Abstract
Anthropogenic land use changes drive a range of infectious disease outbreaks and emergence events and modify the transmission of endemic infections. These drivers include agricultural encroachment, deforestation, road construction, dam building, irrigation, wetland modification, mining, the concentration or expansion of urban environments, coastal zone degradation, and other activities. These changes in turn cause a cascade of factors that exacerbate infectious disease emergence, such as forest fragmentation, disease introduction, pollution, poverty, and human migration. The Working Group on Land Use Change and Disease Emergence grew out of a special colloquium that convened international experts in infectious diseases, ecology, and environmental health to assess the current state of knowledge and to develop recommendations for addressing these environmental health challenges. The group established a systems model approach and priority lists of infectious diseases affected by ecologic degradation. Policy-relevant levels of the model include specific health risk factors, landscape or habitat change, and institutional (economic and behavioral) levels. The group recommended creating Centers of Excellence in Ecology and Health Research and Training, based at regional universities and/or research institutes with close links to the surrounding communities. The centers’ objectives would be 3-fold: a) to provide information to local communities about the links between environmental change and public health; b) to facilitate fully interdisciplinary research from a variety of natural, social, and health sciences and train professionals who can conduct interdisciplinary research; and c) to engage in science-based communication and assessment for policy making toward sustainable health and ecosystems.
Keywords: biodiversity, deforestation, ecosystems, emerging infectious diseases, land use, Lyme disease, malaria, urban sprawl, wildlife, zoonosis
Human-induced land use changes are the primary drivers of a range of infectious disease outbreaks and emergence events and also modifiers of the transmission of endemic infections (Patz et al. 2000). These land use changes include deforestation, road construction, agricultural encroachment, dam building, irrigation, coastal zone degradation, wetland modification, mining, the concentration or expansion of urban environments, and other activities. These changes in turn cause a cascade of factors that exacerbate infectious disease emergence, such as forest fragmentation, pathogen introduction, pollution, poverty, and human migration. These are important and complex issues that are understood only for a few diseases. For example, recent research has shown that forest fragmentation, urban sprawl, and biodiversity loss are linked to increased risk for Lyme disease in the northeastern United States (Schmidt and Ostfeld 2001). Expansion and changes in agricultural practices are intimately associated with the emergence of Nipah virus in Malaysia (Chua et al. 1999; Lam and Chua 2002), cryptosporidiosis in Europe and North America, and a range of food-borne illnesses globally (Rose et al. 2001). Road building is linked to the expansion of bushmeat consumption that may have played a key role in the early emergence of human immunodeficiency virus types 1 and 2 (Wolfe et al. 2000), and simian foamy virus has been found in bushmeat hunters (Wolfe et al. 2004).
In recognition of the complexity of land use change and the risks and benefits to human health that it entails, a special colloquium titled “Unhealthy Landscapes: How Land Use Change Affects Health” was convened at the 2002 biennial meeting of the International Society for Ecosystem Health (6–11 June 2002, Washington, DC) to address this issue. The invited experts worked to establish consensus on the current state of science and identify key knowledge gaps underlying this issue. This article condenses the working group’s report and presents a new research and policy agenda for understanding land use change and its effects on human health. Specifically, we discuss land-use drivers or human activities that exacerbate infectious diseases; the land–water interface, common to many infectious disease life cycles; and conclusions and recommendations for research and training from the working group.
Land-Use Drivers of Infectious Disease Emergence
The emerging infectious diseases (EIDs) resulting from land use change can be entirely new to a specific location or host species. This may occur either from “spillover” or cross-species transmission or simply by extension of geographic range into new or changed habitats. More than 75% of human diseases are zoonotic and have a link to wildlife and domestic animals (Taylor et al. 2001).
The working group developed an extensive list of processes by which land use affects human health (specifically, infectious disease occurrence) and of other factors that contribute to this relationship: agricultural development, urbanization, deforestation, population movement, increasing population, introduction of novel species/pathogens, water and air pollution, biodiversity loss, habit fragmentation, road building, macro and micro climate changes, hydrological alteration, decline in public health infrastructure, animal-intensive systems, eutrophication, military conflict, monocropping, and erosion (ranked from highest to lowest public health impact by meeting participants). The four mechanisms that were felt to have the greatest impact on public health were changes to the physical environment; movement of populations, pathogens, and trade; agriculture; and urbanization. War and civil unrest were also mentioned as a potentially acute and cross-cutting driver. Infectious disease agents with the strongest documented or suspected links to land use change are listed in Table 1.
Table 1.
Agents and infectious diseases with suspected or known links to landscape change.a
| Vector-borne and/or zoonotic | Soil | Water | Human | Other |
|---|---|---|---|---|
| Malaria | Melioidosis | Schistosomiasis | Asthma | Hemorrhagic fevers |
| Dengue | Anthrax | Cholera | Tuberculosis | Foot and mouth |
| Lyme disease | Hookworm | Shigellosis | Influenza | Rice blast |
| Yellow fever | Coccidioidomycosis | Rotavirus | Triachoma | |
| Rift Valley fever | Salmonellosis | |||
| Japanese encephalitis | Leptospirosis | |||
| Onchocerciasis | Cryptosporidiosis | |||
| Trypanosomiasis | ||||
| Plague | ||||
| Filariasis | ||||
| Meningitis | ||||
| Rabies | ||||
| Leishmaniasis | ||||
| Kyasanur Forest fever | ||||
| Hantavirus | ||||
| Nipah virus |
Those with the strongest evidence for a link with land use.
Changes to the biophysical environment.
Deforestation.
Rates of deforestation have grown exponentially since the beginning of the 20th century. Driven by rapidly increasing human population numbers, large swaths of species-rich tropical and temperate forests, as well as prairies, grasslands, and wetlands, have been converted to species-poor agricultural and ranching areas. The global rate of tropical deforestation continues at staggering levels, with nearly 2–3% of forests lost globally each year. Parallel with this habitat destruction is an exponential growth in human–wildlife interaction and conflict. This has resulted in exposure to new pathogens for humans, livestock, and wildlife (Wolfe et al. 2000). Deforestation and the processes that lead to it have many consequences for ecosystems. Deforestation decreases the overall habitat available for wildlife species. It also modifies the structure of environments, for example, by fragmenting habitats into smaller patches separated by agricultural activities or human populations. Increased “edge effect” (from a patchwork of varied land uses) can further promote interaction among pathogens, vectors, and hosts. This edge effect has been well documented for Lyme disease (Glass et al. 1995). Similarly, increased activity in forest habitats (through behavior or occupation) appears to be a major risk factor for leishmaniasis (Weigle et al. 1993). Evidence is mounting that deforestation and ecosystem changes have implications for the distribution of many other microorganisms and the health of human, domestic animal, and wildlife populations.
One example of the effects of land use on human health is particularly noteworthy. Deforestation, with subsequent changes in land use and human settlement patterns, has coincided with an upsurge of malaria and/or its vectors in Africa (Coluzzi 1984, 1994; Coluzzi et al. 1979), in Asia (Bunnag et al. 1979), and in Latin America (Tadei et al. 1998). When tropical forests are cleared for human activities, they are typically converted into agricultural or grazing lands. This process is usually exacerbated by construction of roads, causing erosion and allowing previously inaccessible areas to become colonized by people (Kalliola and Flores Paitán 1998). Cleared lands and culverts that collect rainwater are in some areas far more suitable for larvae of malaria-transmitting anopheline mosquitoes than are intact forests (Charlwood and Alecrim 1989; Jones 1951; Marques 1987).
Another example of the effects of land use on human health involves deforestation and noninfectious disease: the contamination of rivers with mercury. Soil erosion after deforestation adds significant mercury loads, which are found naturally in rainforest soils, to rivers. This has led to fish in the Amazon becoming hazardous to eat (Fostier et al. 2000; Veiga et al. 1994).
Habitat fragmentation.
This alters the composition of host species in an environment and can change the fundamental ecology of microorganisms. Because of the nature of food webs within ecosystems, organisms at higher trophic levels exist at a lower population density and are often quite sensitive to changes in food availability. The smaller patches left after fragmentation often do not have sufficient prey for top predators, resulting in local extinction of predator species and a subsequent increase in the density of their prey species. Logging and road building in Latin America have increased the incidence of cutaneous and visceral leishmaniasis (Desjeux 2001), which in some areas has resulted from an increase in the number of fox reservoirs and sandfly vectors that have adapted to the peridomestic environment (Patz et al. 2000). Foxes, however, are not very important reservoirs for leishmaniasis in Latin America (Courtenay et al. 2002), and a more important factor in the transmission cycle includes domestic dogs.
Ostfeld and Keesing (2000) have demonstrated that smaller fragments in North American forests have fewer small mammal predators. Results suggest that the probability that a tick will become infected depends on not only the density of white-footed mice but also the density of mice relative to that of other hosts in the community. Under this scenario, the density effect of white-footed mice, which are efficient reservoirs for Lyme disease, can be “diluted” by an increasing density of alternative hosts, which are less efficient at transmitting Lyme disease. These results suggest that increasing host diversity (species richness) may decrease the risk of disease through a “dilution effect” (Schmidt and Ostfeld 2001).
Extractive industries.
Gold mining is an extractive industry that damages local and regional environments and has adverse human health effects, because mercury is used to extract gold from riverbeds in the tropical forests. Not only does mercury accumulate in local fish populations, making them toxic to eat (Lebel et al. 1996, 1998), but mercury also suppresses the human immune system. Also, in gold-mining areas, more mosquito-breeding sites and increased malaria risk result from digging gem pits in the forest and from craters resulting from logging; broader disease spread occurs as populations disperse throughout the region (Silbergeld et al. 2002).
Movement of populations, pathogens, and trade.
The movement of humans, domestic animals, wildlife populations, and agricultural products through travel, trade, and translocations is a driver of infectious disease emergence globally. These sometimes inadvertent, sometimes deliberate movements of infectious disease and vectors (e.g., the introduction of smallpox and measles to the Americas by Spanish conquistadors) will continue to rise via continually expanding global travel and by development of Third World populations. Human introduction of pathogens, hosts, or materials into new areas has been termed “pathogen pollution” (Daszak et al. 2000).
Land use changes drive some of these introductions and migrations and also increase the vulnerability of habitats and populations to these introductions. Human migrations also drive land use changes that in turn drive infectious disease emergence. For example, in China’s Yunnan Province, an increase in livestock populations and migration has led to an increase in the incidence of schistosomiasis (Jiang et al. 1997). In Malaysia, a combination of deforestation, drought, and wildfires has led to alterations in the population movements and densities of flying foxes, large fruit bats known to be the reservoir for the newly emergent zoonosis Nipah virus (Chua et al. 1999). It is believed that the increased opportunity for contact between infected bats and pigs produced the outbreak of the disease in pigs, which then was transmitted to people in contact with infected pigs (Aziz et al. 2002).
Another example of human-induced animal movement on a much larger scale is the international pet trade. This movement of animals involves many countries and allows for the introduction of novel pathogens, such as monkeypox, with the potential to damage ecosystems and threaten human and animal health. Monkeypox was originally associated with bushmeat hunting of red colobus monkeys (Procolobus badius); after a localized epidemic emerged in humans, monkeypox persisted for four generations via human-to-human contact (Jezek et al. 1986).
Human movement also has significant implications for public health. Not only are travelers (tourists, businesspeople, and other workers) at risk of contracting communicable diseases when visiting tropical countries, but they also can act as vectors for delivering infectious diseases to another region or, in the case of severe acute respiratory syndrome (SARS), potentially around the world. Refugees account for a significant number of human migrants, carrying diseases such as hepatitis B and tuberculosis and various parasites (Loutan et al. 1997). Because of their status, refugees become impoverished and are more exposed to a wide range of health risks. This is caused by the disruption of basic health services, inadequate food and medical care, and lack of clean water and sanitation (Toole and Waldman 1997). People who cross international boundaries, such as travelers, immigrants, and refugees, may be at increased risk of contracting infectious diseases, especially those who have no immunity because the disease agents are uncommon in their native countries. Immigrants may come from nations where diseases such as tuberculosis and malaria are endemic, and refugees may come from situations where crowding and malnutrition create ideal conditions for the spread of diseases such as cholera, shigellosis, malaria, and measles [Centers for Disease Control and Prevention (CDC) 1998].
Zoonoses.
The importance of zoonotic diseases should be emphasized. Zoonotic pathogens are the most significant cause of EIDs affecting humans, both in the proportion of EIDs that they cause and in the impact that they have. Some 1,415 species of infectious organisms are known to be pathogenic to people, with 61% of them being zoonotic. Of the emerging pathogens, 75% are zoonotic, and zoonotic pathogens are twice as likely to be associated with emerging diseases than are nonzoonotic pathogens (Taylor et al. 2001). More important, zoonotic pathogens cause a series of EIDs with high case fatality rates and no reliable cure, vaccine, or therapy (e.g., Ebola virus disease, Nipah virus disease, and hantavirus pulmonary syndrome). Zoonotic pathogens also cause diseases that have some of the highest incidence rates globally [e.g., acquired immunodeficiency syndrome (AIDS)]. AIDS is a special case, because it is caused by a pathogen that jumped host from nonhuman primates and then evolved into a new virus. Thus, it is in origin a zoonotic organism (Hahn et al. 2000).
Because of the important role of zoonoses in current public health threats, wildlife and domestic animals play a key role in the process by providing a “zoonotic pool” from which previously unknown pathogens may emerge (Daszak et al. 2001). The influenza virus is an example, causing pandemics in humans after periodic exchange of genes among the viruses of wild and domestic birds, pigs, and humans. Fruit bats are involved in a high-profile group of EIDs that includes rabies and other lyssaviruses, Hendra virus and Menangle virus (Australia), and Nipah virus (Malaysia and Singapore), which has implications for further zoonotic disease emergence. A number of species are endemic to both remote oceanic islands and more populous suburban and rural human settlements; these may harbor enzootic and potentially zoonotic pathogens with an unknown potential for spillover (Daszak et al. 2000).
Thus, some of the current major infectious threats to human health are EIDs and reemerging infectious diseases, with a particular emphasis on zoonotic pathogens transferring hosts from wildlife and domestic animals. A common, defining theme for most EIDs (of humans, wildlife, domestic animals, and plants) is that they are driven to emerge by anthropogenic changes to the environment. Because threats to wildlife habitat are so extensive and pervading, many of the currently important human EIDs (e.g., AIDS, Nipah virus disease) are driven partly by human-induced changes to wildlife habitat such as encroachment and deforestation. This is essentially a process of natural selection in which anthropogenic environmental changes perturb the host–parasite dynamic equilibrium, leading to the expansion of those strains suited to the new environmental conditions and facilitating expansion of others into new host species (Daszak et al. 2001).
Agriculture.
Crop irrigation and breeding sites.
Agriculture occupies about half of the world’s land and uses more than two-thirds of the world’s fresh water (Horrigan et al. 2002). Agricultural development in many parts of the world has increased the need for crop irrigation, which reduces water availability for other uses and increases breeding sites for disease vectors. An increase in soil moisture associated with irrigation development in the southern Nile Delta after the construction of the Aswan High Dam has caused a rapid rise in the mosquito Culex pipiens and consequential increase in the arthropod-borne disease Bancroftian filariasis (Harb et al. 1993; Thompson et al. 1996). Onchocerciasis and trypanosomiasis are further examples of vector-borne parasitic diseases that may be triggered by changing land-use and water management patterns. In addition, large-scale use of pesticides has had deleterious effects on farm workers, including hormone disruption and immune suppression (Straube et al. 1999).
Food-borne diseases.
Once agricultural development has expanded and produced food sufficient to meet local need, the food products are exported to other nations, where they can pose a risk to human health. The increase in imported foods has resulted in a rise in food-borne illness in the United States. Strawberries from Mexico, raspberries from Guatemala, carrots from Peru, and coconut milk from Thailand have caused recent outbreaks. Food safety is an important factor in human health, because food-borne disease accounts for an estimated 76 million illnesses, 325,000 hospitalizations, and 5,200 deaths in the United States each year (CDC 2003). Other dangers include antibiotic-resistant organisms, such as Cyclospora, Escherichia coli O157:H7, and other pathogenic E. coli strains associated with hemolytic uremic syndrome in children (Dols et al. 2001).
Secondary effects.
Agricultural secondary effects need to be minimized, such as the emerging microbial resistance from antibiotics in animal waste that is included in farm runoff and the introduction of microdams for irrigation in Ethiopia that resulted in a 7-fold increase in malaria (Ghebreyesus et al. 1999).
Urbanization.
On a global basis, the proportion of people living in urban centers will increase to an unprecedented 65% by the year 2030 (Population Reference Bureau 1998). The 2000 census shows that 80% of the U.S. population now lives in metropolitan areas, with 30% living in cities of 5 million or more. The environmental issues posed by such large population centers have profound impacts on public health beyond the city limits (Knowlton 2001).
Alterations of ecosystems and natural resources contribute to the emergence and spread of infectious disease agents. Human encroachment of wildlife habitat has broadened the interface between wildlife and humans, increasing opportunities for both the emergence of novel infectious diseases in wildlife and their transmission to people. Rabies is an example of a zoonotic disease carried by animals that has become habituated to urban environments. Bats colonize buildings, skunks and raccoons scavenge human refuse, and in many countries feral dogs in the streets are common and the major source of human infection (Singh et al. 2001).
Infectious diseases can also pass from people to wildlife. Nonhuman primates have acquired measles from ecotourists (Wallis and Lee 1999). Also, drug resistance in gram-negative enteric bacteria of wild baboons living with limited human contact is significantly less common than in baboons living with human contact near urban or semiurban human settlements (Rolland et al. 1985).
The Land–Water Interface
Another major driver of infectious disease emergence results from the land–water interface. Land use changes often involve water projects or coastal marine systems in which nutrients from agricultural runoff can cause algal blooms.
Currently the seventh cholera pandemic is spreading across Asia, Africa, and South America. In 1992, a new serogroup (Vibrio cholerae O139) appeared and has been responsible for epidemics in Asia (Colwell 1996). The seasonality of cholera epidemics may be linked to the seasonality of plankton (algal blooms) and the marine food chain. Studies using remote-sensing data of chlorophyll-containing phytoplankton have shown a correlation between cholera cases and sea surface temperatures in the Bay of Bengal. Interannual variability in cholera incidence in Bangladesh is also linked to the El Niño southern oscillation and regional temperature anomalies (Lobitz et al. 2000), and cholera prevalence has been associated with progressively stronger El Niño events spanning a 70-year period (Rodo et al. 2002). This observation on cholera incidence may represent an early health indicator of global climate change (Patz 2002).
Infectious diseases in marine mammals and sea turtles could serve as sentinels for human disease risk. Sea turtles worldwide are affected by fibropapillomatosis, a disease probably caused by one or several viruses and characterized by multiple epithelial tumors. Field studies support the observation that prevalence of this disease is associated with heavily polluted coastal areas, areas of high human density, agricultural runoff, and/or biotoxin-producing algae (Aguirre and Lutz, in press). This represents the breakdown of the land–water interface, to the point that several pathogens typical of terrestrial ecosystems have become established in the oceans. Toxoplasmosis in the endangered sea otter (Enhydra lutris) represents an example of pathogen pollution. Massive mortalities in pinnipeds and cetaceans reaching epidemics of tens of thousands are caused by four morbilliviruses evolving from the canine distemper virus (Aguirre et al. 2002). Additionally, overfishing has myriad ramifications for marine ecosystems and sustainable protein food sources for human populations.
Cryptosporidium, a protozoan that completes its life cycle within the intestine of mammals, sheds high numbers of infectious oocysts that are dispersed in feces. A recent study found that 13% of finished treated water still contained Cryptosporidium oocysts, indicating some passage of microorganisms from source to treated drinking water (LeChevallier and Norton 1995). The protozoan is highly prevalent in ruminants and is readily transmitted to humans. Thus, management of livestock contamination of watersheds is an important public health issue.
One example of how overexploitation of a natural water resource led to infectious disease is that of Lake Malawi in Africa. Overfishing in the lake reduced the population of snail-eating fish to such a level that snail populations erupted. Subsequently, schistosomiasis incidence and prevalence markedly rose after this ecologic imbalance (Madsen et al. 2001).
Recommendations from the Working Group
Conceptual model: bringing land use into public health policy.
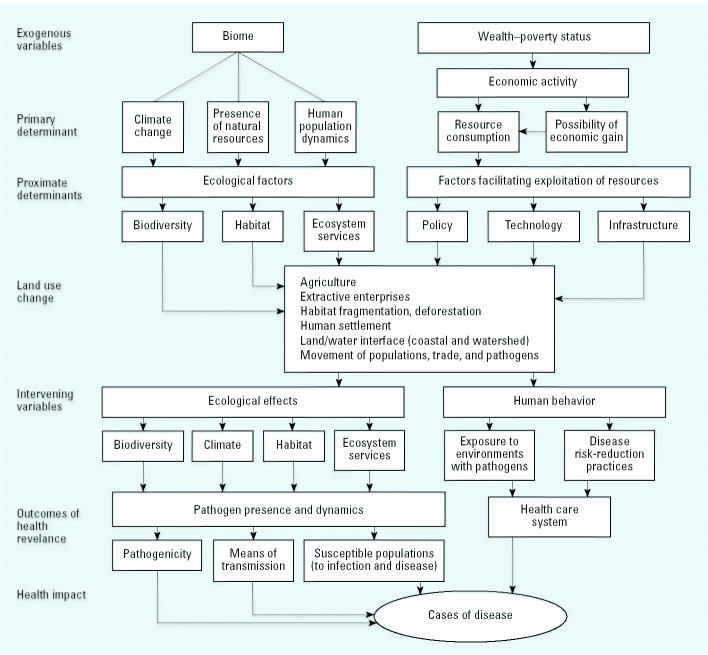
The recommendations stemming from the international colloquium are highly relevant to the Millennium Ecosystem Assessment (MEA), a broad multiagency/foundation-sponsored scientific assessment of degraded ecosystem effects on human well-being. A conceptual framework of the MEA already provides an approach to optimize the contribution of ecosystems to human health (MEA 2003). This framework offers a mechanism to a) identify options that can better achieve human development and sustainable goals, b) better understand the trade-offs involved in environment-related decisions, and c) align response options at all scales, from the local to the global, where they can be most effective. This conceptual framework focuses on human well-being while also recognizing associated intrinsic values. Similar to the MEA, focus is particularly on the linkages between ecosystem services and human health. Workshop participants developed a conceptual model (Figure 1). Like the MEA, it assumes a dynamic interaction between humans and ecosystems that warrants a multiscale assessment (spatial and temporal).
Figure 1. A systems model of land use change that affects public health. This model shows relationships between drivers of land use change and subsequent levels of environmental change and health consequences. Various levels of investigation and intervention are evident and range from specific risks factors and determinants of population vulnerability to larger institutional and economic activity.
By using this framework, policy makers may approach development and health at various levels. These levels include specific health risk factors, landscape or habitat change, and institutional (economic and behavioral) levels. For sound health policy, we must shift away from dealing primarily with specific risk factors and look “upstream” to underlying land-use determinants of infectious disease and ultimately the human behavior and established institutions that are detrimental to sustainable population health. The World Health Organization (WHO) has developed a similar DPSEEA (driving forces, pressures, state, exposure, effect, actions) model that in a similar way describes the interlinkage between human health and different driving forces and environmental change (WHO 1997).
As such understanding increases, it will become more feasible to plan how to prevent new infectious disease emergence. Yet, because these are rare events, accurate predictions will remain daunting. It is already evident that inserting humans into complex ecosystems can lead to a variety of EIDs, but health outcomes depend on the economic circumstances of the human population. In poor and tropical communities, land use change can lead to major shifts in infectious disease patterns. For these situations, many conventional public health interventions can prevent several infectious diseases at relatively low cost. In rich and temperate-climate communities, the infectious disease shifts tend to be more disease specific, for example, in the case of Lyme disease and habitat fragmentation.
Research on deforestation and infectious disease.
Considering the deforestation that usually accompanies agricultural development, new conservation-oriented agriculture should be pursued. As discussed above, water project development and modern livestock management present major health disease risks. However, often the secondary unintended consequences can also wreak havoc; for example, a leaking dam may present greater risks than the reservoir itself. A distressingly large number of development projects not only have adverse effects on human health but also fail to attain their primary economic purposes in a sustainable manner.
Habitat fragmentation, whether caused by forest destruction, desertification, or land-use conversion, affects human and wildlife health and ecosystem processes. There is already much research undertaken by landscape ecologists on the consequences of habitat fragmentation for wildlife, especially larger animals. It would be important to study the effects of landscape fragmentation on public health hazards. Such research could entail three components. The first component consists of gathering baseline data, including using historical data where possible and beginning monitoring programs where necessary. Key data include identifying and quantifying the relevant pathogen load of wildlife, livestock, and human communities in fragmented landscapes. The goals of this data collection are, first, to identify key infectious diseases, both chronic and emergent or reemergent and, second, to document the consequences of fragmentation on relative abundance of wildlife and subsequent pathogen load. For example, the loss of large predators in fragmented habitats in the northeastern United States has led to a superabundance of rodent vectors for Lyme disease.
The second component of the research program would involve health impact modeling, primarily in three areas: a) estimating changes in the relative abundance of organisms, including infectious disease vectors, pathogens, and hosts; b) projecting potential vector or transmission shifts (e.g., should the Nipah virus shift to pulmonary as well as neurologic expression in humans as in swine); and c) projecting the impact of infectious diseases in a region on different geographic scales.
The results of these analyses, if successful, could support the third component of research: development of decision-support tools. Improved decisions on land-use policy could be made from a better understanding of costs and benefits to health and environmental decision makers. In all probability, however, they will be very location specific. For example, to construct an irrigation scheme in India would likely invite a malaria epidemic, whereas the same activity in sub-Saharan Africa may have little effect on malaria transmission. It is worth mentioning that costs and benefits could depend on the time course over which they are assessed. For example, some land-use changes can lead to short-term increases in transmission followed by longer-term decreases (e.g., irrigation and malaria in Sri Lanka) or vice versa (e.g., deforestation and cutaneous leishmaniasis in Latin America).
Policies to reduce microbial traffic/pathogen pollution.
In today’s interconnected world, it becomes very important to invest in the worldwide control of infectious diseases in developing countries, for example. It is also necessary to control transport to stem the flow from one place to the next.
Improved monitoring of trade is warranted in order to target infectious disease introductions. In the attempt to prevent the invasion of a pathogen (and drug-resistant organisms) into the vulnerable areas subject to land use changes, we need to pay greater attention to controls at the sources. We need to document and map these trades and investigate the vectors, the infectious diseases they harbor, and the populations they threaten. Risk assessment should guide surveillance and the development of test kits, targeting point-of-origin intervention to preempt these processes. Assessments must further include nonmarket costs (usually to the detriment of the environment and long-term sustainable health). We should communicate to both the exporters and consumers the need to make their trades clean, economically viable, and certified “clean and green” by an independent scientific agency at the source and/or destination. Additionally, strategies for screening travelers for pathogens that may be introduced to a region should be improved.
Centers of Excellence in Ecology and Health Research and Training.
One approach to developing the issues to which this article draws attention is the creation of a system of regional- or subregional-based interdisciplinary Centers of Excellence in Ecology and Health Research and Training. Based at regional universities and/or research institutes but with very close links to the surrounding communities, these centers would have the following objectives:
Providing information based on good science to local communities about the links between environmental change and public health, including the factors that contribute to specific infectious disease outbreaks. The new research agenda must gather information on household and community perspectives about proposals for the use of their land. These perspectives are key to assessing the cost/benefit of a proposed project. Training local professionals in environmental, agricultural, and health science issues, with a particular focus on granting degrees in a new “trans”-discipline linking health and the environment, would be emphasized.
Acting as centers of integrated analysis of infectious disease emergence, incorporating perspectives and expertise from a variety of natural, social, and health sciences. Research activities would range from taxonomy of pathogens and vectors to identifying best practices for influencing changes in human behavior to reduce ecosystem and health risks.
Incorporating a “health impact assessment” as an important cross-sectorial decision-making tool in overall development planning (parallel to an environmental impact assessment), along with the need for doing more research.
Equipping professionals with the ability to recommend policy toward maintaining ecosystem function and promoting sustainable public health for future generations. For example, the link between forest fragmentation and Lyme disease risk could lead to preserving more intact tracts of forest habitat by planning “cluster” housing schemes.
Implementing research and policy programs.
In selecting areas for research and the placement of centers of excellence, it is important to choose geographically representative, highly diverse areas around the world. In addition, research projects should take place in regions or landscapes that have both well characterized and less characterized patterns of infectious disease emergence or transmission for comparison purposes. Local health and environment professionals, who are in the best position to understand local priorities, should make the choices within each region for initial research areas and sites.
Addressing trade-offs among environment, health, and development.
There are some inherent trade-offs when considering land-use change and health. They are ethical values, environmental versus health choices, and disparities in knowledge and economic class. Trade-offs are between short-term benefit and long-term damage. For example, draining swamps may reduce vector-borne disease hazards but also destroy the wetland ecosystem and its inherent services (e.g., water storage, water filtration, biologic productivity, and habitats for fish and wildlife). Research can help decision making by identifying and assessing trade-offs in different land-use-change scenarios. Balancing the diverse needs of people, livestock, wildlife, and the ecosystem will always be a prominent feature.
Conclusions
When considering issues of land use and infectious disease emergence, the public needs to be attentive to entire ecosystems rather than simply their local environs. Although we may not live within a certain environment, its health may indirectly affect our own. For example, intact forests support complex ecosystems and provide essential habitats for species that are specialized to those flora and that may be relevant to our health. If these complex relationships are disrupted, there may be unforeseen impacts on human health, as the above examples clearly demonstrate.
Encouraging initiatives.
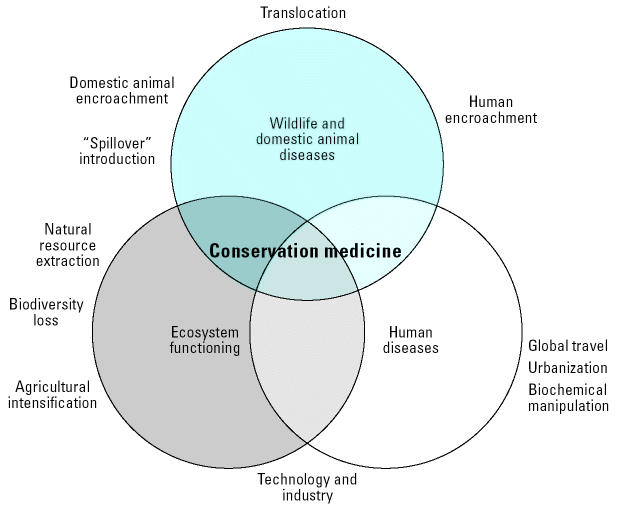
Three new initiatives are rising to the challenges presented above. The first initiative, the Consortium for Conservation Medicine (CCM), was formed recently to address these health challenges at the interface of ecology, wildlife health, and public health (Figure 2). At its core, conservation medicine champions the integration of techniques and partnering of scientists from diverse disciplines, particularly veterinary medicine, conservation biology, and public health. Through the consortium, therefore, these experts work with educators, policy makers, and conservation program managers to devise approaches that improve the health of both species and humans simultaneously [more information is available from the CCM website (CCM 2004)].
Figure 2. The main elements converging under the Consortium for Conservation Medicine. Conservation medicine combines conservation biology, wildlife veterinary medicine, and public health. Adapted from Tabor (2002).
The second initiative, the new international journal EcoHealth, focuses on the integration of knowledge at the intersection of ecologic and health sciences. The journal provides a gathering place for research and reviews that integrate the diverse knowledge of ecology, health, and sustainability, whether scientific, medical, local, or traditional. The journal will encourage development and innovation in methods and practice that link ecology and health, and it will ensure clear and concise presentation to facilitate practical and policy application [more information is available from the EcoHealth website (EcoHealth 2004)].
The third initiative, the MEA, is an international work program designed to meet the needs of decision makers and the public for scientific information concerning the consequences of ecosystem change for human health and well-being and for options in responding to those changes. This assessment was launched by United Nations Secretary-General Kofi Annan in June 2001 and will help to meet the assessment needs of international environmental forums, such as the Convention on Biological Diversity, the Convention to Combat Desertification, the Ramsar Convention on Wetlands, and the Convention on Migratory Species, as well as the needs of other users in the private sector and civil society [more information is available from the Millennium Assessment Working Groups website (Millennium Assessment Working Groups 2004)].
Challenges ahead.
As this working group of researchers continues to work on these topics, we face three challenges. First, strong trans-disciplinary research partnerships need to be forged to approach the research with the degree of creative thinking and comprehensiveness required by the nature of the problems. Second, if the work is to influence policy, the choice of questions and the research must be undertaken collaboratively with the local community and also through discussion with decision makers in government, industry, civil society, and other sectors. Third, investigators must consider how they can integrate their findings into the social, economic, and political dialogue on both the environment and health, globally and locally. As links between land use and health are elucidated, an informed public will more readily use such discoveries to better generate political will for effective change.
References
- Aguirre AA, Lutz P. In press. Marine turtles as sentinels of ecosystem health: is fibropapillomatosis an indicator? EcoHealth.
- Aguirre AA, Ostfeld RS, Tabor GM, House C, Pearl MC. (eds.). 2002. Conservation Medicine: Ecological Health in Practice. New York:Oxford University Press.
- Aziz AJ, Nor SK, Chua KB, Shamshad S. 2002. Emerging Infectious Diseases—A Malaysian Perspective. Tokyo:OIE Regional Representation for Asia and the Pacific. Available: http://www.rr-asia.oie.int/topics/detail011_03.html [accessed 10 April 2003].
- Bunnag T, Sornmani S, Pinithpongse S, Harinasuta C. Surveillance of water-borne parasitic infections and studies on the impact of ecological changes on vector mosquitoes of malaria after dam construction. Southeast Asian J Trop Med Public Health. 1979;10:656–660. [PubMed] [Google Scholar]
- CCM 2004. Conservation Medicine. Palisades, NY:Consortium for Conservation Medicine. Available: http://www.conservationmedicine.org/ [accessed 10 May 2003].
- CDC 1998. Addressing the problem of diseases of travelers, immigrants, and refugees. In: Emerging Infectious Diseases: A Strategy for the 21st Century. Atlanta, GA:National Center for Infectious Diseases, Centers for Disease Control and Prevention. Available: http://www.cdc.gov/ncidod/emergplan/travel/page_2.htm [accessed 10 May 2003].
- CDC 2003. Foodborne Illness. Atlanta, GA:Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention. Available: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/foodborneinfections_g.htm [accessed 10 May 2003].
- Charlwood JD, Alecrim WA. Capture-recapture studies with the South American malaria vector Anopheles darlingi, Root. Ann Trop Med Parasitol. 1989;83:569–576. doi: 10.1080/00034983.1989.11812389. [DOI] [PubMed] [Google Scholar]
- Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control. Bull WHO. 1984;62(suppl):107–113. [PMC free article] [PubMed] [Google Scholar]
- Coluzzi M. Malaria and the Afrotropical ecosystems: impact of man-made environmental changes. Parassitologia. 1994;36:223–227. [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Courtenay O, Quinnell RJ, Garcez LM, Dye C. Low infectiousness of a wildlife host of Leishmania infantum: the crab-eating fox is not important for transmission. Parasitology. 2002;125(pt 5):407–414. doi: 10.1017/s0031182002002238. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95(3):239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- Dols CL, Bowers JM, Copfer AE. Preventing food- and water-borne illnesses. Am J Nurs. 2001;101:24AA–24HH. doi: 10.1097/00000446-200106000-00032. [DOI] [PubMed] [Google Scholar]
- EcoHealth 2004. Conservation Medicine, Human Health, Ecosystem Sustainability. New York:Springer Verlag. Available: http://www.ecohealth.net/ [accessed 4 April 2003].
- Fostier AH, Forti MC, Guimaraes JR, Melfi AJ, Boulet R, Espirito Santo CM, et al. Mercury fluxes in a natural forested Amazonian catchment (Serra do Navio, Amapa State, Brazil) Sci Total Environ. 2000;260:201–211. doi: 10.1016/s0048-9697(00)00564-7. [DOI] [PubMed] [Google Scholar]
- Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes AM, Yohannes M, et al. Incidence of malaria among children living near dams in northern Ethiopia: community based incidence survey. BMJ. 1999;319:663–666. doi: 10.1136/bmj.319.7211.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GE, Schwartz BS, Morgan JM, III, Johnson DT, Noy PM, Israel E. Environmental risk factors for Lyme disease identified with geographic information systems. Am J Public Health. 1995;85:944–948. doi: 10.2105/ajph.85.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Harb M, Faris R, Gad AM, Hafez ON, Ramzy R, Buck AA. The resurgence of lymphatic filariasis in the Nile delta. Bull WHO. 1993;71:49–54. [PMC free article] [PubMed] [Google Scholar]
- Horrigan L, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect. 2002;110:445–456. doi: 10.1289/ehp.02110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zheng QS, Wang XF, Hua ZH. Influence of livestock husbandry on schistosomiasis transmission in mountainous regions of Yunnan Province. Southeast Asian J Trop Med Public Health. 1997;28:291–295. [PubMed] [Google Scholar]
- Jones TW. Deforestation and epidemic malaria in the wet and intermediate zones of Ceylon. Indian J Malariol. 1951;5:135–161. [PubMed] [Google Scholar]
- Kalliola R, Flores Paitán S. (eds.). 1998. Geoecología y Desarrollo Amazónico. Estudio Integrado en la Zona de Iquitos, Perú. Sulkava, Peru:Finnreklama Oy.
- Knowlton K. Urban history, urban health. Am J Public Health. 2001;91:1944–1946. doi: 10.2105/ajph.91.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Chua KB. Nipah virus encephalitis outbreak in Malaysia. Clin Infect Dis. 2002;34(suppl 2):S48–S51. doi: 10.1086/338818. [DOI] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, et al. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Lucotte M, Amorim M, Dolbec J, Miranda D, et al. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methyl-mercury. Neurotoxicology. 1996;17:157–167. [PubMed] [Google Scholar]
- LeChevallier MW, Norton WD. Giardia and cryptosporidium in raw and finished water. J Am Water Works Assoc. 1995;87:54–68. [Google Scholar]
- Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque AS, et al. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutan L, Bierens de Haan D, Subilia L. The health of asylum seekers: from communicable disease screening to post-traumatic disorders [in French] Bull Soc Pathol Exot. 1997;90:233–237. [PubMed] [Google Scholar]
- Madsen H, Bloch P, Phiri H, Kristensen TK, Furu P. Bulinus nyassanus is an intermediate host for Schistosoma haematobium in Lake Malawi. Ann Trop Med Parasitol. 2001;95:353–360. doi: 10.1080/00034980120065813. [DOI] [PubMed] [Google Scholar]
- Marques AC. Human migration and the spread of malaria in Brazil. Parasitol Today. 1987;3:166–170. doi: 10.1016/0169-4758(87)90170-0. [DOI] [PubMed] [Google Scholar]
- MEA (Millennium Ecosystem Assessment) 2003. Ecosystem and Human Well-being: A Framework for Assessment. Washington, DC:Island Press.
- Millennium Assessment Working Groups 2004. Millennium Ecosystem Assessment: Strengthening Capacity to Manage Ecosystems Sustainably for Human Well-being. Washington, DC:Meridian Institute. Available: http://www.millenniumassessment.org [accessed 3 May 2004].
- Ostfeld SR, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000;14:722–728. [Google Scholar]
- Patz JA. A human disease indicator for the effects of recent global climate change. Proc Natl Acad Sci USA. 2002;99:12506–12508. doi: 10.1073/pnas.212467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Population Reference Bureau 1998. 1998 World Population Data Sheet. Washington, DC:Population Reference Bureau.
- Rodo X, Pascual M, Fuchs G, Faruque AS. ENSO and cholera: a nonstationary link related to climate change? Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland RM, Hausfater G, Marshall B, Levy SB. Antibiotic-resistant bacteria in wild primates: increased prevalence in baboons feeding on human refuse. Appl Environ Microbiol. 1985;49:791–794. doi: 10.1128/aem.49.4.791-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JB, Epstein PR, Lipp EK, Sherman BH, Bernard SM, Patz JA. Climate variability and change in the United States: potential impacts on water- and foodborne diseases caused by microbiologic agents. Environ Health Perspect. 2001;109(suppl 2):211–221. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Silbergeld EK, Nash D, Trevant C, Strickland GT, de Souza JM, da Silva RS. Mercury exposure and malaria prevalence among gold miners in Para, Brazil. Rev Soc Bras Med Trop. 2002;35:421–429. doi: 10.1590/s0037-86822002000500001. [DOI] [PubMed] [Google Scholar]
- Singh J, Jain DC, Bhatia R, Ichhpujani RL, Harit AK, Panda RC, et al. Epidemiological characteristics of rabies in Delhi and surrounding areas, 1998. Indian Pediatr. 2001;38:1354–1360. [PubMed] [Google Scholar]
- Straube E, Straube W, Kruger E, Bradatsch M, Jacob-Meisel M, Rose HJ. Disruption of male sex hormones with regard to pesticides: pathophysiological and regulatory aspects. Toxicol Lett. 1999;107:225–231. doi: 10.1016/s0378-4274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Tabor GM. 2002. Defining conservation medicine. In: Conservation Medicine: Ecological Health in Practice (Aguirre AA, Ostfeld RS, Tabor GM, House CA, Pearl MC, eds). New York:Oxford University Press, 8–16.
- Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DF, Malone JB, Harb M, Faris R, Huh OK, Buck AA, et al. Bancroftian filariasis distribution and diurnal temperature differences in the southern Nile delta. Emerg Infect Dis. 1996;2:234–235. doi: 10.3201/eid0203.960313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole MJ, Waldman RJ. The public health aspects of complex emergencies and refugee situations. Annu Rev Public Health. 1997;18:283–312. doi: 10.1146/annurev.publhealth.18.1.283. [DOI] [PubMed] [Google Scholar]
- Veiga MM, Meech JA, Onate N. Mercury pollution from deforestation [Letter] Nature. 1994;368:816–817. doi: 10.1038/368816a0. [DOI] [PubMed] [Google Scholar]
- Wallis J, Lee DR. Primate conservation: the prevention of disease transmission. Int J Primatol. 1999;20:803–826. [Google Scholar]
- Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis. 1993;168:709–714. doi: 10.1093/infdis/168.3.709. [DOI] [PubMed] [Google Scholar]
- WHO 1997. Health and Environment in Sustainable Development: 5 Years after the Earth Summit. Geneva:World Health Organization. Available: http://www.who.int/archives/inf-pr-1997/en/pr97-47.html [accessed 11 October 2003].
- Wolfe ND, Eitel MN, Gockowski J, Muchaal PK, Nolte C, Prosser AT, et al. Deforestation, hunting and the ecology of microbial emergence. Global Change Hum Health. 2000;1:10–25. [Google Scholar]
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet. 2004;363(9413):932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]