacteria survived in soil without developing their degradation capacities. PAH degradation by the autochthonous microflora was even suppressed after introduction of the bacteria with mineral medium. The observed inhibition was not caused by limited bioavailability. Attempts to improve PAH degradation in this soil by addition of compost were successful (17). The inhibition was caused by the introducing medium, which changed the salinity of the pore water in the soil to the range of marine environments. The impact of salinity on PAH degradation in estuarine sediments has already been described (25). No attention has been paid to this detail of soil inoculation in previous publications. The observed effects depended on the soil type used, since the degradation of PAHs in soil from a contaminated site was not inhibited after introducing the same bacteria with mineral medium (20). Therefore, we have to conclude that the inoculation protocol plays an essential role in establishing the degrading activity of specialized strains in soils.
An important factor for the degradation activity of introduced bacteria is the pH of the soil. The shift of the pH from 5.2 to 7.0 enabled PAH degradation by strain BA 2 (Fig. 2). Neutralization of soil is generally discussed to be favorable for the degradation of mineral oil components by bacteria (18). However, a pH of 5.2 should not lead to total inhibition of activity. That PAHs were available in low concentrations might explain the higher impact of pH on the degradation of PAHs. Small pH shifts have dramatic effects on the degradation of low concentrations of xenobiotics in oligotrophic aquatic environments (31). Remarkable differences in the amounts of mineralization from radiolabeled glucose available at high and low concentrations have recently been demonstrated in soils (24). We have to consider that metabolism of the oligotrophic autochthonous (humus-degrading) microflora of soil might be more sensitive to changes in environmental conditions than metabolism of zymogenic (opportunistic) microflora or of introduced bacteria. Therefore, environmental conditions in soil have to be adjusted carefully to develop the degradation potential of introduced bacteria and of the indigenous microflora. If the degradation potential is inherent to the indigenous soil microflora, the addition of “specialists” may only reduce the time necessary for biodegradation.
Acknowledgments
We thank V. Kasche for stimulating discussions.
The research on this subject was financed by the Deutsche Forschungsgemeinschaft (DFG) within the interdisciplinary research project “Remediation of Contaminated Soil” (SFB 188).
REFERENCES
- 1.Acea M J, Moore C R, Alexander M. Survival and growth of bacteria introduced into soil. Soil Biol Biochem. 1988;4:509–515. [Google Scholar]
- 2.Atlas R M. Bioremediation of fossil fuel contaminated soils. In: Hinchee R E, Olfenbuttel R F, editors. In situ bioreclamation. Boston, Mass: Butterworth-Heinemann; 1991. pp. 14–32. [Google Scholar]
- 3.Briglia M, Nurmiaho-Lassila E-L, Vallini G, Salkinoja-Salonen M. The survival of the pentachlorophenol-degrading Rhodococcus chlorophenolicus PCP-1 and Flavobacterium sp. in natural soil. Biodegradation. 1990;1:273–281. [Google Scholar]
- 4.Brodkorb T S, Legge R L. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3117–3121. doi: 10.1128/aem.58.9.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerniglia C E. Microbial degradation of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- 6.Crawford R L, O’Reilly K T, Tao H-L. Microorganism stabilization for in situ degradation of toxic chemicals. In: Kamely D, Chakrabarty A, Omenn G S, editors. Biotechnology and biodegradation. Advances in applied biotechnology series. Vol. 4. Houston, Tex: Gulf Publishing Company; 1989. pp. 203–211. [Google Scholar]
- 7.Dott W, Feidieker D, Kämpfer P, Schleibinger H, Strechel S. Comparison of autochthonous bacteria and commercially available cultures with respect to their effectiveness in fuel oil degradation. J Ind Microbiol. 1989;4:365–374. [Google Scholar]
- 8.Eschenbach A, Kästner M, Bierl R, Schaefer G, Mahro B. Evaluation of a new and more effective method to extract polycyclic aromatic hydrocarbons from soil samples. Chemosphere. 1994;28:683–692. [Google Scholar]
- 9.Goldstein R M, Mallory L M, Alexander M. Reasons for possible failure of inoculation to enhance biodegradation. Appl Environ Microbiol. 1985;50:977–983. doi: 10.1128/aem.50.4.977-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosser R J, Warshawsky D, Vestal J R. Indigeous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl Environ Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkness M R, McDermott J B, Abramowicz D A, Salvo J J, Flanagan W P, Stephens M L, Mondello F J, May R J, Lobos J H, Carroll K M, Brennan M J, Bracco A A, Fish K M, Warner G L, Wilson P R, Dietrich D K, Lin D T, Morgan C B, Gately W L. In situ stimulation of aerobic PCB biodegradation in Hudson River sediments. Science. 1993;259:503–507. doi: 10.1126/science.8424172. [DOI] [PubMed] [Google Scholar]
- 12.Heitkamp M A, Cerniglia C E. Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosms containing sediment and water from a pristine ecosystem. Appl Environ Microbiol. 1989;55:1968–1973. doi: 10.1128/aem.55.8.1968-1973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen C S, Petersen J C. Growth and survival of Pseudomonas cepacia DBO1(pRO101) in soil amended with 2,4-dichlorophenoxyacetic acid. Biodegradation. 1992;2:245–252. doi: 10.1007/BF00114556. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen C S, Petersen J C. Mineralization of 2,4-dichlorophenoxyacetic acid (2,4-D) in soil inoculated with Pseudomonas cepacia DBO1(pRO101), Alcaligenes eutrophus AEO106(pRO101) and Alcaligenes eutrophus JMP134(pJP4): effects of inoculation level and substrate concentration. Biodegradation. 1992;2:253–263. doi: 10.1007/BF00114557. [DOI] [PubMed] [Google Scholar]
- 15.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 16.Kästner M, Lotter S, Heerenklage J, Breuer-Jammali M, Stegmann R, Mahro B. Fate of 14C-labeled anthracene and hexadecane in compost manured soil. Appl Microbiol Biotechnol. 1995;43:1128–1135. doi: 10.1007/BF00166937. [DOI] [PubMed] [Google Scholar]
- 17.Kästner M, Mahro B. Microbial degradation of polycyclic aromatic hydrocarbons in soils affected by the organic matrix of compost. Appl Microbiol Biotechnol. 1996;44:668–675. doi: 10.1007/BF00172501. [DOI] [PubMed] [Google Scholar]
- 18.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S Y, Lu M-H, Bollag J-M. Transformation of metachlor in soil inoculated with Streptomyces sp. Biodegradation. 1990;1:9–17. [Google Scholar]
- 20.Mahro B, Schaefer G, Kästner M. Pathways of microbial degradation of polycyclic aromatic hydrocarbons in soil. In: Hinchee R E, Leeson A, Semprini L, Ong S K, editors. Bioremediation of chlorinated and polycyclic aromatic hydrocarbons. Boca Raton, Fla: Lewis Publishers; 1994. pp. 203–217. [Google Scholar]
- 21.Middledorp P J M, Briglia M, Salkinoja-Salonen M. Biodegradation of pentachlorophenol in natural soil by inoculated Rhodococcus chlorophenolicus. Microb Ecol. 1990;20:123–139. doi: 10.1007/BF02543872. [DOI] [PubMed] [Google Scholar]
- 22.Ogunseitan O A, Delgado I L, Tsai Y-L, Olson B H. Effect of 2-hydroxybenzoate on the maintenance of naphthalene-degrading pseudomonads in seeded and unseeded soil. Appl Environ Microbiol. 1991;57:2873–2879. doi: 10.1128/aem.57.10.2873-2879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramadan M A, El-Tayeb O M, Alexander M. Inoculum size as a factor limiting success of inoculation for biodegradation. Appl Environ Microbiol. 1990;56:1392–1396. doi: 10.1128/aem.56.5.1392-1396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J, Bartha R. Metabolic efficiency and turnover of soil microbial communities in biodegradation tests. Appl Environ Microbiol. 1996;62:2411–2415. doi: 10.1128/aem.62.7.2411-2415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiaris M P. Seasonal biotransformation of naphthalene, phenanthrene, and benzo[a]pyrene in surficial estuarine sediments. Appl Environ Microbiol. 1989;55:1391–1399. doi: 10.1128/aem.55.6.1391-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevors J T, Kuikman P, van Elsas J D. Review article. Release of bacteria into soil: cell numbers and distribution. J Microbiol Methods. 1994;19:247–259. [Google Scholar]
- 27.Wagner-Döbler I, Pipke R, Timmis K N, Dwyer D F. Evaluation of aquatic sediment microcosms and their use in assessing possible effects of introduced microorganisms on ecosystem parameters. Appl Environ Microbiol. 1992;58:1249–1258. doi: 10.1128/aem.58.4.1249-1258.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir S C, Dupuis S P, Providenti M A, Lee H, Trevors J T. Nutrient-enhanced survival of and phenanthrene mineralization by alginate-encapsulated and free Pseudomonas sp. UG14Lr cells in creosote-contaminated soil slurries. Appl Microbiol Biotechnol. 1995;43:964–951. doi: 10.1007/BF02431932. [DOI] [PubMed] [Google Scholar]
- 29.Wiesel I, Wübker S M, Rehm H-J. Degradation of polycyclic aromatic hydrocarbons by an immobilized mixed bacterial culture. Appl Microbiol Biotechnol. 1993;39:110–116. [Google Scholar]
- 30.Wislocki P G, Lu A Y H. Carcinogenicity and mutagenicity of proximate and ultimate carcinogens of polycyclic aromatic hydrocarbons. In: Yang S K, Silverman B D, editors. Polycyclic aromatic hydrocarbon carcinogenesis: structure-activity relationships. Vol. 1. Boca Raton, Fla: CRC Press; 1988. pp. 1–30. [Google Scholar]
- 31.Zaidi B R, Stucki G, Alexander M. Low chemical concentration and pH as factors limiting the success of inoculation to enhance biodegradation. Environ Toxicol Chem. 1988;7:143–151. doi: 10.1021/es00177a005. [DOI] [PubMed] [Google Scholar]