Abstract
Amphibians can be exposed to contaminants in nature by many routes, but perhaps the most likely route is agricultural runoff in amphibian breeding sites. This runoff results in high-level pulses of pesticides. For example, atrazine, the most widely used pesticide in the United States, can be present at several parts per million in agricultural runoff. However, pesticide levels are likely to remain in the environment at low levels for longer periods. Nevertheless, most studies designed to examine the impacts of contaminants are limited to short-term (~ 4 days) tests conducted at relatively high concentrations. To investigate longer-term (~ 30 days) exposure of amphibians to low pesticide levels, we exposed tadpoles of four species of frogs—spring peepers (Pseudacris crucifer), American toads (Bufo americanus), green frogs (Rana clamitans), and wood frogs (Rana sylvatica)—at early and late developmental stages to low concentrations of a commercial preparation of atrazine (3, 30, or 100 ppb; the U.S. Environmental Protection Agency drinking water standard is 3 ppb). We found counterintuitive patterns in rate of survivorship. Survival was significantly lower for all animals exposed to 3 ppb compared with either 30 or 100 ppb, except the late stages of B. americanus and R. sylvatica. These survival patterns highlight the importance of investigating the impacts of contaminants with realistic exposures and at various developmental stages. This may be particularly important for compounds that produce greater mortality at lower doses than higher doses, a pattern characteristic of many endocrine disruptors.
Keywords: amphibian, atrazine, endocrine disruption, NMDRC, nonmonotonic dose–response curve
Although pesticides are used on a local scale, they are ubiquitous and spread regionally and globally. In some areas, the transport and deposition of pesticides from agriculturally intensive areas to adjacent nonagricultural areas are well documented (Davidson et al. 2002; LeNoir et al. 1999; Zabik and Seiber 1993). Moreover, pesticides have been found in the bodies of frogs from areas where pesticide use has not occurred historically or in the past 25 years (Cory et al. 1970; Datta et al. 1998; Russell et al. 1995, 1997). Agricultural runoff of pesticides can also have an effect on amphibians because pesticides have been detected at amphibian sites almost a year after being applied (Hayes et al. 2003). This measure of persistence combined with their high susceptibility to exposure, because they have a complex life cycle and permeable skin (Cooke 1981; Hall and Henry 1992), demonstrates that amphibians are excellent model organisms for testing pesticide exposure.
Runoff from agricultural lands can expose pond-breeding amphibians to various levels of pesticides. For example, atrazine, the most widely used pesticide in the United States [U.S. Department of Agriculture (USDA) 2002], has been reported at levels from 0.1 to 6.7 ppb in amphibian breeding ponds in mid- to late July (Hayes et al. 2003), but during storm events agricultural runoff has been reported to be as high as 480 ppb (Huber 1993).
Moreover, it has been measured in rainfall at levels up to 40 ppb in agricultural areas (Nations and Hallberg 1992). Atrazine can be present at several parts per million in agricultural runoff for short periods of time (days). However, atrazine levels are likely to remain relatively low (parts per billion) for longer periods of time (Huber 1993). Nevertheless, most studies designed to examine the impacts of contaminants are limited to short-term (~ 4 days) tests conducted with relatively high concentrations (parts per million). Studies that have examined larval amphibian exposure to atrazine have found effects on plasma thyroxine, plasma corticosterone, larval size (Larson et al. 1998), developmental stage (Howe et al. 1998; Larson et al. 1998), body condition (Allran and Karasov 2000; Howe et al. 1998), hermaphrodism and demasculinized larynges (Hayes et al. 2002), and increased susceptibility to infection (Kiesecker 2002).
Atrazine has been suggested to exhibit endocrine-disrupting effects via inhibition of androgen receptors in mammals (Danzo 1997) and by inducing aromatase, the enzyme that converts androgen to estrogen, in mammals (Sanderson et al. 2000, 2001), amphibians (Hayes et al. 2002, 2003), and potentially reptiles (Crain et al. 1997). Studies have linked endocrine disruptors to changes in both humans and wildlife (Bigsby et al. 1999; Colborn and Thayer 2000; Colborn et al. 1993; Taylor and Harrison 1999). However, it is the careful examination of wildlife studies that has brought widespread awareness of endocrine disruption to the forefront. These chemicals display several unique characteristics contrary to traditional toxicologic thinking. For example, many endocrine disruptors do not have a definable toxicologic threshold level below which effects are negligible or nonexistent (Welshons et al. 2003). We now know that endocrine disruptors commonly exhibit a nonmonotonic dose–response curve (NMDRC) in which the response reverses as concentration increases in a U-shaped or inverted U-shaped curve. A classic NMDRC was illustrated for the estrogen mimic diethylstilbestrol in a study of prostate weight in mice (vom Saal et al. 1997) and continues to be evident in both vertebrate (Cavieres et al. 2002; Gupta 2000) and invertebrate (Oehlmann et al. 2000) responses to endocrine disruptors.
Because endocrine disruptors often display effects at low levels, it is important to examine low, ecologically relevant concentrations of contaminants. Further, these effects can vary considerably depending on the period of the life cycle during which an organism is exposed (Bigsby et al. 1999). To investigate long-term exposure of amphibians to low pesticide concentrations, we exposed four local species of frogs, spring peepers (Pseudacris crucifer), American toads (Bufo americanus), green frogs (Rana clamitans), and wood frogs (Rana sylvatica), at early (Gosner stages 25–27; Gosner 1960) and late (stages 29–36) developmental stages to low concentrations of atrazine (3, 30, or 100 ppb).
Materials and Methods
Animal collection.
Spring peepers (P. crucifer) and wood frogs (R. sylvatica) were collected from State Game Lands #176 (Centre County, PA) as either embryos or larvae. American toads (B. americanus) were collected as larvae from a wading pool where eggs were voluntarily laid. Green frogs (R. climitans) were collected as embryos from Colyer Lake (Centre County, PA). Collection date and stage as well as larval period for each species are summarized in Table 1. Wood frogs to be used as late-stage animals were held in cattle watering tanks outdoors, whereas American toads and green frogs to be used as late-stage animals were held in glass aquaria in the laboratory. All other animals were collected from the field and placed immediately into the experiment.
Table 1.
Animals collected.
| Date(s) | Stage | Larval period | Reference | |
|---|---|---|---|---|
| P. crucifer | 30 March–2 May | Embryo | 45 days | Gosner and Rossman 1960 |
| R. sylvatica | 24 March–6 April | Embryo | 67 days | Stebbins 1951 |
| B. americanus | 17 July | Hatchling | 39 days | Wilbur 1977 |
| R. clamitans | 31 July | Embryo | 92 days to 1 year | Richmond 1964 |
Date(s) of collection do not indicate the only time embryos are available; the breeding period for each species varies depending on temperature and rainfall across the home range of each species.
Dosing.
We exposed animals to 0, 3, 30, or 100 ppb of commercial-grade atrazine [Aatrex Nine-O, 85.5% atrazine (Syngenta, Greensboro, NC); generally applied during April and May]. The lowest concentration (3 ppb) was based on the drinking water standard for atrazine set by the U.S. Environmental Protection Agency (U.S. EPA 2002). This concentration was chosen to reflect a conservative exposure level for larval amphibians. To achieve the concentrations, we dissolved commercial-grade atrazine (water-soluble granules) in tap water to create a stock solution of 30 mg/L as needed. The stock solution was diluted in dechlorinated tap water to make approximate treatment concentrations. All water used for treatments in the experiment was allowed to reach room temperature before being mixed with atrazine stock solution. Every 3 days, treatment water in the tadpole containers and food was replaced (Hayes et al. 2002). Every 3 days, survival and/or date of metamorphosis (Gosner stage 42; Gosner 1960) was recorded for all individuals. Dead animals were removed from the experiment and preserved in 70% ethanol.
Two samples of water from each concentration of atrazine were collected within 1 hr of being mixed and taken to an outside laboratory (Exygen, Inc., State College, PA) to be tested for concentration accuracy. On average, low, medium, and high concentrations were 2.84 ± 0.05 ppb, 25.20 ± 1.82 ppb, and 64.80 ± 2.88 ppb (mean ± SD), respectively.
Exposures.
Exposures lasted for approximately 30 days. Experiments for each species were performed separately when animals became available. Tadpoles were haphazardly assigned to individual experimental units in a randomized block design containing one of four treatments of atrazine (0, 3, 30, and 100 ppb). Smaller animals (spring peepers, American toads, and early-stage green frogs) were kept in 120 mL polypropylene cups in 100 mL dechlorinated water (with appropriate treatment), whereas larger animals (late wood frogs and late green frogs) were kept in 750 mL high-density polyethylene cups in 500 mL dechlorinated water. Exposures began at either early larval stages (Gosner stages ~ 25–27; Gosner 1960) or late larval stages (stages ~ 29–36) under controlled laboratory conditions. We examined a subsample of animals to determine the developmental stage at which exposure began. At the time of experimentation, early-stage wood frogs were not available.
Animals that metamorphosed during the experiment were eliminated from the data set; therefore, sample size for each treatment for late-stage animals varied. Although averaged for all species, the control had the highest proportion of metamorphs (41.7%) compared with low (26.0%), medium (20.4%), and high (26.5%) treatments. Sample size for each treatment was as follows: early spring peepers [control (C) = 111, low (L) = 112, medium (M) = 101, high (H) = 106], late spring peepers (C = 14, L = 20, M = 21, H = 19), early American toads (C = 30, L = 27, M = 30, H = 30), late American toads (C = 6, L = 7, M = 7, H = 7), early green frogs (C = 15, L = 15, M = 15, H = 15), late green frogs (C = 15, L = 15, M = 15, H = 15), late wood frogs (C = 8, L = 10, M = 10, H = 10).
Experiments ran from 30 March 2002 to 7 November 2002 under controlled laboratory conditions of a 12-hr light/dark cycle and a temperature of 22°C. All animals were fed crushed alfalfa every 3 days when the water was changed. The use of animals in this study was approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University (IACUC #02R011-00)
Statistical analysis.
Survivorship is a function of the days until death and overall mortality at the end of the experiment. These variables were incorporated into a nonparametric survival analysis using Minitab statistical software (Release 13.1; Minitab Inc., State College, PA) to obtain Kaplan-Meier survival probabilities. In turn, these probabilities were used to create survivorship curves (Figure 1). The survivorship curves represent the days until death for animals that died during the experiment; they also take into account the number of animals that lived past the experimental period. A log-rank test value was used to evaluate statistical significance at α = 0.05. Because tests were done separately and under different conditions (e.g., varying water volume) for each species, statistical comparisons could not be made between species or stages of the same species.
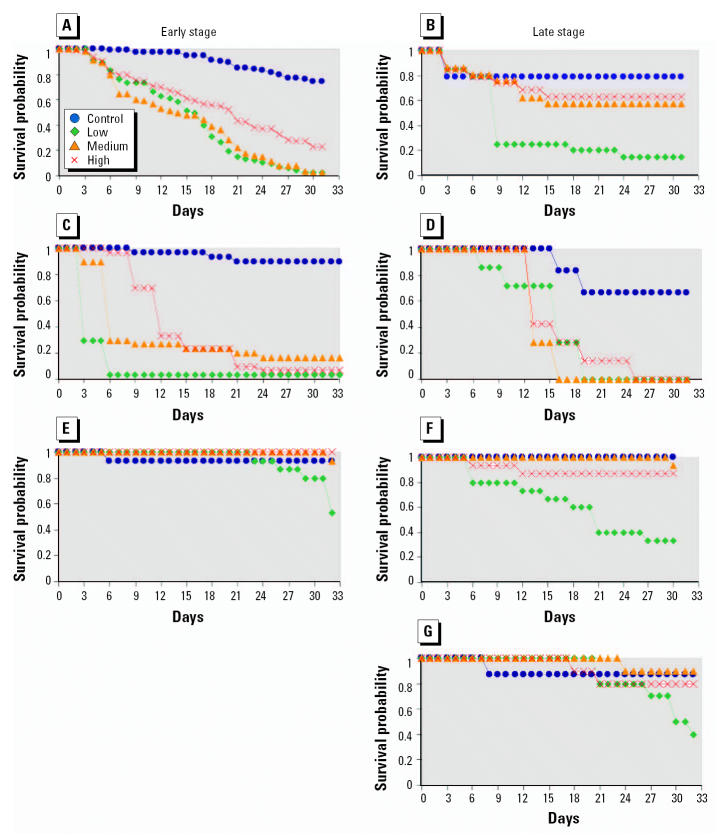
Figure 1. Survivorship curves for early and late-stage animals; all experiments ran at least 30 days. (A) Early spring peepers (p < 0.001, p-value for overall significance). (B) Late spring peepers (p = 0.002). (C) Early American toads (p < 0.001). (D) Late American toads (p = 0.003). (E) Early green frogs (p < 0.001). (F) Late green frogs (p < 0.001). (G) Late wood frogs (p = 0.069). Early wood frogs were not available for experimentation.
Results
Generally, survival was lowest at the low concentrations of atrazine and highest at the high concentrations. This survivorship pattern was seen for early American toads and for both early and late stages of spring peepers and green frogs (Figure 1).
Early spring peepers.
Although survivorship at low and medium atrazine concentrations was not significantly different (p = 0.597) for early spring peepers (Figure 1A), survivorship increased significantly (p < 0.001) with each increasing concentration.
Late spring peepers.
Late spring peepers (Figure 1B) showed significantly reduced survivorship (p < 0.01) for low exposure compared with all other atrazine concentrations. However, neither medium (p = 0.252) nor high (p = 0.392) exposures were significantly different from the control.
Early American toads.
For early American toads (Figure 1C), survivorship at medium and high atrazine concentrations was not significantly different (p = 0.207). Survivorship increased significantly (p < 0.001) with each increasing concentration of atrazine.
Late American toads.
The survivorship pattern for late American toads (Figure 1D) revealed that each individual concentration of atrazine was significantly different from the control (p < 0.01); however, none of the survivorship curves for the individual levels of atrazine was significantly different compared with the others (low and medium, p = 0.203; low and high, p = 0.792; medium and high, p = 0.240).
Early green frogs.
For early green frogs (Figure 1E) there was a significantly reduced survivorship period for the low atrazine concentration compared with all other treatments (low and control, p = 0.021; low and medium, p = 0.013; low and high, p = 0.003).
Late green frogs.
Late green frogs (Figure 1F) exposed to low atrazine concentrations had a significantly reduced survivorship compared with all other treatments (low and control, p < 0.001; low and medium, p < 0.001; low and high, p = 0.005).
Late wood frogs.
For late wood frogs (Figure 1G) there was a significant difference only between the survivorship of low and medium atrazine concentrations (p = 0.027). The difference between control and low treatments approached significance (p = 0.07). All other comparisons were not significantly different (p > 0.05).
Discussion
Our results suggest that in some situations contaminants can have a greater impact at lower concentrations that at higher concentrations. In these laboratory experiments, animals exposed to the low concentration of atrazine died sooner (Figure 1) than did animals exposed to the medium or high concentrations. This pattern is evident in all species except late American toads; the difference in pattern seen here may be attributed to the small sample size of that test.
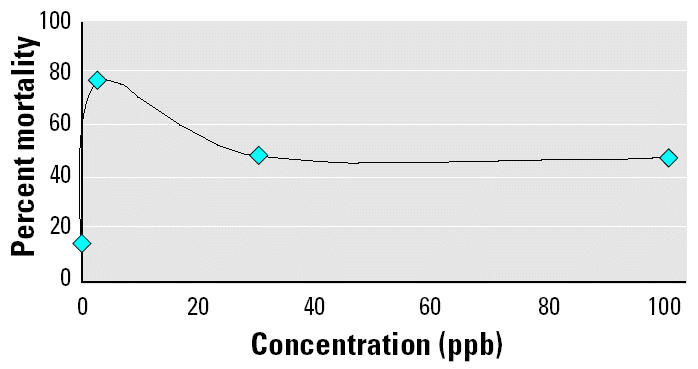
The nature of the pattern is similar to the NMDRC commonly seen in studies examining the effects of endocrine disruptors (Cavieres et al. 2002; Gupta 2000; vom Saal et al. 1997). Traditional toxicology studies use LC50 values (lethal concentrations whereby 50% of the population dies) and linearly extrapolate a predicted response from the LC50 high concentration to low concentrations (Welshons et al. 2003). In our study, mortality was high at the low concentration and low at the high concentration, which is indicative of a U-shaped curve (Figure 2). For example, Birge et al. (1980) reported an LC50 of 48 ppm for atrazine in the American toad 4-days posthatching. When we converted our survival results to 4-day data and combined them with the data from Birge et al. (1980), the pattern in the present case fit the NMDRC pattern as illustrated by a U-shaped curve. The overall pattern (Figure 2) emerging from such low levels suggests that great care should be taken when attempting to elucidate the effects of endocrine disruptors on amphibians at various points in their life cycle. Our results also demonstrate the importance of testing at levels below traditional toxicologic methodologies.
Figure 2. Heuristic model of the NMDRC for atrazine. Data are plotted as the average mortality of all species exposed to ecologically relevant concentrations of atrazine regardless of developmental stage.
In our study, both early-stage (Gosner stages 25–27) and late-stage (Gosner stages 29–36) animals were exposed to reveal possible differences in amphibian response at different points in the life cycle. Pesticide exposure at different life stages can yield significantly different survival rates (Harris et al. 2000). Moreover, exposure to endocrine disruptors during critical times of development can lead to irreversible changes (Bigsby et al. 1999). In our study, it appeared that early and late American toads responded differently to atrazine exposures in terms of survival (Figure 1C, D).
The Rana species (green frogs and wood frogs) seem to be more robust in that they experienced higher survivorship (Figure 1E–G) than the other families represented (Hylidae and Bufonidae; Figure 1A–D). Unlike survivorship patterns of the other species, which consistently dropped below 15.00%, the probability of survival for Rana species never dropped below 33.33% (Figure 1F).
Others have studied the effects of atrazine on larval amphibians using atrazine levels ranging from 0.01 to 47,600 ppb (Allran and Karasov 2000; Detenbeck et al. 1996; Hayes et al. 2002; Howe et al. 1998; Larson et al. 1998), but none have reported the survivorship patterns observed in our study. Hayes et al. (2002) exposed Xenopus laevis to concentrations lower than those in the present study and reported hermaphrodism and decreased larynx size, yet no mortality was found.
Species, grade of atrazine, concentration of atrazine, or experimental setup may have caused the dissimilarities between the present study and others. For example, in the present study, we used a commercial preparation of atrazine (Aatrex Nine-O) containing 85.5% atrazine to create the atrazine concentrations. The remaining 14.5% of this field-grade atrazine contains other chemicals (e.g., surfactants) that may have an impact on survivorship patterns of larval amphibians. Also, in other experiments different grades of atrazine were used; for example, Howe et al. (1998) used atrazine 4L containing 40.8% atrazine.
Although atrazine was originally created for plant use, it has been linked to several hormonal impacts in animals. The discovery of hermaphrodism in X. laevis exposed to atrazine in the laboratory (Hayes et al. 2002) has been extended to R. pipiens exposed in a field setting (Hayes et al. 2003). Atrazine has also been linked to reproductive abnormalities in both the testis and ovary of X. laevis (Tavera-Mendoza et al. 2002a, 2002b), decreased reproductive success of R. temporaria (Hazelwood 1970), and decreased reproductive output in bluegill sunfish, L. macrochirus, (Kettle et al. 1987). Several mammalian studies have shown links between atrazine and reproductive abnormalities including decrease in testosterone production (Friedmann 2002) and altered onset of puberty (Laws et al. 2000). Atrazine may affect mammals (Sanderson et al. 2000, 2001), amphibians (Hayes et al. 2002), and potentially reptiles (Crain et al. 1997) by altering aromatase, the enzyme responsible for converting an androgen to an estrogen.
Although someone may interpret our results such that high levels of atrazine contamination are actually beneficial (Figure 2), it is very likely that such high levels of any chemical would have other, unmeasured, negative direct effects or indirect effects on species interactions (Relyea and Mills 2001), disease susceptibility (Kiesecker 2002), or the community in general (Mills 2002).
Individual applications of pesticides affect amphibian environments locally—mode of application, persistence, and modes of action all differ; however, the use of pesticides in general is worldwide. Given the pattern of significantly reduced survival at low concentrations, the results of our study suggest that low-level testing is imperative when considering contaminant exposure of amphibians. Not only are these results contrary to traditional thinking but they also occur at ecologically relevant levels (Hayes et al. 2003; Huber 1993). Atrazine is generally applied between April and May and corresponds well with the larval period of the species tested (Table 1). Moreover, the exposures lasted for at least 30 days, highlighting the importance of longer term exposure experiments. For example, the survival patterns for the early green frogs and late wood frogs only became apparent after being exposed for 24 days. As populations of amphibians and other wildlife decline, it is critical to examine all possible mechanisms for their decrease (Blaustein and Kiesecker 2002).
References
- Allran JW, Karasov WH. Effects of atrazine and nitrate on northern leopard frog (Rana pipiens) larvae exposed in the laboratory from posthatch through metamorphosis. Environ Toxicol Chem. 2000;19:2850–2855. [Google Scholar]
- Bigsby R, Chapin RE, Daston GP, Davis BJ, Gorski J, Gray LE, et al. Evaluating the effects of endocrine disruptors on endocrine function during development. Environ Health Perspect. 1999;107(suppl 4):613–618. doi: 10.1289/ehp.99107s4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge WJ, Black JA, Kuehne RA. 1980. Effects of Organic Compounds on Amphibian Reproduction. Research Report 21. Lexington, KY:University of Kentucky Water Resources Institute.
- Blaustein AR, Kiesecker JM. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol Lett. 2002;5:597–608. [Google Scholar]
- Cavieres MF, Jaeger J, Porter W. Developmental toxicity of a commercial herbicide mixture in mice: I. Effects on embryo implantation and litter size. Environ Health Perspect. 2002;110:1081–1085. doi: 10.1289/ehp.021101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, Thayer K. Aquatic ecosystems: harbingers of endocrine disruption. Ecol Appl. 2000;10:949–957. [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke AS. Tadpoles as indicators of harmful levels of pollution in the field. Environ Pollut Ser A. 1981;25:123–133. [Google Scholar]
- Cory L, Fjeld P, Serat W. Distribution patterns of DDT residues in the Sierra Nevada mountains. Pestic Monit J. 1970;3:204–211. [PubMed] [Google Scholar]
- Crain DA, Guillette LJ, Jr, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzo BJ. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ Health Perspect. 1997;105:294–301. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Hansen L, McConnell L, Baker J, LeNoir J, Seiber JN. Pesticides and PCB contaminants in fish and tadpoles from the Kaweah River basin, California. Bull Environ Contam Toxicol. 1998;60:829–836. doi: 10.1007/s001289900702. [DOI] [PubMed] [Google Scholar]
- Davidson C, Shaffer HB, Jennings MR. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypothesis for California amphibian declines. Conserv Biol. 2002;16:1588–1601. [Google Scholar]
- Detenbeck NE, Hermanutz R, Allen K, Swift MC. Fate and effects of the herbicide atrazine in flow-through wetland mesocosms. Environ Toxicol Chem. 1996;15:937–946. [Google Scholar]
- Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetelogica. 1960;16:183–190. [Google Scholar]
- Gosner KL, Rossman DA. Eggs and larval development of the treefrogs Hyla crucifer and Hyla ocularis. Herpetelogica. 1960;16:225–232. [Google Scholar]
- Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224:61–68. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- Hall RJ, Henry PFP. Assessing effects of pesticides on amphibians and reptiles: status and needs. Herpetol J. 1992;2:65–71. [Google Scholar]
- Harris ML, Chora L, Bishop CA, Bogart JP. Species- and age-related differences in susceptibility to pesticide exposure for two amphibians, Rana pipiens, and Bufo americanus. Bull Environ Contam Toxicol. 2000;64:263–270. doi: 10.1007/s001289910039. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haefelle C, Vonk A. Atrazine-induced hermaphrodism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect. 2003;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood E. Frog pond contaminated. Br J Herpetol. 1970;4:177–185. [Google Scholar]
- Howe GE, Gillis R, Mowbray RC. Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ Toxicol Chem. 1998;17:519–525. [Google Scholar]
- Huber W. Ecotoxicological relevance of atrazine in aquatic systems. Environ Toxicol Chem. 1993;12:1865–1881. [Google Scholar]
- Kettle WD, deNoyelles F, Jr, Heacock BD, Kadoum AM. Diet and reproductive success of bluegill from experimental ponds treated with atrazine. Bull Environ Contam Toxicol. 1987;38:47–52. doi: 10.1007/BF01606556. [DOI] [PubMed] [Google Scholar]
- Kiesecker JM. Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? Proc Natl Acad Sci USA. 2002;99:9900–9904. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DL, McDonald S, Fivizzani AJ, Newton WE, Hamilton SJ. Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiol Zool. 1998;71:671–679. doi: 10.1086/515999. [DOI] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female Wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci. 2000;58:366–376. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- LeNoir JS, McConnell LL, Fellers MG, Cahill TM, Seiber JN. Summertime transport of current-use pesticides from California’s Central Valley to the Sierra Nevada mountain range, USA. Environ Toxicol Chem. 1999;18:2715–2722. [Google Scholar]
- Mills NE. 2002. Direct and Indirect Effects of an Insecticide on Rana sphenocephela Tadpoles [PhD Thesis]. Columbia, MO:University of Missouri.
- Nations BK, Hallberg GR. Pesticides in Iowa precipitation. J Environ Qual. 1992;21:486–492. [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: Bisphenol A and octylphenol as xenoestrogens. Ecotoxicology. 2000;9:383–397. doi: 10.1023/a:1008972518019. [DOI] [PubMed] [Google Scholar]
- Relyea RA, Mills N. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor) Proc Natl Acad Sci USA. 98:2491–2496. doi: 10.1073/pnas.031076198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond ND. The green frog (Rana clamitans melanota) developing in one season. Herpetelogica. 1964;20:132. [Google Scholar]
- Russell RW, Gillan KA, Haffner GD. Polychlorinated biphenyls and chlorinated pesticides in southern Ontario, Canada, green frogs. Environ Toxicol Chem. 1997;16:2258–2263. [Google Scholar]
- Russell RW, Hecnar SJ, Haffner GD. Organochlorine pesticide-residues in southern Ontario spring peepers. Environ Toxicol Chem. 1995;14:815–817. [Google Scholar]
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, Van den Berg M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Stebbins RC. 1951. Amphibians of Western North America. Berkeley, CA:University of California Press.
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the testis. Environ Toxicol Chem. 2002a;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ Toxicol Chem. 2002b;21:1264–1267. [PubMed] [Google Scholar]
- Taylor MR, Harrison PTC. Ecological effects of endocrine disruption: current evidence and research priorities. Chemosphere. 1999;39:1237–1248. doi: 10.1016/s0045-6535(99)00191-5. [DOI] [PubMed] [Google Scholar]
- USDA 2002. Agricultural Chemical Usage: 2001 Field Crops Summary. Washington, DC:U.S. Department of Agriculture.
- U.S. EPA 2002. List of Contaminants and Their MCLs. EPA 816-F-02-013. Washington, DC:U.S. Environmental Protection Agency.
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur HM. Density-dependent aspects of growth and metamorphosis in Bufo americanus. Ecology. 1977;58:196–200. [Google Scholar]
- Zabik JM, Seiber JN. Atmospheric transport of organophosphate pesticides from California’s Central Valley to the Sierra Nevada Mountains. J Environ Qual. 1993;22:80–90. [Google Scholar]