Abstract
We assessed gastrointestinal effects in 1,365 adults exposed to either < 0.01 (controls), 2, 4, or 6 mg copper/L of drinking water for 2 months in a randomized, double-blind community-based study. The risk of symptoms increased with increasing Cu exposure and decreased with time. The best model by counting-process analysis included Cu concentration and sex. The risk of symptoms remained significantly higher in women than in men during weeks 1–4 for all concentrations tested; at week 1 comparison with the < 0.01-mg/L group showed that differences became significant in women at 4 mg/L [relative risk (RR) = 1.53; 95% confidence interval (CI), 1.02–2.05), and in men at 6 mg/L (RR = 1.9; 95% CI, 1.02–2.79). At week 2 for men and week 4 in women, the Cu concentration required to obtain significant differences on symptom report was > 6 mg Cu/L. We conclude that exposure to Cu in drinking water results in gastrointestinal symptoms, which are modulated by Cu concentration, time, and sex.
Keywords: adults, copper, drinking water, exposure, nausea
Copper is relevant to human nutrition because it is both essential and toxic depending on the dose and duration of exposure. Ingestion of high Cu doses induces acute effects in the gastrointestinal tract, mainly in the stomach (Furukawa and Hatano 1998; Kayashima et al. 1978; Niijima et al. 1987; Wang and Borison 1951), whereas chronic effects from long-term overexposure results mainly on Cu accumulation in the liver and liver damage (Bremmer 1998). Reports of acute Cu intoxication in humans are infrequent (National Research Council 2000; Ross 1955; Spitalny et al. 1984; Wyllie 1957); the possibility that low Cu concentrations, such as those contained in drinking water, may induce acute adverse effects in humans was raised in the early 1980s and 1990s and quickly became a concern of health authorities and regulators. Most natural drinking waters have Cu concentrations not exceeding a few milligrams per liter; however, soft, acidic waters, especially when going through new Cu pipes, may deliver higher amounts of Cu (National Research Council 1980). Anecdotal and accidental random events where variable concentrations of Cu was related to acute gastrointestinal symptoms have appeared in the literature (National Research Council 2000; Ross 1955; Spitalny et al. 1984; Wyllie 1957), but the exact responses and their distribution at a given dose within a population were unknown. The current World Health Organization (WHO) provisional guideline value for drinking water of 2 mg Cu/L is based on acute gastrointestinal symptoms that are reversible in nature (WHO 1993, 2003).
Over the past decade, systematic controlled randomized studies have characterized the full response to acute Cu exposure in drinking water, defining the first adverse effect rather than toxic effects (Araya et al. 2001; Olivares et al. 2001; Pizarro et al. 1999). In these studies, clinical assays using controlled exposure were performed including asymptomatic participants 18–60 years of age, balanced by sex, who were exposed to a single bolus of different waters containing Cu sulfate in concentrations ranging from 0.01 to 12 mg Cu/L. The first and most frequent symptom reported was nausea, which was transient, appearing mainly within 15 min after ingestion (Araya et al. 2001; Gotteland et al. 2001; Olivares et al. 2001; Pizarro et al. 1999). The no observed effect level (NOEL) was 2 mg Cu/L, and the lowest observed adverse effect level (LOAEL) for nausea was 4 mg Cu/L (Olivares et al. 2001). At testing concentrations of up to 12 mg Cu/L, the authors reported that nearly one-third of the subjects remained asymptomatic. Vomiting was observed in 11.5% of the study subjects and was first reported at 6 mg/L, showing a 2-fold increase when the Cu concentration reached 10–12 mg Cu/L. Diarrhea and abdominal cramps were rare within the range of concentrations studied (Araya et al. 2001; Gotteland et al. 2001; Olivares et al. 2001; Pizarro et al. 1999). Using these dose–response curves and the 95% confidence intervals (CI), the Cu concentration at which 5% of the population would experience nausea was 2.0 mg Cu/L for the crude initial response and 4.2 mg Cu/L for the nausea response confirmed by repeat testing (Olivares et al. 2001). Another study emphasizing the interindividual variability of responses across countries included volunteers from the United States, Northern Ireland, and Chile. Using the pooled data obtained in the three countries and statistical significance to define a level, the NOEL and LOAEL for water were determined to be 4 and 6 mg Cu/L (Araya et al. 2001).
Because an epidemiologic study using natural exposure to Cu in water would be difficult to carry out, we decided to conduct a controlled exposure study in a community whose members maintained living conditions as close to real life as possible.
Materials and Methods
Study design.
This randomized, double-blind community intervention trial was designed to evaluate differences in the report of gastrointestinal symptoms in subjects exposed during 2 months to Cu concentrations of < 0.01 mg/L [usual Cu concentration in tap water in Santiago, Chile (Troncoso et al. 1997)], 2 mg/L [WHO provisional-guideline-value set in 1998 (WHO 2003)], 4 mg/L [concentration at which gastrointestinal symptoms were significantly increased in previous controlled clinical trials (Araya et al. 2001; Olivares et al. 2001)], or 6 mg/L [concentration at which vomiting was first reported (Araya et al. 2001; Olivares et al. 2001)]. Nausea, vomiting, diarrhea, and abdominal pain were defined outcome variables. The 2-month exposure allowed for assessing the effect of time, and it was considered safe from potential chronic adverse effects. Participating families continued living at home, carrying out customary activities. Daily water consumption and symptoms were recorded in diaries once a day; therefore, strict relation between time of Cu consumption and appearance of symptoms was not controlled. One person per household (usually the mother) ensured that diaries were filled every night. Twelve trained field workers visited each family every second day, reviewing data recorded and delivering bottles containing the stock solution to be used for water preparation.
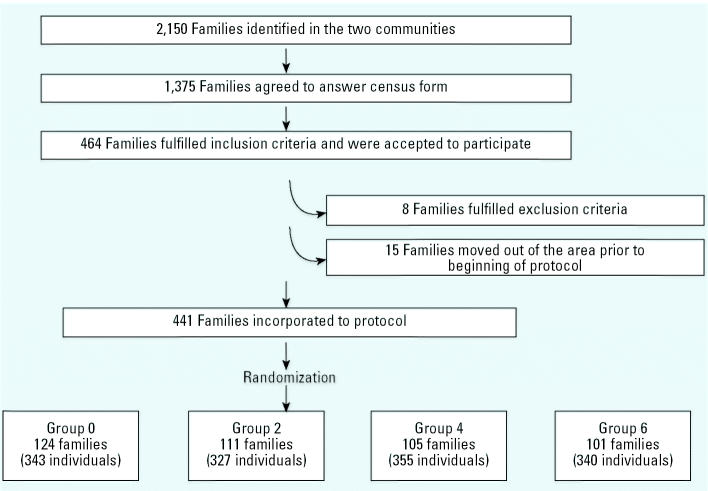
Two communities were selected in southeastern Santiago on the basis of their sizes, and all houses were censused, including gathering general information and identifying potential candidates for the study. Houses in both communities were built as a group more than 13 years before; they shared the city water source and all had Cu pipes that had not been modified or changed in the last 5 years. Because the intervention consisted of preparing at home a “test water” to be consumed by all family members, sample selection was by family, including population ≥ 18 years of age (Figure 1). Exclusion criteria were defined a priori: a) severe chronic illnesses requiring multiple chronic medication; b) alcoholism (> 120 mL alcohol/day); c) smoking more than 40 cigarettes/day; and d ) consumption of drugs. Eight families met these criteria; also, 15 additional families moved to another area. After two meetings with the community in which we explained the protocol and invitited them to participate, 441 families signed an informed consent (one per participant) and were randomized (using a computer-generated random list) to receive< 0.01, 2, 4, or 6 mg Cu/L, representing a total of 1,365 individuals. No families dropped out of the study during the 2-month observation period.
Figure 1. Flow chart showing procedure used to generate the study groups.
Two persons knew the randomization list, the one who generated it and the one who prepared Cu stock solutions. Because of difficulties in masking Cu taste in water, some individuals were expected to taste the Cu at higher concentrations; participants were carefully instructed not to share their perceptions with field workers or other persons. During a 2-month pilot phase, families received placebo water (< 0.01 mg Cu/L), and we validated procedures and forms. Data obtained during this period provided basal information for sample size calculation. The Committee of Ethics for Research in Humans of the Institute of Nutrition and Food Technology (INTA), University of Chile, approved this protocol. An International Technical Advisory Committee also reviewed ethical aspects of the protocol. All volunteers received written and oral information about the protocol and were free to refuse continuing in the study at any time.
Test waters.
Twice per week, a box containing eight 80-mL screw-cap bottles of similar external appearance, coded by color, was delivered to each family; bottles contained a stock solution of Cu sulfate (Merck, Darmstadt, Germany; pro analysis grade) in amounts to reach the concentration 2, 4, or 6 mg Cu/L when diluted to 10 L with tap water. Water for home consumption was prepared by pouring the stock solution into a graduated 20-L container (provided by the researchers) and filling it to 10 L with tap water. Participants were instructed to agitate the container before drinking the water, drink the water when they were at home, and not share it with visitors. The same person that prepared the test water was also responsible for maintaining stock solutions out of reach of children and adults.
The actual Cu content in stock solutions was measured daily, whereas in home-prepared water this was performed once per week by means of unexpected visits to the households, either early in the morning, at mid-day, or in the evening. Cu was measured by atomic absorption spectrophotometry (model 2280; Perkin Elmer, Norwalk, CT, USA). The national system responsible for tap water in Santiago, Empresa Metropolitana de Obras Sanitarias (EMOS), provided tap waters used at INTA laboratories and in the community. Quality of tap water was tested at the INTA once, following U.S. Environmental Protection Agency protocol (Troncoso et al. 1997).
Because water was prepared and maintained at home, potential bacteriologic contamination was investigated in a subsample of 179 randomly chosen households, once per household. Sampling was distributed along the 2-month survey. We used mesophilic aerobic counts (MAC), total coliform counts (TCC), and fecal coliforms as indicators of bacterial contamination. These parameters were determined by means of routine procedures (Downes et al. 2001; Eaton et al. 1995).
Water consumption.
Recording of consumption of fluids indicated the number and size of glasses, cups, and soup bowls, including the approximate amount left over; 300-mL mugs were given to participants, and soup bowls and cups used were measured at the beginning of the survey.
Health survey.
For symptom recording, participants filled out the diary choosing from a list based on previous studies, validated during the pilot phase, that included the four symptoms defined as outcomes and the following symptoms that blinded the subject as to the variables of interest [being energetic (a positive effect assigned to Cu), lack of energy, cough, headache, backache, chest pain, others). Operational definitions of symptoms were provided during the initial meeting and again during home visits. Participants were instructed to discontinue the test water and use plain tap water for 48 hr when they experienced any symptom included in the list. At the end of the 48-hr period, they could continue drinking the assigned water. If symptoms appeared again, subjects were instructed to discontinue the assigned test water permanently and consult the full-time (research) physician located in the community. If symptoms persisted, they should seek help at the local emergency service. Two physicians belonging to the research team were always on call during the study period.
Quality assurance of field operation and data entry.
In addition to field workers, a field supervisor worked every day in the community. Data collected were taken to INTA daily and reviewed to detect missing values. Three times per week, data were reviewed again with a computational supervisor to detect missing data and errors and to correct them whenever possible. Data were entered into the computer at the end of the collection period, corrected, and validated before analysis.
Sample size.
Pilot phase data showed a basal prevalence of total gastrointestinal symptoms (the four outcomes defined for this study) of 5%. Using a power of 80% and a probability of 5%, the sample size required to detect a change in frequency from 5% to 15% was 141 families/group (EPI INFO 6.0; Centers for Disease Control and Prevention, Atlanta, GA, USA). Estimating 5% dropout, we set the final number of families per group at 150.
Analysis of results.
We used Cu concentration in drinking water as an independent variable. Daily volume of water ingested was analyzed as “total water,” “mixed water” (infusions, soups, and others), and “plain water” (test water). Dependent variables were nausea, vomiting, diarrhea, abdominal pain, and total gastrointestinal symptoms. All subjects were included in the analyses; when diaries were not completed, the data were labeled as “missing.” Statistical analysis included analysis of variance (ANOVA), chi-square test, and Fisher’s test. Hazard associated with Cu exposure was calculated by counting-process analysis using S-Plus 6.0 software for Windows (Insightful Corporation, Seattle WA, USA).
Our initial approach was to analyze only those subjects in the randomized groups that followed the protocol instructions, thus generating data under quasi-experimental conditions. However, this omitted an important proportion of the information because many participants’ registered information was incomplete. We sought expert advice, and a post hoc data assessment led us to choose the counting process (S-Plus 6.0). This analysis did not follow the randomized groups but improved the assessment of the dose–response curve after controlled exposure, allowing individuals to remain present every time they recorded data; in the counting process, each subject is treated as an observation of a Poisson process. A censored subject is counted per event, that is, the time of symptom report, even when the rest of the data are incomplete; thus, all participants remained in the analyses and were counted as many times as they were at home, ingested test waters, and filled out their diaries. Proportional hazard models derived by this process allowed predicting hazard ratios for different exposures (Cu concentration measured in water at home) and covariate conditions. Model information used for dependent variable was start–stop for an event; the censoring variable was the report of outcome(s). Because the risk of event decreased after repeated exposure, data were stratified by the variable “time.” Seven-day risk intervals were generated; Cu concentration assigned to each interval and to each occurring event was the value obtained in the weekly measurement in home-prepared waters. Thus, the 1,365 individuals surveyed for 63 days yielded 85,995 person-days of exposure; the number of events detected resulted in a total of 13,354 risk intervals, which were used for the counting-process analysis. Covariates were sex, age, total daily fluid volume, volume ingested as plain water (on the day of event), volume ingested as mixed fluids (on the day of event), and the weekly Cu concentration value obtained from measures of home-prepared water. Multiple models were calculated to assess the potential effect of different covariate sets on the relative risk (RR) estimates for a given Cu concentration. In order to express the risk due to Cu exposure, holding other variables constant, we estimated the RR [95% confidence interval (CI)] due to an increase of Cu exposure and kept the no-exposure groups as the reference risk value.
Results
Comparability of enrolled and censused families.
Families and individuals that fulfilled entry criteria were assigned to the intervention groups as shown in Figure 1. There were no significant differences in sociodemographic indicators measured between families who participated and those who did not. All enrolled families and subjects (n = 1,365) provided data throughout the study period. No individuals were withdrawn because of violations to the protocol.
Comparability of intervention groups.
Randomization resulted in similar groups for the analyzed variables. Baseline demographic characteristics and behaviors that would modify patterns of drinking water were similarly distributed in the four groups (Table 1).
Table 1.
Characteristics of the group, Cu concentration in test waters, Cu intake in waters, and test water consumption.
| Groups by Cu concentration in water (mg/L)
|
||||
|---|---|---|---|---|
| 0 (n = 343) | 2 (n = 327) | 4 (n = 355) | 6 (n = 340) | |
| Age (years; mean ± SD) | 37.4 ± 14.5 | 36.9 ± 13.8 | 37.0 ± 15.0 | 38.5 ± 15.3 |
| Female [n (%)] | 184 (53.6) | 161 (49.2) | 166 (46.8) | 162 (47.6) |
| Out of home (%)a | 73.8 | 70.3 | 71.0 | 71.5 |
| Schooling < 8 years (%)b | 46.7 | 45.0 | 46.5 | 45.6 |
| [Cu] (mg/L; mean ± SD)c | 0.05 ± 0.16 | 2.02 ± 0.25 | 3.71 ± 0.8 | 5.77 ± 1.0 |
| Cu intake (mg/day; mean ± SD)d | 0.08 ± 0.31 | 3.6 ± 1.4 | 6.9 ± 2.8 | 11.0 ± 4.4 |
| Fluid intake [L/day; median (25th percentile–75th percentile)]e | ||||
| Total | 1.7 (1.3–2.3) | 1.7 (1.4–2.2) | 1.7 (1.3–2.2) | 1.8 (1.4–2.3) |
| Mixed water | 1.3 (1.0–1.7) | 1.4 (1.1–1.7) | 1.3 (1.0–1.7) | 1.4 (1.1–1.8) |
| Plain water | 0.4 (0.1–0.7) | 0.4 (0.1–0.6) | 0.35 (0.1–0.6) | 0.41 (0.2–0.7) |
There were no statistically significant differences in the general characteristic among groups.
Percentage of subjects ≤ 8 hr out of home, obtained from the median of hours absent from home per individual per weekdays.
Percentage of subjects with < 8 years of schooling (eight years of education represents a complete basic school education in the Chilean educational system).
Cu concentration in water prepared at home.
Cu intake from water prepared at home.
Fluid intake was calculated from average daily fluid consumption for each start–stop period (counting-process analysis).
Bacteriologic study.
Water was clean by EMOS contemporary data for the study area. Fecal coliforms were not detected; 22 of 179 (12.3%) home water samples had either MAC or TCC positive, and in 5 of 179 (2.8%) both indicators were positive (2, 2, and 1 families in 0, 2, and 6 mg Cu/L groups, respectively). Because bacteriologic evaluations were done once per family and symptom report had low frequency, it was not possible to analyze results using data obtained during the week of bacteriologic sampling. We found no association between the proportion of individuals that reported symptoms or the total number of symptoms reported and at least one positive parameter in the bacteriologic study. In families in whom both parameters were positive, TCC was significantly associated with total symptom report (p = 0.0012, Fisher’s test).
Adherence to the assigned group.
Mean Cu concentration measured in test waters prepared at home, fluid intake, and mean daily dose of Cu received by the study subjects are shown in Table 1. Individual daily water consumption had significant day-to-day variability (ranging from 0 to 6 L/day, in one person), but intergroup differences were not significant, expressed either as total, plain water, or mixed waters. Week-to-week fluid intake along the 9 study weeks did not reveal significant differences (ANOVA). On one occasion, weekly measurement of Cu concentration in home-prepared waters showed two families whose water Cu concentrations were different from the expected values: one had 2 mg Cu/L instead of < 0.01 mg Cu/L, and the other had 2 mg Cu/L instead of 4 mg Cu/L. Information obtained from these families revealed that they decided to interchange one stock solution bottle in order to share the potential “benefits” of having more Cu in their drinking water. The Cu concentration measured in test waters at home was > 6 mg Cu/L (range, 7–13 mg/L) in 8,043 (out of 85,996) occasions, affecting 417 of 1,365 participants.
Health survey.
The number of families enrolled were fewer than the estimated sample size; however, the greater frequency of symptoms reported in the survey (in comparison with the prevalence obtained in the pilot phase) allowed statistical analysis and detection of significant differences. Traditional analysis of this type of data consists of determining the proportion of responders in the study group, because persons that repeat their responses cannot be treated as independent observations. In turn, analysis of responses does not take into consideration the fact that one individual may report more than one symptom, and symptoms more than once. Characteristics of the individuals, Cu concentration in test waters, and Cu and water consumption are shown in Table 1, whereas the proportion of responding individuals appear in Table 2. Of the 1,365 individuals, 222 reported at least one symptom in 665 occasions, providing a total of 794 symptoms; 16.3% of surveyed individuals were “responders,” that is, they reported at least one symptom at least once. Individuals of group 0 represented 2.9% of these responders, providing 18% of the total number of symptoms reported during the study. “Responders” were more frequently women (64.2%, χ2 = 70.84, p = 0.0000). Only 2.6% of responses were obtained from individuals ≥ 60 years of age; although there were no significant differences by age interval, comparison of responses obtained in the first and third terciles for age gave an odds ratio of 0.41. Individuals who ingested < 500 mL test waters/day represented < 1% in all four study groups. Using chi-square analysis, symptom report increased significantly over the basal prevalence at 4 and 6 mg Cu/L, whereas there were no differences between 4 and 6 mg Cu/L (Table 2). Abdominal pain and nausea were the most frequently reported symptoms. Distribution of the total number of responses reported during the 2-month controlled Cu exposure expressed as percentage of total symptom report per group is shown in Table 3. Symptoms reported yielded eight “combinations” throughout the survey (individuals tended to repeat their pattern of report, with 182 of 222 responders reporting the same “combination” of symptoms throughout the survey). Symptom report significantly decreased during the 9 weeks (χ2 = 486.909, p = 0.0000), yielding an odds ratio for symptom report of 0.06 and 0.09 in the last 2 weeks of study, respectively. This did not coincide with decreasing fluid consumption over time. Reports of total “unrelated symptoms” (used to distract the participants) were similar in the four study groups (χ2 = 2.32, p = 0.5083).
Table 2.
Symptoms reported per individual [n (%)] during the 2-month controlled Cu exposure.
| Groups by Cu concentration in water (mg/L)
|
|||||
|---|---|---|---|---|---|
| Symptoms | 0 (n = 343) | 2 (n = 327) | 4 (n = 355) | 6 (n = 340) | Total |
| Personsa | 40 (11.7)*,** | 50 (15.3) | 65 (18.3)* | 67 (19.7)** | 222 (16.3) |
| Occasionsb | 105/21,609 (0.5) | 153/20,601 (0.7) | 216/22,365 (1.0) | 191/21,420 (0.9) | 665/85,995 (0.8) |
| Totalc | 123/21,609 (0.6) | 177/20,601 (0.9) | 230/22,365 (1.0) | 264/21,420 (1.2) | 794/85,995 (0.9) |
Persons reporting at least one symptom at least once.
Number of occasions of symptom report.
Total number of symptoms reported (participants may have reported one or more symptoms on one or more occasion); results expressed as proportion of the total persons per day exposed.
p < 0.02;
p < 0.005.
Table 3.
Symptoms reported per group [n (%)] during the 2-month controlled Cu exposure.a
| Groups by Cu concentration in water (mg/L)
|
|||||
|---|---|---|---|---|---|
| 0 (n = 343) | 2 (n = 327) | 4 (n = 355) | 6 (n = 340) | Total | |
| Nausea | 29/123 (23.6) | 32/177 (18.1) | 57/230 (24.8) | 108/264 (40.9) | 226/794 (28.5) |
| Abdominal pain | 74/123 (60.2) | 100/177 (56.5) | 136/230 (37.1) | 98/264 (37.1) | 408/794 (51.4) |
| Diarrhea | 20/123 (16.3) | 43/177 (24.3) | 34/230 (14.8) | 47/264 (17.8) | 144/794 (18.1) |
| Vomiting | 0/123 (0.0) | 2/177 (1.1) | 3/230 (1.3) | 11/264 (4.2) | 16/794 (2.0) |
Participants may have reported one or more symptoms on one or more occasion.
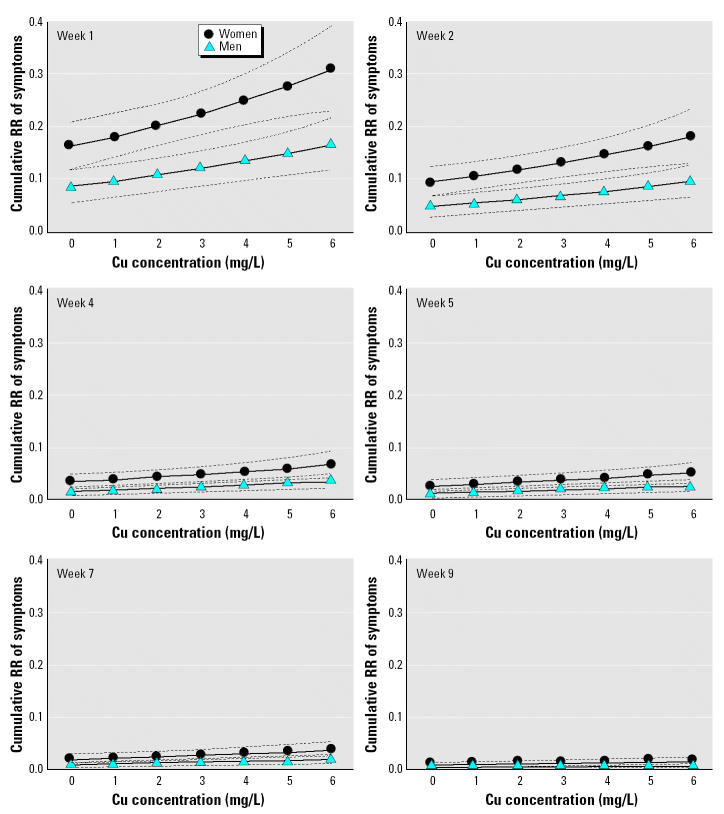
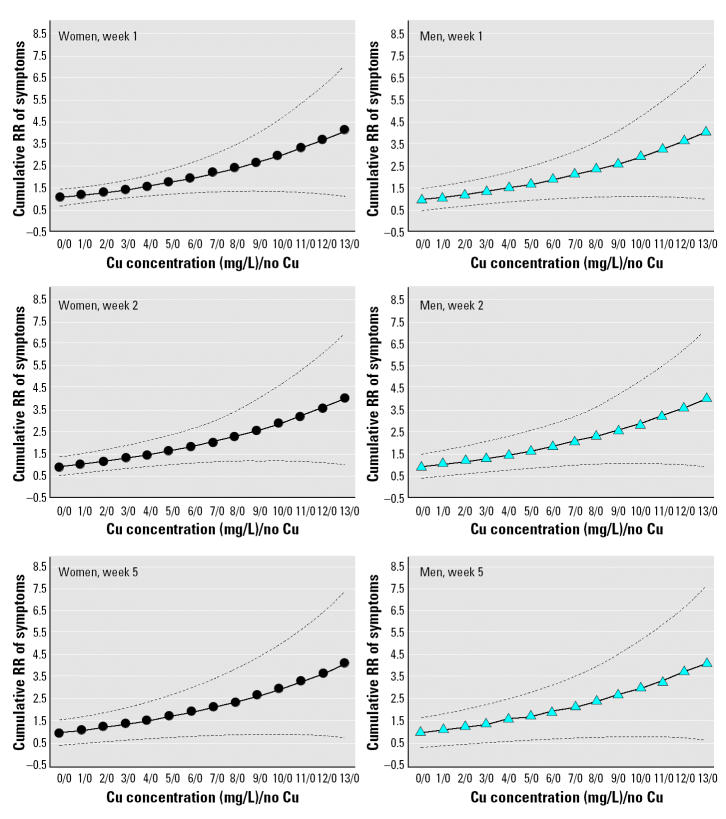
Counting-process analysis using the stepwise method yielded a model that included sex (p < 0.0001) and Cu concentration received on the day of the event (p < 0.0001). The covariates of age, volume of fluid intake, and water consumed as total, plain, and mixed did not have a significant effect on the model. Cumulative hazard curves for increasing Cu concentrations in test water by sex and stratified by weeks showed a progressive decrease of risk over time (Figure 2). Counting-process analysis confirmed results of the preliminary analysis, which showed an increased risk of symptoms associated with increasing Cu concentration and with female sex. Analysis of risk differences between sexes using the 95% CI of the cumulative hazard ratio showed that the risk remains significantly higher from week 1 to week 4 for all Cu concentrations (except basal 0). Figure 3 shows the RR of symptoms against exposure to increasing Cu concentration in test water (compared with no exposure); on week 1 the RR became significant at 4 mg/L in women (RR = 1.53; 95% CI, 1.02–2.05)] and at 6 mg/L in men (RR = 1.9; 95% CI, 1.02–2.79). With advancing time, the significance shifted to higher Cu exposure, such that in week 2 for men and week 4 for women, the Cu concentration required to obtain significant differences on symptom report was > 6 mg Cu/L. Mistakes made while preparing test waters at home created the opportunity to evaluate the effect of Cu exposure > 6 mg/L (actual range, 6–13 mg/L), using the same risk analysis with counting process; this analysis involves a low number of families (n = 126) and individuals (n = 417). The resulting cumulative hazard curves suggest an exponential increment of symptoms associated to rising Cu concentration in test water (Figure 3).
Figure 2. Cumulative RR of symptoms reported during the 2-month controlled Cu exposure. Dotted lines indicate 95% CI.
Figure 3. Cumulative RR of symptoms reported after exposure to different Cu concentrations in drinking water compared to no Cu exposure. Dotted lines indicate 95% CI.
Discussion
This study assessed for the first time the effect of controlled Cu exposure in individuals maintaining conditions close to real daily life, providing information both on the acute gastrointestinal responses and the effect of time in a 2-month interval. Systematic review of Cu effects on human health recently performed by the National Research Council (1980) did not reveal enough information to conclude on the acute and subacute effects of Cu. Previous studies intending to evaluate Cu effects on the population failed to clarify the relation of exposure Cu in tap water and digestive symptoms (Buchanan et al. 1994; CDC 2000; Fitzgerald 1998; Knobeloch et al. 1994; Petterson and Rasmussen 1999; Vergara et al. 1999; Zietz et al. 2003). In the present study, two different approaches for statistical analysis, changing Cu concentration in drinking water from 0 to 4 mg/L, resulted in a significant increment of report of gastrointestinal symptoms. This concentration range also agrees with others previously obtained using controlled clinical trials (Araya et al. 2001; Olivares et al. 2001).
Counting-process analysis represented a significant improvement in the analysis because it treated all participants as present on every occasion that they registered data; this represented a major concern because participating families did not follow instructions strictly, leaving days without registering information when they were out of the home for 2–3 days. A main limitation of this study is the lack of control of the exact timing of exposure and appearance of symptoms. This was indeed performed in the above-mentioned clinical trials that led to the dose–response curve (Araya et al. 2001; Gotteland et al. 2001; Olivares et al. 2001; Pizarro et al. 1999). Instead, in the present study the main objective was to assess to what extent this dose–response curve is applicable when individuals are exposed to Cu in a more realistic fashion. Only Cu concentration and sex were chosen for the model, whereas total volume, daily Cu dose, and quality of the test waters ingested (“plain” or “mixed infusions”) were left out of the model. This result is most relevant because it indicates that Cu concentration and volume are the main determinants of response, and dose and vehicle for Cu ingestion are less important.
Nausea has proved to be a good marker of early response to acute Cu exposure; however, the high frequency of abdominal pain observed in this study was unexpected because abdominal pain was infrequently reported in previous studies. It is difficult to explain this finding; it is possible that repeated acute Cu exposure may be part of the explanation, but this cannot be established by the present study.
Predominance of women in the reporting group is another relevant finding of the study (Figure 2); this difference was suspected in previous studies (Araya et al. 2001; Olivares et al. 2001) and was obtained in the present study by using both chi-square analysis and the counting process. Acute response to Cu exposure is triggered very specifically in the stomach through mechanisms that result in vagal stimulation. Sex differences for vagal phenomena have not been described. In gastric physiology, another sex-related observation is that females have lower gastric alcoholic dehydrogenase activity (Frezza et al. 1990); this has been interpreted as being responsible for the different response to alcohol of women compared to men (Frezza et al. 1990). Whether other sex differences occur and how they may relate to response to Cu remain to be clarified.
Symptom reporting clearly decreased over time both in men and women (Figure 3). Although misreporting of symptoms due to decreasing motivation cannot be ruled out, we found no proof of this using several controls. Therefore, we interpret these results as suggesting an adaptive response to repeated Cu exposure.
In this study we have established the symptoms, range of responses, and relevant variables associated with repeated acute Cu exposure in human adults. Even considering the findings in women (who appeared to be more sensitive to Cu exposure) and results obtained during the first 2 weeks of exposure (which yielded the highest incidence of effects), the current provisional guideline for drinking water (2 mg Cu/L) set by the WHO (WHO 1993, 2003) is safe; these data represent relevant information for regulators who must decide on the relationship of Cu exposure and the safety of drinking water for human health.
References
- Araya M, McGoldrick MC, Klevay L, Strain JJ, Robson P, Neilsen, et al. Determination of an acute no-observed-adverse-effect-level (NOAEL) for copper in water. Regul Toxicol Pharmacol. 2001;34:137–1345. doi: 10.1006/rtph.2001.1492. [DOI] [PubMed] [Google Scholar]
- Bremmer I. Manifestations of copper excess. Am J Clin Nutr. 1998;67:1069S–1073S. doi: 10.1093/ajcn/67.5.1069S. [DOI] [PubMed] [Google Scholar]
- Buchanan SD, Diseker R, Sinks T, Daniel J, Floodman T. 1994. Evaluating Human Gastrointestinal Irritation among Humans from Copper in Drinking Water, Lincoln, Nebraska. Draft. Epi-E94-73. Atlanta, GA:National Center for Environmental Health, Centers for Disease Control and Prevention, and Lincoln, NE:Division of Drinking Water and Environmental Sanitation, Nebraska Department of Health.
- CDC 2000. Surveillance for Waterborne-Disease Outbreaks—United States, 1997–1998. Atlanta, GA:Centers for Disease Control and Prevention. [PubMed]
- Downes FP, Ito K. eds. 2001. Compendium of Methods for the Microbiological Examination of Foods. 4th ed. Washington, DC:American Public Health Association.
- Eaton AD, Clesceri LS, Greenberg AE, Franson MH. eds. 1995. Standard Methods for the Examination of Water and Wastewater. 19th ed. Washington, DC:American Public Health Association.
- Fitzgerald DJ. Safety guidelines for copper in water. Am J Clin Nutr. 1998;67:1098S–1102S. doi: 10.1093/ajcn/67.5.1098S. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Hatano M. An acute experiment on retrograde intestinal peristalsis with emesis using decerebrated dogs. J Auton Nerv Syst. 1998;70:56–65. doi: 10.1016/s0165-1838(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Gotteland M, Araya M, Pizarro F, Olivares M. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig Dis Sci. 2001;46:1909–1914. doi: 10.1023/a:1010683014390. [DOI] [PubMed] [Google Scholar]
- Kayashima N, Tanka M, Iwasaki M, Hayama T. Site of oral copper sulfate in dogs. (I) Threshold of various portions of gastrointestinal tract to locally applied copper sulfate. Jpn J Pharmacol. 1978;28:775–781. doi: 10.1254/jjp.28.775. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Ziarnik M, Howard J, Theis B, Farmer D, Anderson H, et al. Gastrointestinal upsets associated with ingestion of copper-contaminated water. Environ Health Perspect. 1994;102:958–961. doi: 10.1289/ehp.94102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 1980. Drinking Water and Health. Vol. 3. Washington, DC:National Academy Press.
- National Research Council 2000. Copper in Drinking Water. Washington, DC:National Academy Press.
- Niijima A, Jiang ZY, Dauton NG, Fox RA. Effect of copper sulphate on the rate of afferent discharge in gastric branch of the vagus nerve in the rat. Neurosci Lett. 1987;80:71–74. doi: 10.1016/0304-3940(87)90497-6. [DOI] [PubMed] [Google Scholar]
- Olivares M, Araya M, Pizarro F, Uauy R. Nausea threshold in apparently healthy individuals who drink fluids containing graded concentrations of copper. Regul Toxicol Pharmacol. 2001;33:271–275. doi: 10.1006/rtph.2000.1440. [DOI] [PubMed] [Google Scholar]
- Petterson R, Rasmussen F. Daily intake of copper from drinking water among young children in Sweden. Environ Health Perspect. 1999;107:441–446. doi: 10.1289/ehp.99107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro F, Olivares M, Uauy R, Contreras P, Rebelo A, Gidi G. Acute gastrointestinal effects of graded levels of copper in drinking water. Environ Health Perspect. 1999;107:117–121. doi: 10.1289/ehp.99107117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AI. Vomiting and diarrhea due to copper in stewed apples. Lancet. 1955;9:87–88. doi: 10.1016/s0140-6736(55)92187-5. [DOI] [PubMed] [Google Scholar]
- Spitalny KC, Brondum J, Vogt RL, Sargent HE, Kappel S. Drinking water induced intoxication in a Vermont family. Pediatrics. 1984;74:1103–1106. [PubMed] [Google Scholar]
- Troncoso M, Toledo MS, Figueroa G, Guzmán E, Oyarzún MT, Yánez CG, et al. 1997. Estudio Químico-Microbiológico Comparativo de Aguas. Santiago, Chile:Informe Técnico, Secretaría Técnica, Instituto de Nutrición y Tecnología de los Alimentos (INTA), Universidad de Chile.
- Vergara JD, Zietz B, Schneider HB, Dunkelberg H. Determination of the extent of excessive copper concentrations in the tap water of households with copper pipes and assessment of possible health hazards for infants. Eur J Med Res. 1999;4:475–482. [PubMed] [Google Scholar]
- Wang SC, Borison HL. Copper sulfate emesis: a study of afferent pathways for the gastrointestinal tract. Am J Physiol. 1951;164:520–526. doi: 10.1152/ajplegacy.1951.164.2.520. [DOI] [PubMed] [Google Scholar]
- WHO 1993. Guidelines for Drinking Water Quality. Vol. 1. Recommendations. 2nd ed. Geneva:World Health Organization.
- WHO 2003. Guidelines for Drinking Water Quality. 3rd ed. Geneva:World Health Organization. Available: http://www.who.int/water_sanitation_health/GDWQ/Updating/3rdedition.htm [accessed 14 March 2004].
- Wyllie J. Copper poisoning at a cocktail party. Am J Public Health. 1957;47:617. [Google Scholar]
- Zietz BP, Dieter H, Lakomek M, Schneider H, Kessler-Gaedtke B, Dunkelberg H. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci Total Environ. 2003;302:127–144. doi: 10.1016/s0048-9697(02)00399-6. [DOI] [PubMed] [Google Scholar]