Abstract
Immunomagnetic separation is a useful enrichment method selective for Escherichia coli O157 cells against non-O157 E. coli cells from a preenrichment culture. However, E. coli cells are adsorbed onto a solid surface nonspecifically. With the conventional immunomagnetic separation method, this nonspecific adsorption interfered with immunomagnetic separation. It was found that this interference could be reduced with a low-ionic-strength solution. When immunomagnetic separation was carried out with this solution, the proportion of E. coli O157 cells to non-O157 E. coli cells increased from 9.6 to 31.4 times compared to the proportion obtained by the conventional immunomagnetic separation method. The effectiveness of this solution was successfully evaluated by the use of E. coli O157-spiked samples.
Outbreaks of Escherichia coli O157 posed serious health threats in 1996 in Japan. Several mass infections in schools involving more than 6,000 patients and three deaths in Sakai City occurred, and school lunches were identified as the possible source of infection on the basis of epidemiological research (7). In almost all cases, the causative foods were not identified.
Conventional methods with selective enrichment broths for the detection and isolation of fecal coliforms in food samples require incubation at 44.5°C. However, E. coli O157:H7 cannot grow at this temperature, and as a result, these selective methods cannot be applied to differentiate this organism from most other nonpathogenic strains (8). Foods such as raw ground beef can be contaminated with a considerable number of coliforms (1,000 per g or more) (9) and can contain an average of six E. coli strains (12). The infectious dose of E. coli O157:H7 is believed to be very low (4). The recovery of the pathogen from the contaminated food might be hampered by the presence of large numbers of nonpathogenic E. coli strains in the enrichment culture for E. coli.
The effectiveness of the immunomagnetic separation (IMS) method with magnetic beads coated with antibodies against E. coli O157 as a selective enrichment method for E. coli O157 strains (1, 13), especially when the method is applied to fecal specimens (3, 5), has been well established. However, it was difficult to identify the vehicle of infection in most of the E. coli O157 outbreaks in Japan, suggesting that more sensitive methods are needed to isolate the small number of organisms present in suspected food.
It was known that Vibrio parahaemolyticus strains were adsorbed onto immunomagnetic beads nonspecifically and that the ability of adsorption differed among the strains. I established a method to prevent this nonspecific adsorption in the case of IMS selective for V. parahaemolyticus serotype K (10). In the IMS selective for E. coli O157 strains, non-O157 E. coli strains which contaminate food may also be present in large numbers in the enrichment culture and adsorb onto immunomagnetic beads nonspecifically, consequently reducing the sensitivity of the IMS method.
Initial bacterial adsorption to a solid surface was found to be affected by the presence of electrolytes in a suspending medium (6, 11). The bacteria were adsorbed in the presence of an electrolyte, and it was shown that a divalent electrolyte is more effective than a monovalent electrolyte. The adsorption decreased as the electrolyte concentration decreased. The mechanism of adsorption was interpreted in terms of the balance between the electrostatic repulsion forces and van der Waals attraction forces (2).
In this study, a method to prevent nonspecific adsorption of E. coli strains was devised and applied for the improvement of IMS sensitivity. For this purpose, the effect of electrolyte concentration on adsorption was investigated with low-ionic-strength water. Ultrapure water with a resistivity of 18.2 MΩ (Milli-Q [MQ] SP; Nihon Millipore Ltd.) was treated with cation-exchange resin to make low-ionic-strength water. Five grams of analytical grade Chelex 100 chelating ion-exchange resin (Bio-Rad Laboratories) was mixed with 100 ml of ultrapure water (MQ) and stood overnight at room temperature to chelate ions and settle the resin. The supernatant (chelex-treated MQ [CMQ]) was used as the low-ionic-strength solution. Brain heart infusion broth (BHI; Difco Laboratories) was treated in the same way with Chelex 100. E. coli could not grow or grew very poorly in this medium and could not grow in CMQ either. Whether or not the CMQ was sterilized did not affect the results, so CMQ was used without sterilization.
IMS is conventionally carried out by mixing Dynabeads (coated with polyclonal antibody against E. coli O157 [anti-E. coli O157]) (∼108 beads/ml; Dynal A.S., Oslo, Norway) with a preenrichment culture of E. coli. The same experiment was carried out with CMQ. There were several possible choices for the preenrichment medium, but BHI was used in this experiment because it has no selectivity for E. coli growth. The degree of bacterial adsorption onto Dynabeads was compared with that of cells suspended in BHI and in CMQ. The following four non-O157 E. coli strains were employed to examine the effectiveness of the low-ionic-strength water on bacterial adsorption onto Dynabeads. E. coli strains V8 Nalr, V9 Nalr, V28 Nalr, and V29 Nalr were initially isolated from clinical specimens, and then nalidixic-acid-resistant spontaneous mutant strains were isolated from the colonies grown on nalidixic-acid (12.5 μg/ml)-supplemented MacConkey agar plates on which each E. coli strain was densely plated (0.5 ml of BHI overnight culture) and cultured 24 to 48 h at 37°C.
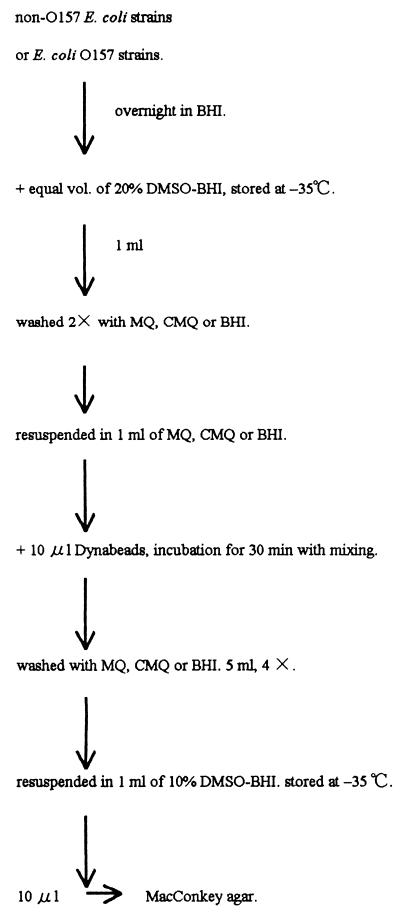
The experimental design for bacterial adsorption is illustrated in Fig. 1. Cells were cultured overnight in BHI at 37°C. Each culture was mixed with an equal volume of 20% dimethyl sulfoxide (DMSO) (vol/vol) containing BHI (20% DMSO-BHI) and stored at −35°C until use. One milliliter of each culture was washed twice with MQ, CMQ, or BHI (as a control) by centrifugation (8 ml for 10 min at 3,500 rpm) and then resuspended in 1.0 ml of MQ, CMQ, or BHI, respectively. Polystyrene round-bottom tubes (16 by 125 mm; Falcon) were used. Ten microliters of Dynabeads was added to the suspension, and the suspension was incubated for 30 min at room temperature with occasional shaking. Five milliliters of MQ, CMQ, or BHI was added to the mixture. The nonadsorbed cells were separated from the Dynabead-adsorbed cells by placing the tube on a magnetic particle concentrator (model MPC-1; Dynal A.S.). The nonadsorbed cells were discarded, and the Dynabead-adsorbed cell fraction was washed four times by the same magnet procedure. The washed Dynabeads were resuspended in 1 ml of 10% DMSO-BHI and stored at −35°C. At the same time, 10 μl of these suspensions was plated onto MacConkey agar plates following serial 10-fold dilution in CMQ to find the appropriate dilution range. Colonies were counted after overnight incubation at 37°C. Then the stored suspension was thawed, and after appropriate dilution, the number of colonies in each suspension was determined (Table 1).
FIG. 1.
Procedure for measurement of adsorption ability of E. coli onto Dynabeads.
TABLE 1.
Effect of solution upon adsorption of E. coli to Dynabeads (coated with anti-E. coli O157)
| Strain | Cell concn in medium (cells/ml ± SD) | Solution used for adsorption and washing | No. of cells adsorbed (cells/ml ± SD) |
|---|---|---|---|
| V8 Nalr | (5.5 ± 3.0) × 107 | BHI | (5.3 ± 0.6) × 103 |
| MQ | (7.5 ± 0.7) × 103 | ||
| CMQ | (6.4 ± 4.8) × 101 | ||
| V9 Nalr | (4.0 ± 0.3) × 108 | BHI | (6.5 ± 0.4) × 106 |
| MQ | (3.3 ± 0.2) × 106 | ||
| CMQ | (4.2 ± 0.1) × 104 | ||
| V28 Nalr | (3.9 ± 0.1) × 108 | BHI | (1.8 ± 0.2) × 104 |
| MQ | (1.8 ± 0.3) × 105 | ||
| CMQ | (2.6 ± 0.5) × 102 | ||
| V29 Nalr | (3.9 ± 0.9) × 108 | BHI | (2.4 ± 0.4) × 104 |
| MQ | (5.5 ± 0.4) × 106 | ||
| CMQ | (2.0 ± 0.9) × 102 |
These Dynabead suspensions contain more than 106 beads/ml. There is a possibility that more than one cell attached to a single bead, especially in the cases of V9 Nalr (in BHI and in MQ) and V29 Nalr (in MQ). However, the number of resultant colonies which were treated in CMQ was far lower than the number of beads in the plating sample. This result means that for CMQ, the possibility that more than one cell adsorbed onto a single bead was remote. Thus, the experiments were performed with the assumption that most of the colonies originated from a single cell attached to a single bead when the number of colonies was far lower than the number of beads.
Adsorption of non-O157 E. coli onto Dynabeads decreased by 1/69 (V28 Nalr strain) to 1/828 times (V8 Nalr strain) in CMQ compared to BHI. However, MQ was not effective and slightly stimulated adsorption, suggesting that the presence of the remaining electrolytes affected adsorption in MQ (Table 1). The ability of adsorption differed among the strains, but the reduction of adsorption in CMQ was remarkable among all strains.
Nonspecific adsorption of O157 strains onto beads in CMQ was also examined with Dynabeads (M280 sheep anti-rabbit immunoglobulin G [IgG]) (6 × 108 to 7 × 108 beads/ml), which is equivalent to Dynabeads (anti-E. coli O157) except for the coated antibody. The four E. coli O157:H7 strains used, O157KB0 Rifr, O157KB1 Rifr, O157KB2 Rifr, and O157KB3 Rifr, were spontaneous rifampin-resistant mutants from the collection of the Kobe Institute of Health. These mutants were isolated from the colonies on rifampin (100 μg/ml)-supplemented MacConkey agar plates in the same manner as the nalidixic-acid-resistant mutants. The O157KB0 Rifr strain was isolated from bovine stomachs, and other strains were isolated from hemorrhagic colitis patients in different years. Overnight BHI cultures of these strains were examined for adsorption onto Dynabeads the same way non-O157 strains were (Fig. 1). The V9 Nalr strain was also employed as a control to examine the difference of adsorption between Dynabeads (M280 sheep anti-rabbit IgG) and Dynabeads (anti-E. coli O157). Unexpectedly, all these O157 strains were adsorbed onto the beads 37 times (O157KB2 Rifr strain) to 6,200 times (O157KB3 Rifr strain) more efficiently in CMQ than in BHI (Table 2). This result was confirmed by a mixed cell suspension. E. coli O157KB0 cells were mixed with a 2,000-times-larger number of V9 Nalr cells. The ratio of E. coli O157KB0 Rifr cells to V9 Nalr cells was compared before and after adsorption onto the beads. After adsorption in CMQ, the adsorbed O157KB0 Rifr cell-to-V9 Nalr cell ratio increased 3,500 times compared to the initial ratio in the mixed cell suspension (Table 3). It is not clear at present whether all O157 strains have the same characteristics and what the mechanism related to adsorption onto the beads is. However, this characteristic is beneficial for selecting O157 strains against non-O157 strains.
TABLE 2.
Effect of solution upon adsorption of E. coli O157 strains to Dynabeads (M280; coated with sheep anti-rabbit IgG)
| Strain | Cell concn in the medium (cells/ml ± SD) | Solution used for adsorption and washing | No. of cells adsorbed (cells/ml ± SD) |
|---|---|---|---|
| O157KB0 Rifr | (2.7 ± 1.1) × 108 | BHI | (6.8 ± 3.1) × 102 |
| CMQ | (1.6 ± 0.3) × 106 | ||
| O157KB1 Rifr | (3.3 ± 0.9) × 107 | BHI | (3.0 ± 1.4) × 102 |
| CMQ | (2.1 ± 0.6) × 105 | ||
| O157KB2 Rifr | (5.8 ± 1.6) × 108 | BHI | (2.0 ± 0.3) × 104 |
| CMQ | (7.4 ± 1.1) × 105 | ||
| O157KB3 Rifr | (4.8 ± 1.5) × 108 | BHI | (4.5 ± 0.7) × 102 |
| CMQ | (2.8 ± 0.3) × 106 | ||
| V9 Nalr | (5.5 ± 0.3) × 108 | BHI | (1.3 ± 0.1) × 105 |
| CMQ | (3.7 ± 0.5) × 103 |
TABLE 3.
Effect of solution upon adsorption of E. coli O157KB0 Rifr and V9 Nalr in the mixed cell suspension to Dynabeads (M280; coated with sheep anti-rabbit IgG)
| Before adsorption (cells/ml ± SD)
|
Solution used for:
|
No. of cells adsorbed (cells/ml ± SD)
|
|||||
|---|---|---|---|---|---|---|---|
| Rifampin selection | Nalidixic acid selection | Ratio of cells (Rifr:Nalr) | Adsorption | Washing | Rifampin selection | Nalidixic acid selection | Ratio of cells (Rifr:Nalr) |
| (2.7 ± 1.1) × 105 | (5.5 ± 0.3) × 108 | 4.9 × 10−4:1 | BHI | BHI | 5 ± 10 | (4.5 ± 1.1) × 104 | 1.1 × 10−4:1 |
| CMQ | CMQ | (1.5 ± 0.4) × 103 | (8.9 ± 2.2) × 102 | 1.7:1 | |||
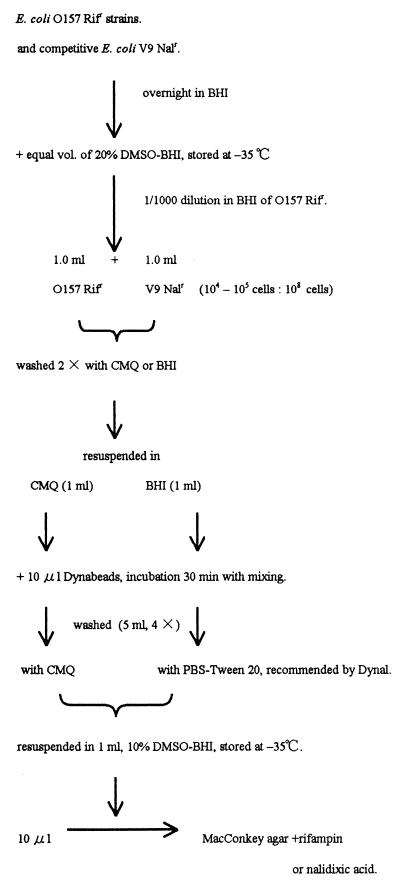
Accordingly, CMQ was applied to IMS (Fig. 2). The V9 Nalr strain, which showed the highest adsorption ability among non-O157 E. coli (Table 1), was selected as a competitive background strain on the assumption that nonpathogenic strains were usually abundant in the E. coli enrichment culture. Four E. coli O157:H7 strains, O157KB0 Rifr, O157KB1 Rifr, O157KB2 Rifr, and O157KB3 Rifr, were employed. The E. coli O157 strains and the competitive V9 Nalr strain were grown overnight in BHI. An equal volume of 20% DMSO-BHI was mixed and stored at −35°C until use. E. coli O157 cultures were thawed and diluted 1,000 times in BHI, and 1 ml of this diluted culture was mixed with 1 ml of thawed undiluted V9 Nalr culture. This mixed cell suspension contained 104 to 105 E. coli O157 cells per ml and about a 103-times-larger number of V9 Nalr cells (5.5 × 108 cells per ml). Two milliliters of the mixed cell suspension was washed twice with CMQ or BHI by centrifugation, and the pelleted cells were resuspended in 1 ml of CMQ or BHI, respectively. IMS was carried out conventionally with a preenrichment culture. In these experiments, BHI was used as the preenrichment medium. Then 10 μl of Dynabeads was added to each cell mixture and incubated for 30 min with occasional mixing. The nonbinding cells in the suspension in CMQ were washed with CMQ, and the nonbinding cells in the control BHI medium were washed with phosphate-buffered saline (PBS), pH 7.4, with Tween 20 (0.05% [vol/vol]) (PBS-Tween 20) the same way as in the adsorption experiments (5 ml, four times). The efficacy of washing with CMQ was compared with that of washing with PBS-Tween 20 because the manufacturer (Dynal) recommended the latter.
FIG. 2.
Procedure for IMS selective for E. coli O157.
After being washed, the cells were resuspended in 1 ml of 10% DMSO-BHI and stored at −35°C. Then an appropriate preliminary dilution range for Rifr and Nalr cells was determined with a portion of these suspensions. Ten microliters was plated onto a MacConkey agar plate supplemented with rifampin (100 μg/ml) or nalidixic acid (12.5 μg/ml). Following overnight incubation, the magnitude of the enrichment of E. coli O157 Rifr strains against the competitive V9 Nalr strain was measured by comparing the number of Rifr colonies and Nalr colonies that resulted (Table 4).
TABLE 4.
IMS of E. coli O157 cells from a large number of competitive V9 Nalr cells
| E. coli O157 strain selected | Before IMS
|
Solution used for:
|
After IMS
|
|||||
|---|---|---|---|---|---|---|---|---|
| Cells/ml ± SD
|
IMS | Washing | Cells/ml ± SD
|
Ratio of cells (Rifr:Nalr) | ||||
| Rifampin selection | Nalidixic acid selection | Ratio of cells (Rifr:Nalr) | Rifampin selection | Nalidixic acid selection | ||||
| O157KB0 Rifr | (2.7 ± 1.1) × 105 | (5.5 ± 0.3) × 108 | 1:2.0 × 103 | BHI | PBS-Tween 20 | (2.7 ± 0.4) × 104 | (6.6 ± 1.0) × 103 | 4.1:1 |
| CMQ | CMQ | (2.9 ± 1.0) × 103 | (7.2 ± 5.4) × 101 | 40:1 | ||||
| O157KB1 Rifr | (3.3 ± 0.9) × 104 | (5.5 ± 0.3) × 108 | 1:1.7 × 104 | BHI | PBS-Tween 20 | (1.4 ± 0.2) × 104 | (7.7 ± 0.8) × 103 | 1.8:1 |
| CMQ | CMQ | (1.3 ± 0.1) × 104 | (2.3 ± 0.9) × 102 | 56.5:1 | ||||
| O157KB2 Rifr | (5.8 ± 1.6) × 105 | (5.5 ± 0.3) × 108 | 1:9.5 × 102 | BHI | PBS-Tween 20 | (2.9 ± 1.0) × 104 | (5.1 ± 0.6) × 103 | 5.7:1 |
| CMQ | CMQ | (9.3 ± 3.8) × 103 | (1.7 ± 0.5) × 102 | 54.7:1 | ||||
| O157KB3 Rifr | (4.8 ± 1.5) × 105 | (5.5 ± 0.3) × 108 | 1:1.1 × 103 | BHI | PBS-Tween 20 | (3.2 ± 1.2) × 104 | (3.4 ± 0.8) × 103 | 9.4:1 |
| CMQ | CMQ | (5.7 ± 1.2) × 104 | (2.0 ± 0.8) × 102 | 285:1 | ||||
The initial proportion of Rifr cells to Nalr cells in the mixed cell suspension (1:9.5 × 102 to 1:1.7 × 104) increased by 5.4 × 103 to 3.0 × 104 times after IMS when the conventional IMS method was applied.
When IMS was carried out in and washed away by CMQ, the proportion of Rifr cells to Nalr cells increased 9.6 (O157KB2 Rifr) to 31.4 (O157KB1 Rifr) times compared to the proportion obtained by the conventional IMS method. In conventional routine work, several colonies which look like E. coli on the MacConkey agar plate are directly examined with an E. coli O157 latex test kit (Oxoid) or isolated and inoculated in an appropriate medium such as triple sugar iron agar and sulfide indole motility medium for further biotyping and serotyping for identification of E. coli O157. It is essential to examine a large number of colonies to find O157 colonies that are present in small numbers. The results obtained for IMS which was carried out in and washed away by CMQ indicate that the chances of finding O157 colonies are 9 to 31 times better than for conventional methods. This improved IMS would help isolate E. coli O157 strains which may be present in food or environmental samples in small numbers.
Isolation of E. coli O157 from artificially inoculated minced beef was carried out to verify the effectiveness of CMQ. Five samples of minced beef were purchased from five different retailers, and equal weights of these samples were mixed well. Portions of 10 g were stored until use at −35°C in a sterile stomacher bag which was recommended as a preenrichment culture container by Dynal. Ten grams of the sample was mixed with 90 ml of buffered peptone water (BPW; Oxoid), which was also recommended by Dynal as a preenrichment medium for food samples, and then mixed in the stomacher. After serial dilution with saline, the total number of viable bacterial cells was counted by plating onto a plate count agar, and the number of coliform bacteria was estimated by plating onto a desoxycholate agar plate (Table 5). Species identification was made for nine coliform colonies on the plate of the highest dilution (ID test EB-20 for the identification of lactose-fermenting, gram-negative, rod-shaped bacteria; Nissui). Eight colonies were Enterobacter spp., and one colony was Klebsiella spp. MacConkey agar was used exclusively to isolate E. coli in these experiments. These colonies, which were grown on MacConkey agar plates, were difficult to distinguish from E. coli colonies. Colony counts were also performed on MacConkey agar plates and on nalidixic-acid-supplemented and rifampin-supplemented MacConkey agar plates. MacConkey agar suppressed the growth of some bacterial species. There were fewer viable colonies on this plate than on the plate count agar. The number of lactose-fermenting (red-colored) colonies grown on MacConkey agar plates was about the same as that of the coliform colonies on the desoxycholate agar plates. No lactose-fermenting colonies in 0.02 g of the sample grew on rifampin- or nalidixic-acid-supplemented MacConkey agar plates. These observations indicated that there was no rifampin- or nalidixic-acid-resistant coliform in this minced-beef sample.
TABLE 5.
Viable cell count in minced beef
| Medium and cell type | Cells/g ± SD |
|---|---|
| Plate count agar | |
| Total viable cells | (1.8 ± 0.1) × 106 |
| Desoxycholate agar | |
| Coliform cells | (5.1 ± 0.1) × 103 |
| MacConkey agar | |
| Total | (6.2 ± 0.2) × 104 |
| Lactose fermenting | (7.0 ± 2.9) × 103 |
| Rifampin-supplemented MacConkey agar | |
| Lactose fermenting | 0a |
| Lactose nonfermenting | (1.7 ± 0.5) × 102 |
| Nalidixic acid supplemented MacConkey agar | |
| Lactose fermenting | 0a |
| Lactose nonfermenting | (2.5 ± 0.5) × 103 |
0 indicates no cells in 0.02 g of the minced beef.
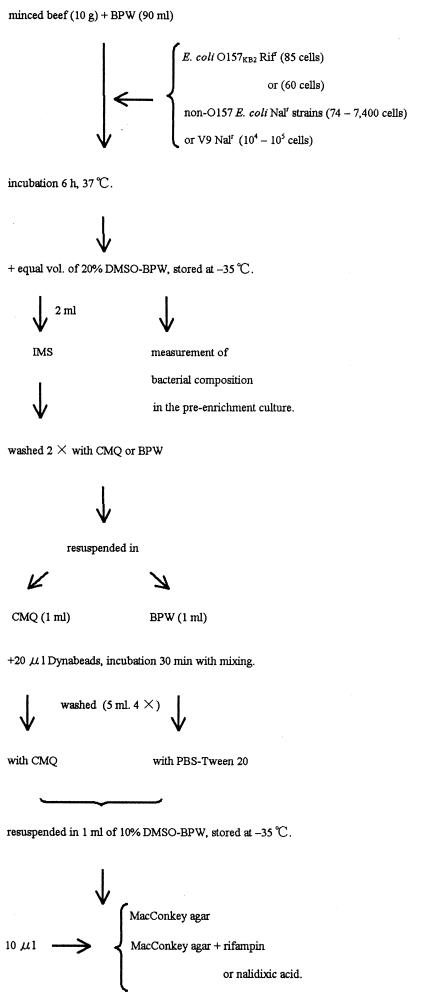
For comparison of the relative effectiveness of the IMS technique with the CMQ treatment and the conventional method, E. coli O157 cells were inoculated along with non-O157 E. coli Nalr cells to measure the degree of improvement (Fig. 3). Ten-gram portions of the minced beef were mixed with 90 ml of BPW. A small number of E. coli O157KB1 Rifr cells (85) and a different number of competitive non-O157 E. coli Nalr cells (74 to 7,400) were inoculated into these mixtures and mixed briefly in the stomacher. Non-O157 E. coli Nalr cells were a mixture of equal volumes of overnight BHI culture of V8 Nalr, V9 Nalr, V28 Nalr, and V29 Nalr strains. Preenrichment cultures were performed at 37°C for 6 h. These cultures were mixed with an equal volume of 20% DMSO containing BPW (20% DMSO-BPW) and stored at −35°C until use. The number of colonies was counted, and the composition of each of the Rifr and Nalr E. coli colonies in the preenrichment culture was examined with a portion of frozen culture (Table 6). Since this minced beef contained no lactose-fermenting rifampin-resistant or nalidixic-acid-resistant cells, only E. coli Rifr and Nalr grew as red-colored colonies on rifampin- or nalidixic-acid-supplemented MacConkey agar plates. The small number of lactose-nonfermenting cells which grew as translucent colonies did not interfere with the counting of E. coli colonies.
FIG. 3.
Procedure for isolation of E. coli O157 from the spiked minced beef.
TABLE 6.
Recovery of E. coli O157 strain from the spiked minced beef by IMS
| Expt no. | No. of E. coli cells inoculated
|
Preenrichment culture
|
Solution used for:
|
After IMS
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MacConkey agar (cells/ml ± SD)
|
MacConkey agar (cells/ml ± SD)
|
|||||||||||
| O157KB2 Rifr | Non-O157 E. coli cellsb | Total cells | Rifampin selection | Nalidixic acid selection | Ratio of cells (Rifr:Nalr) | IMS | Washing | Total cells | Rifampin selection | Nalidixic acid selection | Ratio of cells (Rifr:Nalr) | |
| A | 85 ± 28 | 74 ± 3 | (1.9 ± 0.4) × 107 | (4.8 ± 1.4) × 105 | (3.0 ± 0.8) × 105 | 1.6:1 | BPW | PBS-Tween 20 | (4.2 ± 0.1) × 105 | (5.4 ± 1.4) × 104 | (1.0 ± 0.1) × 103 | 5.4 × 101:1 |
| CMQ | CMQ | (1.6 ± 0.2) × 104 | (5.6 ± 1.3) × 103 | 0a | >5.6 × 103:1 | |||||||
| B | 85 ± 28 | (74 ± 3) × 101 | (3.8 ± 0.2) × 107 | (4.4 ± 0.6) × 104 | (2.6 ± 0.2) × 105 | 1.7 × 10−1:1 | BPW | PBS-Tween 20 | (1.4 ± 0.4) × 105 | (7.6 ± 1.5) × 103 | (9.4 ± 2.3) × 102 | 1.8 × 101:1 |
| CMQ | CMQ | (1.1 ± 0.1) × 104 | (1.6 ± 0.3) × 103 | 5 ± 10 | 3.2 × 102:1 | |||||||
| C | 85 ± 28 | (74 ± 3) × 102 | (2.2 ± 0.2) × 107 | (8.0 ± 1.0) × 104 | (3.4 ± 0.4) × 106 | 2.5 × 10−2:1 | BPW | PBS-Tween 20 | (8.1 ± 1.1) × 104 | (1.2 ± 0.04) × 104 | (6.8 ± 0.3) × 103 | 1.7:1 |
| CMQ | CMQ | (6.3 ± 0.7) × 103 | (2.7 ± 1.0) × 102 | 0a | >2.7 × 102:1 | |||||||
| D | 60 ± 2 | (97 ± 2) × 102 | (4.0 ± 0.6) × 107 | (9.0 ± 4.6) × 104 | (1.8 ± 0.4) × 107 | 5.1 × 10−3:1 | BPW | PBS-Tween 20 | (2.2 ± 0.4) × 105 | (5.4 ± 1.0) × 104 | (4.2 ± 0.3) × 104 | 1.3:1 |
| CMQ | CMQ | (4.3 ± 0.6) × 103 | (3.2 ± 0.7) × 102 | 5 ± 10 | 6.4 × 101:1 | |||||||
| E | 60 ± 2 | (97 ± 2) × 103 | (9.4 ± 0.6) × 107 | (4.6 ± 1.0) × 104 | (1.1 ± 0.1) × 108 | 4.3 × 10−4:1 | BPW | PBS-Tween 20 | (4.2 ± 0.4) × 105 | (1.9 ± 0.1) × 104 | (3.7 ± 0.02) × 105 | 5.0 × 10−2:1 |
| CMQ | CMQ | (7.3 ± 0.3) × 103 | (2.1 ± 0.1) × 103 | (3.1 ± 0.9) × 102 | 6.8:1 | |||||||
| F | 0 | 0 | NDc | NDc | NDc | NDc | BPW | PBS-Tween 20 | (8.5 ± 0.9) × 104 | 0a | 0a | |
There were no cells in the 0.2-ml cell suspension.
In experiments D through F, the non-O157 E. coli strain used was V9 Nalr.
ND, not determined.
These frozen cultures were applied to IMS selective for E. coli O157 strains. Two milliliters of the frozen culture was thawed and washed twice with CMQ or BPW by centrifugation. The cell pellets were resuspended in 1 ml of CMQ or BPW, respectively. By the Dynal method, IMS was carried out with a preenrichment BPW culture. Accordingly, BPW was adopted as the solution for IMS, and 20 μl of Dynabeads was added to the preenrichment culture. This volume of Dynabeads was as recommended in the Dynal protocol.
CMQ was also effective when applied to minced-beef samples (Table 6). By the Dynal method, the number of recovered O157KB2 Rifr strains decreased as the competitive E. coli Nalr strains increased (the Rifr-to-Nalr ratio decreased from 54:1 [Table 6, experiment A] to 1.7:1 [Table 6, experiment C]). However, when the CMQ solution was applied, nonspecifically adsorbed E. coli Nalr colonies were negligible.
The V9 Nalr strain was highly adsorptive onto Dynabeads. This strain was adopted as the more tenacious competitive E. coli strain for the spiked experiment. Again in this case, E. coli O157KB2 Rifr strain could be isolated even when the number of inoculated V9 Nalr cells was more than 1,000 times larger (Table 6, experiment E). These results were reconfirmed by examining 25 colonies on a MacConkey agar plate after IMS (Table 7). These colonies were selected by comparison with O157KB2 Rifr colonies to avoid any preference for the appearance of a colony. The presence of O157 antigens was determined by a slide agglutination test with an E. coli O157 latex test kit. The same colonies were inoculated onto nalidixic-acid- and rifampin-supplemented MacConkey agar plates to confirm resistance to these drugs. The ratio of recovered O157 strains to non-O157 coliforms was similar to the Rifr-to-Nalr colony ratio after IMS. Six O157 antigen-negative and rifampin-sensitive colonies (Table 7, experiment C) were selected as representatives of naturally contaminated coliform bacteria in these experiments and were identified as Hafnia alvei.
TABLE 7.
Serotype examination of the E. coli-like coloniesa grown on MacConkey agar plate after IMS
| Expt no. | Solution used for:
|
No. of O157 antigen-positive and rifampin-resistant colonies | No. of O157 antigen-negative colonies
|
Recovered O157 colonies (%) | ||
|---|---|---|---|---|---|---|
| IMS | Washing | Nalidixic acid resistant | Rifampin sensitive | |||
| A | BPW | PBS-Tween 20 | 15 | 2 | 8 | 60 |
| CMQ | CMQ | 23 | 0 | 2 | 92 | |
| B | BPW | PBS-Tween 20 | 20 | 1 | 4 | 80 |
| CMQ | CMQ | 24 | 0 | 1 | 96 | |
| C | BPW | PBS-Tween 20 | 8 | 11 | 6b | 32 |
| CMQ | CMQ | 25 | 0 | 0 | 100 | |
| D | BPW | PBS-Tween 20 | 14 | 11 | 0 | 56 |
| CMQ | CMQ | 24 | 1 | 0 | 96 | |
| E | BPW | PBS-Tween 20 | 3 | 22 | 0 | 12 |
| CMQ | CMQ | 22 | 3 | 0 | 88 | |
A total of 25 colonies were examined.
All six colonies were H. alvei.
The absence of naturally contaminated verocytotoxin-producing E. coli O157 strains in these minced-beef samples was confirmed by PCR assay. The preenrichment culture of minced beef, in which there were no inoculated E. coli strains, was used for IMS. The recovered beads were assayed by the PCR method for the detection of verocytotoxin genes. The number of beads examined was equivalent to 0.5 ml of bead suspension after IMS and contained 4.3 × 104 cells (Table 6, experiment F). No verocytotoxin gene was detected in this sample, while 0.5 ml of equivalent beads in the experiment (Table 6, experiment B: BPW used for IMS and PBS-Tween 20 used for washing; 7 × 104 cells), which was adopted as the positive control, showed the presence of VT1 and VT2 genes (data not shown).
CMQ proved to be efficient to prevent nonspecific adsorption of other non-O157 E. coli cells onto Dynabeads. This improved IMS method is applicable for the isolation of many other pathogenic bacteria for which antibodies are available.
I previously described a method for selective enrichment for Vibrio parahaemolyticus serotype K, using a rabbit antiserum kit for V. parahaemolyticus and Dynabeads (M280 sheep anti-rabbit IgG) (10). However, an attempt to select a specified serotype of V. parahaemolyticus was not successful by this method because halophilic V. parahaemolyticus cells lysed immediately after exposure to CMQ. The bacteria selected for this test must be hardy enough to withstand low-ionic-strength solutions.
ACKNOWLEDGMENTS
I am very grateful to T. Hatta, K. Akiyoshi, and I. Fuke for their helpful discussion and critical reading of the manuscript. I also thank C. Smith (Kyoto University of Foreign Studies) for his careful reading of the manuscript.
This work was supported in part by a grant from Daido Life Welfare Foundation, Osaka, Japan.
REFERENCES
- 1.Bennett A R, MacPhee S, Betts R P. The isolation and detection of Escherichia coli O157 by use of immunomagnetic separation and immunoassay procedures. Lett Appl Microbiol. 1996;22:237–243. doi: 10.1111/j.1472-765x.1996.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 2.Busscher H J, Weerkamp A H. Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiol Rev. 1987;46:165–173. [Google Scholar]
- 3.Chapman P A, Wright D J, Siddons C A. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J Med Microbiol. 1994;40:424–427. doi: 10.1099/00222615-40-6-424. [DOI] [PubMed] [Google Scholar]
- 4.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 5.Karch H, Janetzki-Mittmann C, Aleksic S, Datz M. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J Clin Microbiol. 1996;34:516–519. doi: 10.1128/jcm.34.3.516-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall K C, Stout R, Mitchell R. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol. 1971;68:337–348. [Google Scholar]
- 7.National Institute of Health, Japan. A large-scale outbreak of diarrheal disease due to EHEC O157:H7 among schoolchildren—Sakai City. Outbreaks of EHEC O157 infection in Japan. Infect Agents Surveillance Rep. 1996;17:180–181. [Google Scholar]
- 8.Padhye N V, Doyle M P. Escherichia coli O157:H7: epidemiology, pathogenesis, and methods for detection in food. J Food Prot. 1992;55:555–565. doi: 10.4315/0362-028X-55.7.555. [DOI] [PubMed] [Google Scholar]
- 9.Restaino L, Lyon R H. Efficacy of petrifilm VRB for enumerating coliforms and Escherichia coli from frozen raw beef. J Food Prot. 1987;50:1017–1022. doi: 10.4315/0362-028X-50.12.1017. [DOI] [PubMed] [Google Scholar]
- 10.Tomoyasu T. Development of the immunomagnetic enrichment method selective for Vibrio parahaemolyticus serotype K and its application to food poisoning study. Appl Environ Microbiol. 1992;58:2679–2682. doi: 10.1128/aem.58.8.2679-2682.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uyen H M, van der Mei H C, Weerkamp A H, Busscher H J. Comparison between the adhesion to solid substrata of Streptococcus mitis and that of polystyrene particles. Appl Environ Microbiol. 1988;54:837–838. doi: 10.1128/aem.54.3.837-838.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter E H, Ferreira J L, Payne W L, Jones V M. Probability of recovering pathogenic Escherichia coli from foods. Appl Environ Microbiol. 1985;49:1374–1378. doi: 10.1128/aem.49.6.1374-1378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright D J, Chapman P A, Siddons C A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol Infect. 1994;113:31–39. doi: 10.1017/s0950268800051438. [DOI] [PMC free article] [PubMed] [Google Scholar]