Abstract
Fatty acid biomarker analysis coupled with gas chromatography-isotope ratio mass spectrometry was used to confirm the presence of methanotrophic and thiotrophic bacterial endosymbionts in the tissues of a hydrothermal vent mussel (Bathymodiolus sp.), collected from the Menez Gwen vent field on the mid-Atlantic ridge. Monounsaturated (n-8) fatty acids, which are diagnostic of methanotrophic bacteria, were detected in all three types of tissues examined (gill, posterior adductor, and mantle), although levels were highest in gill tissues where the bacteria were found. Stable-carbon-isotope compositions (δ-13C per mille relative to that of Peedee belemnite) of fatty acids for all three tissues ranged from −24.9 to −34.9‰, which encompasses the range predicted for both thiotroph- and methanotroph-based nutrition. The data suggest that these thio- and methanotrophic bacterial endosymbionts are equally important in the nutrition of the vent mussel at this particular vent site.
Symbioses between deep-sea bivalves and either thio- (4, 18) or methanotrophic bacteria (5, 8, 18) have been widely reported. More recently, it has been established that some bivalves from hydrothermal vents on the mid-Atlantic ridge (MAR) contain both types of symbiotic bacteria in their gills (6), and this has been interpreted as being advantageous for the bacteria in colonizing a wider range of geochemical environments (17).
Fatty acid biomarkers, diagnostic for thio- and methanotrophic bacteria, have proven to be a useful tool in the study of host-symbiont relationships in deep-sea faunas (33–35). When such analyses are coupled with isotope ratio mass spectrometry (IRMS), the technique becomes even more powerful and it may be possible to identify both the source of carbon and trophic transfer within chemoautolithotrophic ecosystems (1, 16, 28, 33, 36).
In contrast to deeper MAR sites (3,000 to 3,650 m), where alvinocaridid shrimps tend to dominate (38, 41), the Menez Gwen (850 m) and Lucky Strike (1,650 m) vent fields at the Azores triple junction are dominated by an undescribed bivalve mussel, Bathymodiolus sp. (14, 21, 42). Stable-carbon-isotope (δ-13C) values of −24.1‰ for the mussels at Lucky Strike led to speculation that methanotrophic metabolism could be a significant source of nutrition (17). In the present study we conducted gas chromatography (GC)-IRMS analysis of various tissue types in the mussel species from Menez Gwen in an attempt to establish, from the stable-isotope compositions of their fatty acid biomarkers, the relative importance of thio- and methanotrophic bacterial endosymbionts in its nutrition.
Station location and description.
Three specimens of Bathymodiolus sp. were collected from the same locality by using the IFREMER submersible vessel Nautile, during the DIVA 2 cruise (dive PL 16), in June 1994 from the Menez Gwen hydrothermal vent field at 37°50′N, 31°31′W (850 m) on the MAR. As with other vent sites, the mussels at Menez Gwen were found in areas typified by diffuse-flow, low-temperature venting. A full description of the Menez Gwen site and ecology is given in references 14, 21, and 22. Mussels were frozen at −70°C shortly after collection. Specimens were measured (greatest anterior-to-posterior-shell lengths) and weighed in the laboratory before removal of mantle, gill, and posterior adductor tissues. Thin films of dark-brown epibiotic material which coated the outside of the shells were scraped off with a scalpel for analysis.
Lipid analyses.
Immediately after dissection, mussel tissue samples and epibiotic material were homogenized in chloroform-methanol (2:1, vol/vol) before being filtered through a prewashed (chloroform-methanol [2:1, vol/vol]) Whatman no. 1 paper filter. Total lipid was extracted by following the method of Folch et al. (20) and dried under nitrogen. Aliquots of total lipid were transesterified in methanol containing 1.5% (vol/vol) sulfuric acid for 16 h at 50°C (9), and the fatty acid methyl esters were purified by thin-layer chromatography. Component fatty acid methyl esters were analyzed by GC on a Canberra 436 GC fitted with a BP20 fused silica capillary column (50 m by 0.32 mm, inside diameter; SGE) with hydrogen as a carrier gas.
GC-MS.
To reduce coelution of fatty acids, methyl esters were first separated into saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), diunsaturated fatty acid (DUFA), and polyunsaturated fatty acid (PUFA; three or more double bonds) fractions by argentation high-performance thin-layer chromatography with hexane-diethyl ether (90:10, vol/vol) (43). MUFA positional isomers were determined with dimethyl disulfide adducts (30) and the double-bond positions of DUFAs and PUFAs as both diethylamide (31) and picolinyl (10) derivatives with a Fisons MD 800 GC-MS. The GC was fitted with a DB-5MS column (15 m by 0.25 mm, inside diameter; J & W Scientific) and the MS was operated in the electron-impact-negative mode with helium as the carrier gas.
GC-IRMS.
Stable-carbon-isotope ratios (13C/12C) were measured by GC-combustion IRMS with a VG Isochrom II instrument equipped with a column similar to that described above (15). Conventional quartz closed-tube combustion (39) was employed to determine the δ-13C composition of the methanol used to prepare the methyl esters, and the contribution of derivatized carbon to specific fatty acids was calculated as described previously (33).
The three mussels used in this study had wet masses, including their shells, of 17.5, 12.6, and 10.3 g. Maximum anterior-to-posterior-shell dimensions of these same specimens were 6.7, 5.4, and 4.9 cm, respectively.
Fatty acid composition.
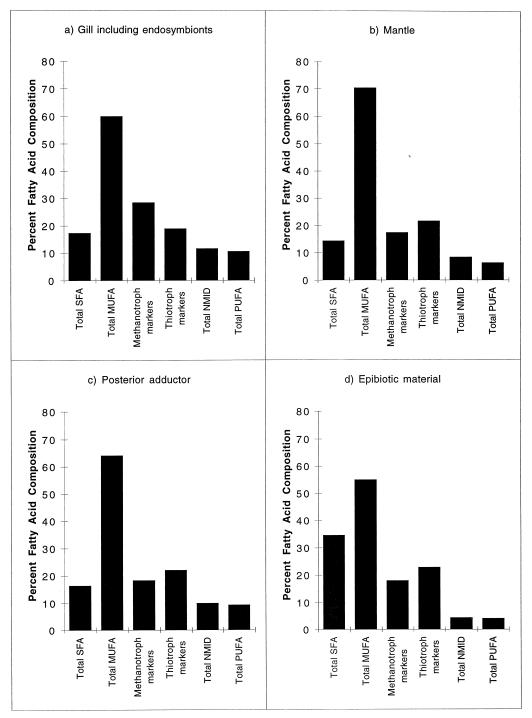
Overall, the fatty acid compositions of gill, mantle, and posterior adductor tissues were similar and dominated by the SFA 16:0 and (n-7) MUFAs (Table 1; Fig. 1). Lesser amounts of 16:1(n-9) and 16:1(n-8) MUFAs were also detected, although these could not be adequately resolved for accurate quantification by GC and hence had to be combined (Table 1). Notably, there were significantly higher proportions of 16:1(n-9+n-8) and 20:1(n-13) MUFAs in the gill tissue fractions than in mantle and adductor tissue fractions (analysis of variance [ANOVA], P < 0.001 and P < 0.01, respectively) (Fig. 1). Gill tissue also contained a significantly lower proportion of 18:1(n-7) than mantle and adductor tissues (ANOVA, P < 0.001 and P < 0.003, respectively).
TABLE 1.
Fatty acid compositions of various tissues and epibiotic material from hydrothermal vent mussels
| Fatty acid | Biomarker designationa | Mean weight % (range) fatty acid compositionb of:
|
|||
|---|---|---|---|---|---|
| Mantle tissue | Posterior adductor tissue | Gill tissue including endosymbionts | Epibiotic material | ||
| 14:0 | T | 0.5 (0.4–0.8) | 0.6 (0.5–0.8) | 2.0 (1.8–2.4) | 5.4 (3.5–9.2) |
| 14:1 | Tr. | ||||
| 15:0 | 1.6 (1.0–2.6) | ||||
| 16:0 | T + M | 12.2 (11.1–13.4) | 13.7 (13.2–14.0) | 12.7 (11.1–15.0) | 21.3 (16.6–27.6) |
| 16:1(n-9+n-8) | M | 2.3 (1.9–2.7) | 2.6 (2.2–3.0) | 16.3 (13.4–17.7) | 5.0 (2.8–7.7) |
| 16:1(n-7) | T + M | 39.7 (37.5–44.4) | 34.5 (31.5–37.6) | 27.6 (24.4–29.3) | 28.9 (19.8–35.5) |
| 18:0 | T | 1.5 (1.2–1.6) | 1.9 (1.8–2.0) | 2.4 (2.2–2.6) | 5.7 (4.4–8.0) |
| 18:1(n-13+n-9+n-8) | M | 5.4 (4.8–6.2) | 5.1 (4.8–5.3) | 2.8 (2.1–3.0) | 8.0 (6.6–8.7) |
| 18:1(n-7) | T | 8.2 (7.5–8.7) | 7.4 (6.7–8.8) | 2.4 (1.9–3.1) | 6.8 (6.1–7.6) |
| 18:2(n-6) | T + M | 1.0 (0.8–1.1) | 1.0 (0.8–1.1) | 0.6 (0.6–0.8) | 2.7 (1.0–3.8) |
| 18:3(n-7) | T | 3.6 (3.2–4.3) | 4.7 (4.3–5.2) | 6.5 (6.3–6.8) | 2.0 (0.7–2.7) |
| 20:0 | Tr. | Tr. | Tr. | 0.4 (0–0.8) | |
| 20:1(n-13) | M | 2.5 (2.1–3.1) | 3.4 (2.8–4.2) | 5.3 (4.6–6.2) | 1.6 (0.6–2.4) |
| 20:1(n-9+n-8) | M | 4.5 (3.9–4.8) | 3.7 (3.4–4.1) | 1.5 | 1.8 (0.7–2.7) |
| 20:1(n-7) | T | 7.7 (7.1–8.4) | 7.1 (6.1–8.5) | 5.5 (5.1–5.7) | 2.7 (0.7–4.1) |
| 20:2 Δ5,11 | T + M | 2.6 (2.5–2.8) | 3.1 (2.8–3.7) | 3.5 (3.2–3.7) | 1.2 (0.3–1.8) |
| 20:2 Δ5,13 | T + M | 3.6 (2.8–4.1) | 4.0 (3.6–4.2) | 5.4 (5.3–5.7) | 1.9 (0.5–2.9) |
| 20:3(n-9) | M | 0.8 (0.6–0.8) | 1.0 (0.9–1.1) | 1.2 (1.0–1.3) | 0.4 (0.2–0.6) |
| 20:3(n-7) | T | 1.8 (1.5–2.1) | 2.3 (2.1–2.5) | 2.8 (2.4–3.2) | 1.0 (0.4–1.4) |
| 20:5(n-3) | 0.5 (0.2–1.3) | 0.7 (0.5–1.0) | |||
| 22:2 Δ7,13 | Tr. | ||||
| 22:2 Δ7,15 | T + M | 2.0 (1.4–2.5) | 2.6 (2.4–3.0) | 2.5 (2.3–2.8) | 1.0 (0.2–1.5) |
| 22:6(n-3) | 0.6 (0.2–1.5) | ||||
T, thiotrophic fatty acid; M, methanotrophic fatty acid.
n = 3. Tr., trace (<0.1%).
FIG. 1.
Proportions of different groups of fatty acids from gill tissue containing endosymbionts (a), mantle tissue (b), posterior adductor tissue (c), and epibiotic material (d). Thiotrophic and methanotrophic fatty acid markers comprise only those fatty acids considered to be specific to either group (Table 1).
The non-methylene-interrupted dienes (NMIDs) 20:2 Δ5, 11; 20:2 Δ5, 13; and 22:2 Δ7, 15 were moderately abundant in all three tissues and, when combined, accounted for 8.2 to 11.4% of the total fatty acids (Fig. 1). Gill tissue contained significantly higher proportions of the triunsaturated fatty acid 18:3(n-7) than mantle and adductor tissues (ANOVA, P < 0.001, and P < 0.004, respectively) (Table 1), while 20:3(n-7) was only significantly higher in gill tissue than in mantle tissue (ANOVA, P < 0.03). The PUFAs 20:5(n-3) and 22:6(n-3) were detected only in posterior adductor tissue in very small amounts (Table 1; Fig. 1).
Compared to the mussel tissues, epibiotic material from the shell surfaces contained larger amounts of SFAs (14:0, 16:0, and 18:0) and smaller amounts of NMIDs and 20:3(n-7) (Table 1; Fig. 1). When combined, the (n-13) and (n-9+n-8) MUFAs were present in proportions comparable to those observed in mussel tissue and accounted for approximately 16% of both mussel tissue and epibiotic fatty acids. 20:5(n-3) was also detected in the epibiotic fatty acids, although in very small amounts (Table 1).
Stable-isotope composition.
The methanol used to prepare methyl esters had a δ-13C value of −41.8‰ relative to that of Peedee belemnite (PDB), and GC-IRMS values of fatty acids were corrected (33). Mean stable-carbon-isotope compositions (δ-13C per mille relative to that of PDB) of fatty acids in the mussel tissues ranged from −24.9‰ for the NMID 20:2 Δ5,11 to −34.9‰ for 14:0 (in gill tissue), although the majority of fatty acids were in the general range −28.5 to −32.0‰ (Table 2). Although stable-isotope ratios for specific fatty acids in the three tissue types were similar, the gill SFAs 14:0, 16:0, and 18:0 were all significantly 12C enriched compared to the corresponding fatty acids from mantle and adductor tissues (ANOVA, P < 0.05) (Table 2).
TABLE 2.
δ-13C values of fatty acids in various tissues and epibiotic material of the hydrothermal vent mussel Bathymodiolus sp.
| Fatty acid | Mean δ-13C value (range)a of:
|
|||
|---|---|---|---|---|
| Mantle tissue | Posterior adductor tissue | Gill tissue including endosymbiots | Epibiotic material | |
| 14:0 | −29.7 (−29.7 to −29.7) | −26.4 (−26.3 to −26.4) | −34.9 (−34.5 to −35.6) | −26.0 (−25.7 to −26.2) |
| 16:0 | −27.5 (−26.7 to −28.8) | −25.9 (−25.3 to −26.7) | −29.5 (−28.7 to −30.3) | −26.9 (−25.3 to −27.9) |
| 16:1(n-9+n-8+n-7) | −28.8 (−28.0 to −30.3) | −28.2 (−27.3 to −29.1) | −28.5 (−27.7 to −29.2) | −26.2 (−24.9 to −27.4) |
| 18:0 | −27.5 (−26.0 to −29.0) | −28.1 (−27.6 to −28.3) | −31.1 (−27.8 to −33.0) | −29.0 (−27.1 to −31.4) |
| 18:1(n-13+n-9+n-8) | −28.6 (−25.8 to −30.8) | −29.3 (−28.1 to −30.2) | −28.8 (−25.3 to −33.7) | −29.0 (−27.6 to −30.7) |
| 18:1(n-7) | −30.2 (−27.7 to −32.1) | −28.7 (−28.4 to −29.2) | −31.5 (−29.3 to −34.6) | −26.4 (−25.9 to −27.1) |
| 18:2(n-6) | −28.7 (−27.6 to −29.8) | −28.7 (−28.5 to −28.8) | −28.9 (−28.3 to −29.5) | |
| 18:3(n-7) | −28.6 (−27.1 to −30.0) | −31.9 (−29.7 to −33.7) | −34.5 (−34.1 to −34.8) | −30.1 |
| 20:1(n-13+n-9+n-8) | 28.6 (−24.7 to −30.9) | −31.3 (−28.9 to −35.3) | −30.8 (−30.4 to −31.0) | −28.8 |
| 20:1(n-7) | −29.7 (−28.1 to −31.7) | −29.3 (−28.0 to −30.6) | −31.3 (−30.0 to −32.5) | −30.1 |
| 20:2 Δ5,11 | −24.9 (−19.8 to −28.3) | −29.7 (−29.1 to −30.1) | −27.9 (−26.5 to −30.5) | |
| 20:2 Δ5,13 | −25.9 (−21.3 to −30.2) | −28.7 (−28.4 to −29.0) | −28.6 (−26.3 to −30.2) | |
| 20:3(n-7) | −30.4 (−30.2 to −30.6) | −30.5 (−29.3 to −31.7) | ||
| 22:2 Δ7,15 | −28.4 (−23.7 to −31.0) | −30.3 (−28.6 to −31.8) | ||
All values are corrected for the −41.8‰ δ-13C value of the derivatization reagent. n = 3.
Stable-isotope compositions of epibiont fatty acids were within the range of values determined for the mussel tissues (−26.0 to −30.1‰) (Table 2). The fatty acids 14:0, 16:1(n-9+n-8+n-7), and 18:1(n-7) had significantly higher levels of δ-13C in epibiotic material than in all mussel tissues, while 16:0 was only significantly 13C enriched compared to gill tissue (ANOVA, P < 0.01).
In order to gain further information on the contribution of the thiotrophic and methanotrophic endosymbionts to the nutrition of the mussel, the stable-carbon-isotope composition of total free fatty acids of mantle tissue was measured; the value was determined to be −28.3‰ by closed-tube combustion. Mantle tissue is not known to contain endosymbionts, and all fatty acids contained in this tissue can be attributed to the mussel.
Discussion.
The occurrence of deep-sea invertebrate-bacterium symbioses has previously been well-established by techniques such as electron microscopy (6, 18, 40), enzyme assays diagnostic of particular bacterial symbionts (6, 17, 18), and analyses of bulk-carbon-isotope ratios (6, 19). However, these techniques offer limited quantitative information and do not discriminate between material derived from the host, bacterial endosymbiont(s), and exogenous sources. By contrast, analyses of fatty acid biomarkers often allow a fuller evaluation of host-symbiont energy relationships and carbon sources (23, 26, 33, 34, 36).
Electron microscopy of gill tissues from mussels at the adjacent Lucky Strike site has indicated the presence of endosymbiotic bacteria with stacked intracellular membranes, which are characteristic of type I methanotrophs (17). Mussels at Menez Gwen also appear to have endosymbiotic bacteria with stacked intracellular membranes, as was confirmed by the detection of 16:1(n-8), a fatty acid which is considered to be a definitive marker for this bacterial group (3, 29). Furthermore, the occurrence of this same fatty acid in all tissues, gill, mantle, and adductor muscle, confirmed that (i) methane-oxidizing metabolism is active in the mussel and (ii) the type I methanotroph-derived fatty acids contribute directly to the overall nutrition of the bivalve host. It is notable that the 18:1(n-8) fatty acid, which is diagnostic of type II methanotrophic bacteria (3, 29), was also detected in all the three tissue types. It is established that 16:1(n-7) is a major component of thiotrophic bacteria, and hence this fatty acid has been used as a marker for the presence and abundance of thiotrophic bacteria both in sediments and deep-sea invertebrates (24, 35). However, as 16:1(n-7) can also be a major component of both type I methanotrophs and conventional heterotrophic bacteria (3, 29, 37), its use as a marker for thiotrophs is not definitive. Similarly, 18:1(n-7) has been reported as indicative of the presence of thiotrophic bacteria (24), but this fatty acid is also a substantial component of type II methanotrophs (3). Thiotrophic bacteria have been detected previously in the gill tissues of mussels from the adjacent Lucky Strike vent field (6, 17, 41) and are likely to be present in the animals analyzed in the present study, but the low proportions of 18:1(n-7) in the gill tissues of the Menez Gwen mussels (2.4%) does allow the conclusion that type II methanotroph fatty acids contribute in only a limited way to the overall nutrition of the mussel. The larger amounts of 18:1(n-7) in mantle and adductor tissues (7 to 8%) may indicate active elongation of 16:1(n-7) by the host species, although the presence of a functional gut (28) makes an exogenous bacterial source also a possibility.
The exceptionally low levels of the PUFAs 20:5(n-3) and 22:6(n-3) in all samples implies that (i) there is not a significant source of these compounds at Menez Gwen, either from the endosymbionts or from suspended particles, and (ii) these are not essential fatty acids for the mussel, as they are for higher marine vertebrates and possibly hydrothermal vent shrimps (32–34). It has been proposed that animals whose diets predominantly consist of bacteria, i.e., diets rich in 16:0, 16:1(n-7), and 18:1(n-7) fatty acids, while being relatively deficient in (n-3) PUFAs, produce NMIDs from monenoic fatty acids (2, 27) which effectively substitute for the low levels of (n-3) PUFAs. The presence of circa 10% NMIDs in mussel tissues is consistent with this hypothesis. The higher levels of the triunsaturated fatty acids 18:3(n-7) and 20:3(n-7) in gill tissue suggest that bacterial symbionts may be the source of these compounds. Several studies have demonstrated that thiotrophic bacteria are capable of synthesizing polyunsaturated fatty acids (25, 34), although this ability has not been identified to any extent in methanotrophs (3, 29).
The fatty acid profile of the epibiotic material, while exhibiting large amounts of (n-8) MUFAs, contained substantially higher levels of the SFAs 14:0, 16:0, and 18:0. This is consistent with the epibiotic samples comprising predominantly detrital material, since these fatty acids are least prone to auto-oxidation and tend to accumulate in marine sediments. Furthermore, the detection of (n-8) and NMID fatty acids, albeit in small amounts, suggests this detrital material is derived from the mussel, most probably via feces and pseudofeces. However, it is possible that some of the epibiotic fatty acids originated from bacteria living on the shell, a substratum which is known to be colonized by methanotrophs (12).
Different groups of chemolithotrophic bacteria (thio- and methanotrophs) associated with hydrothermal ecosystems utilize different carbon sources with distinct carbon isotope ratios. Furthermore, these different bacterial groups utilize different enzyme systems for carbon fixation which discriminate by various degrees against 13C. Thiotrophic bacteria oxidize hydrogen sulfide and utilize the energy released to fix carbon dioxide into organic compounds. Similarly, methanotrophs also use a reduced compound as an energy source, in this case methane, which they also use as a carbon source (18). Thus, the isotope signatures in specific carbon-containing compounds can give a valuable indication as to the nature of the original carbon source and the organism(s) responsible for their synthesis.
The carbon isotope ratio of methane in the seawater at Menez Gwen has recently been determined to be circa −13‰ (7), a value which is also within the range reported for thiotrophs (11). Methanotrophic bacteria in deep-sea bivalves discriminate against 13CH4 by approximately 6 to 12‰ (8), and as lipid is isotopically lighter than total organic carbon by 3‰ (due to discrimination against 13C during the synthesis of acetyl coenzyme A by pyruvate decarboxylase [13]), we can predict that fatty acids derived from a methane carbon source would have isotope values in the range −22 to −28‰. This range agrees well with the value of −24.1‰ determined previously from a total carbon analysis of mussels from Lucky Strike, which also contain methanotrophic and thiotrophic endosymbionts (17). In contrast, the carbon isotope ratio of hydrothermal bivalves containing only thiotrophic endosymbionts is usually somewhat lower, with δ-13C values typically in the range −30 to −36‰ (6, 11, 19).
If first we consider gill tissue, where the endosymbionts are known to be located (17), it is apparent that the fatty acids 14:0, 18:0, 18:1(n-7), 20:1(n-7), 18:3(n-7), and 20:3(n-7) are comparatively depleted of 13C, which is consistent with a thiotrophic source for these compounds. By contrast, the fatty acids which are markers for methanotrophs, i.e., 16:1(n-9+n-8+n-7) and 18:1(n-13+n-9+n-8), are relatively enriched with 13C and are at the top end of the range predicted for a methanotrophic carbon source. Unfortunately, it was not possible to distinguish the individual MUFAs, as the GC-IRMS technique necessitates the use of helium as a carrier gas, thus reducing resolution. Therefore, the slightly higher than predicted isotope ratios for MUFAs suggest that at least a proportion of the (n-7) and (n-9) fatty acids are synthesized by the thiotrophic endosymbionts. The isotopically light values for the fatty acids 18:3(n-7) and 20:3(n-7) support the earlier assertion that these compounds are of thiotrophic origin.
Although some fatty acids are characteristic of either a thio- or a methanotrophic origin, others are synthesized by both types of bacteria and hence their isotope ratios should reflect dual biosynthetic pathways. Based on information from the literature (3, 6, 17, 28, 29) and data from the present study, each fatty acid was assigned a biomarker designation in accordance with its origin (Table 1). It was then possible to calculate the potential minimum and maximum percent contributions of the two symbiont pathways to the overall host-symbiont fatty acid pool (Table 3). Minimum estimates include only fatty acids which are recognized specific thio- or methanotroph markers and also those fatty acids whose isotope ratios are typical of one group only. Maximum estimates for each group were based on these same fatty acids, plus those capable of being produced by both symbiont types. Clearly, the maximum value may exaggerate the contributions of those fatty acids which are synthesized by both symbionts. Given that the carbon isotope composition for total fatty acids in mantle tissue is −28.3‰, i.e., a value midway between the range expected for thio- and methanotroph metabolism, it is reasonable to conclude that the two types of symbiont are more or less equally important for the nutrition of the mussel host at this particular vent site. Such nutritional plasticity clearly facilitates the colonization of more than one type of chemical environment (17). It may explain the dominance of mussels at both Menez Gwen and other “shallow” sites on the MAR (e.g., Lucky Strike at 1,700 m), where vent effluent is richer in methane than it is at deeper sites and where thiotrophic bacteria-shrimp symbioses dominate (33, 38, 41).
TABLE 3.
Estimated minimum and maximum percent contributions of thiotrophic and methanotrophic fatty acids to the mussel tissues and epibiotic materiala
| Origin of fatty acids | Minimum, maximum % contributions to:
|
|||
|---|---|---|---|---|
| Gill tissue including endosymbionts | Mantle tissue | Posterior adductor tissue | Epibiotic material | |
| Thiotroph | 19.9, 71.2 | 22.3, 82.7 | 22.8, 80.7 | 23.1, 79.7 |
| Methanotroph | 24.4, 80.8 | 14.7, 78.4 | 14.8, 77.0 | 16.4, 74.8 |
Minimum estimates include only fatty acids which are recognized to be specific thio- or methanotroph markers (Table 1) and also those fatty acids whose isotope ratios are typical of one group only. Maximum estimates for each group were based on these same fatty acids plus those capable of being produced by both symbiont types. See the text for details.
Acknowledgments
We thank Anne-Marie Alayse (IFREMER, Brest, France), chief scientist of the DIVA 2 cruise, for providing the mussel specimens. We also thank C. Taylor for conducting GC-IRMS analysis and J. R. Dick for GC-MS analysis of fatty acids.
REFERENCES
- 1.Abrajano T A, Murphy D E, Fang J, Comet P, Brooks J M. 13C/12C ratios in individual fatty acids of marine mytilids with and without bacterial symbionts. Org Geochem. 1994;21:611–617. [Google Scholar]
- 2.Ackman R G, Hooper S N. Non-methylene interrupted fatty acids in lipids of shallow-water marine invertebrates: a comparison of the two molluscs (Littorina littorea and Lunatia trisseriata) with the sand shrimp (Crangon septemspinous) Comp Biochem Physiol B. 1973;46:153–165. [Google Scholar]
- 3.Bowman J P, Skerratt J H, Nichols P D, Sly L I. Phospholipid fatty acid and lipopolysaccharide fatty acid signature lipids in methane-utilizing bacteria. FEMS Microbiol Ecol. 1991;85:15–22. [Google Scholar]
- 4.Cavanaugh C M. Symbioses of chemoautotrophic bacteria and marine invertebrates from hydrothermal vents and reducing sediments. Bull Biol Soc Wash. 1985;6:373–388. [Google Scholar]
- 5.Cavanaugh C M, Levering P R, Maki J S, Mitchell R, Lidstrom M E. Symbiosis of methylotrophic bacteria and deep-sea mussels. Nature. 1987;325:346–348. [Google Scholar]
- 6.Cavanaugh C M, Wirsen C O, Jannasch H W. Evidence for methylotrophic symbionts in a hydrothermal vent mussel (Bivalvia: Mytilidae) from the Mid-Atlantic Ridge. Appl Environ Microbiol. 1992;58:3799–3803. doi: 10.1128/aem.58.12.3799-3803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlou, J. L., J. P. Donval, Y. Fouquet, E. Douville, J. Knoery, P. Jean-Baptiste, A. Dapoigny, and M. Stievenard. Geochemistry of fluids collected at Lucky Strike (37°17′N) and Menez Gwen (37°50′N) hydrothermal fields, south of the Azores Triple Junction on the Mid-Atlantic Ridge (DIVA 1 cruise—May 1994). J. Geophys. Res., in press.
- 8.Childress J J, Fisher C R, Brooks J M, Kennicutt II M C, Bidigare R, Anderson A E. A methanotrophic marine molluscan (Bivalvia, Mytilidae) symbiosis: mussels fueled by gas. Science. 1986;233:1306–1308. doi: 10.1126/science.233.4770.1306. [DOI] [PubMed] [Google Scholar]
- 9.Christie W W. Lipid analyses. 2nd ed. Oxford, United Kingdom: Pergamon Press; 1982. [Google Scholar]
- 10.Christie W W, Stefanov K. Separation of picolinyl ester derivatives of fatty acids by high-performance liquid chromatography for identification by mass spectrometry. J Chromatogr. 1987;392:259–265. [Google Scholar]
- 11.Conway N, Kennicutt M C, Van Dover C L. Stable isotopes in the study of marine chemosynthetically-based ecosystems. In: Lajtha K, Michener R, editors. Stable isotopes in ecology. New York, N.Y: Blackwell Scientific Publications; 1994. pp. 158–186. [Google Scholar]
- 12.de Angelis M A, Reysenbach A L, Baross J A. Surfaces of hydrothermal vent invertebrates: sites of elevated microbial CH4 oxidation activity. Limnol Oceanogr. 1991;36:570–577. [Google Scholar]
- 13.DeNiro M J, Epstein S. Mechanisms of carbon isotope fractionation associated with lipid synthesis. Science. 1977;197:261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- 14.Desbruyères D, Alyase A-M, Antoine E, Barbier G, Barriga F, Biscoito M, Briand P, Brulport J P, Comtet T, Cornec L, Crassous P, Dando P, Fabri M C, Felback H, Lallier F, Fiala-Médioni A, Gonçalves J, Mérnard F, Kerdoncuff J, Patching J, Saldanha L, Sarradin P-M. New information on the ecology of deep-sea vent communities in the Azores triple junction area: preliminary results of the DIVA 2 cruise (31 May–4 July 1994) Inter Ridge News. 1994;3:18–19. [Google Scholar]
- 15.Eakin P A, Fallick A E, Gerc J. Some instrumental effects in the determination of stable carbon isotope ratios by gas chromatography-isotope ratio mass spectrometry. Chem Geol. 1992;101:71–79. [Google Scholar]
- 16.Fang J P, Abrajano T A, Comet P A, Brooks J M, Sassen R, MacDonald I R. Gulf of Mexico hydrocarbon seep communities. XI. Carbon isotope fractionation during fatty acid biosynthesis of seep organisms and its implications for chemosynthetic processes. Chem Geol. 1993;109:271–279. [Google Scholar]
- 17.Fiala-Médioni A, Cavanaugh C, Dando P, Van Dover C. Symbiotic mussels from the mid-Atlantic ridge: adaptations to trophic resources. J Conf Abstr. 1996;1:788. [Google Scholar]
- 18.Fisher C R, Childress J J, Oremland R S, Bidigare R R. The importance of methane and thiosulphate in the metabolism of the bacterial symbionts of two deep-sea mussels. Mar Biol. 1987;96:59–71. [Google Scholar]
- 19.Fisher C R, Childress J J, Arp A J, Brooks J M, Distel D, Favuzzi J A, Felbeck H, Hessler R, Johnson K S, Kennicutt II M C, Macko S A, Newton A, Powell M A, Somero G N, Soto T. Microhabitat variation in the hydrothermal vent mussel, Bathymodiolus thermophilus, at the Rose Garden vent on the Galapagos Rift. Deep-Sea Res. 1988;35:1769–1791. [Google Scholar]
- 20.Folch J, Lees N, Sloan-Stanley G H. A simple method for the isolation and purification of total lipid. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Fouquet Y, Charlou J-L, Costa I, Donval J-P, Radford-Knoery J, Pellé H, Ondréas H, Louren N, Segonzac M, Tivey M K. A detailed study of the Lucky Strike hydrothermal vent site and discovery of a new hydrothermal site: Menez Gwen; preliminary results of the DIVA 1 cruise (5–29 May 1994) Inter Ridge News. 1994;3:14–17. [Google Scholar]
- 22.Fouquet Y, Ondréas H, Charlou J-L, Donval J-P, Radford-Knoery J, Costa I, Louren N, Tivey M K. Atlantic larva lakes and hot vents. Nature. 1995;377:201. [Google Scholar]
- 23.Fullarton J G, Dando P R, Sargent J R, Southward A J, Southward E C. Fatty acids of hydrothermal vent Ridgeia piscesae and inshore bivalves containing symbiotic bacteria. J Mar Biol Assoc U K. 1995;75:455–468. [Google Scholar]
- 24.Guezennec J, Fiala-Medioni A. Bacterial abundance and diversity in the Barbados Trench determined by phospholipid analysis. FEMS Microbiol Ecol. 1996;19:83–93. [Google Scholar]
- 25.Jacq E, Prieur D, Nichols P, White D C, Porter T, Geesey G G. Microscopic examination and fatty acid characterization of filamentous bacteria colonizing substrata around subtidal hydrothermal vents. Arch Microbiol. 1989;152:64–71. [Google Scholar]
- 26.Jahnke L L, Summons R E, Dowling L M, Zahiralis K D. Identification of methanotrophic lipid biomarkers in cold-seep mussel gills: chemical and isotope analysis. Appl Environ Microbiol. 1995;61:576–582. doi: 10.1128/aem.61.2.576-582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingensmith J S. Distribution of non-methylene-interrupted dienoic fatty acids in polar lipids and triacylglycerols of selected tissues of the hardshell clam (Mercenaria mercenaria) Lipids. 1982;17:976–981. doi: 10.1007/BF02534595. [DOI] [PubMed] [Google Scholar]
- 28.Le Pennec M, Donval A, Herry A. Nutritional strategies of the hydrothermal ecosystem bivalves. Prog Oceanogr. 1990;24:71–80. [Google Scholar]
- 29.Nichols P D, Smith G A, Antworth C P, Hanson R S, White D C. Phospholipid and lipopolysaccharide normal and hydroxy fatty acids as potential signatures for methane-oxidising bacteria. FEMS Microbiol Ecol. 1985;31:327–335. [Google Scholar]
- 30.Nichols P D, Gurkert J B, White D C. Determinations of monounsaturated fatty acid double-bond position and geometry for microbial monocultures and complex consortia by capillary GC-MS of their dimethyl sulphide adducts. J Microbiol Methods. 1986;5:49–55. [Google Scholar]
- 31.Nilsson R, Liljenberg C. The determination of double bond positions in polyunsaturated fatty acids—gas chromatography/mass spectrometry of the diethylamide derivative. Phytochem Anal. 1991;2:253–259. [Google Scholar]
- 32.Pond D W, Dixon D R, Sargent J R. Wax-ester reserves facilitate dispersal of hydrothermal vent shrimps. Mar Ecol Prog Ser. 1997;146:289–290. [Google Scholar]
- 33.Pond D W, Dixon D R, Bell M V, Fallick A E, Sargent J R. Occurrence of 16:2(n-4) and 18:2(n-4) fatty acids in the lipids of the hydrothermal vent shrimps Rimicaris exoculata and Alvinocaris markensis: nutritional and trophic implications. Mar Ecol Prog Ser. 1997;156:167–174. [Google Scholar]
- 34.Pond D W, Segonzac M, Bell M V, Dixon D R, Fallick A E, Sargent J R. Lipid and lipid carbon stable isotope composition of the hydrothermal vent shrimp Mirocaris fortunata: evidence for nutritional dependence on photosynthetically fixed carbon. Mar Ecol Prog Ser. 1997;157:221–231. [Google Scholar]
- 35.Pranal V, Fiala-Medioni A, Guezennec J. Fatty acid characteristics in two symbiotic gastropods from a deep hydrothermal vent of the West Pacific. Mar Ecol Prog Ser. 1996;142:175–184. [Google Scholar]
- 36.Rieley G, Van Dover C L, Hedrick D B, White D C, Eglinton G. Lipid characteristics of hydrothermal vent organisms from 9°N East Pacific Rise. In: Parson L M, Walker C L, Dixon D R, editors. Hydrothermal vents and processes. London, United Kingdom: The Geological Society; 1995. pp. 329–342. [Google Scholar]
- 37.Sargent J R, Parkes R J, Mueller-Harvey I, Henderson J. Lipid biomarkers in marine ecology. In: Sleigh M A, editor. Microbes in the sea. Chichester, United Kingdom: Ellis Horwood; 1987. pp. 119–138. [Google Scholar]
- 38.Segonzac M, De-Saint-Laurent M, Casanova B. L’énigme du comportement trophique des crevettes Alvinocarididae des sites hydrothermaux de la dorsale médio-atlantique. Cah Biol Mar. 1993;34:535–571. [Google Scholar]
- 39.Sofer Z. Preparation of carbon dioxide for stable isotope analysis of petroleum fractions. Anal Chem. 1980;52:1389–1391. [Google Scholar]
- 40.Vacelet J, Fiala-Médioni A, Fisher C R, Boury-Esnault N. Symbiosis between methane-oxidizing bacteria and a deep-sea carnivorous cladorhizid sponge. Mar Ecol Prog Ser. 1996;145:77–85. [Google Scholar]
- 41.Van Dover C. Ecology of mid-Atlantic ridge hydrothermal vents. In: Parson L M, Walker C L, Dixon D R, editors. Hydrothermal vents and processes. London, United Kingdom: The Geological Society; 1995. pp. 257–294. [Google Scholar]
- 42.Van Dover C, Desbruyères D, Segonzac M, Comtet T, Saldanha L, Fiala-Médioni A, Langmuir C. Biology of the Lucky Strike site hydrothermal field. Deep-Sea Res. 1996;43:1509–1529. [Google Scholar]
- 43.Wilson R, Sargent J R. High-resolution separation of polyunsaturated fatty acids by argentation thin-layer chromatography. J Chromatogr. 1992;623:403–407. [Google Scholar]