Abstract
We reported previously that insecticide exposures were widespread among minority women in New York City during pregnancy and that levels of the organophosphate chlorpyrifos in umbilical cord plasma were inversely associated with birth weight and length. Here we expand analyses to include additional insecticides (the organophosphate diazinon and the carbamate propoxur), a larger sample size (n = 314 mother–newborn pairs), and insecticide measurements in maternal personal air during pregnancy as well as in umbilical cord plasma at delivery. Controlling for potential confounders, we found no association between maternal personal air insecticide levels and birth weight, length, or head circumference. For each log unit increase in cord plasma chlorpyrifos levels, birth weight decreased by 42.6 g [95% confidence interval (CI), −81.8 to −3.8, p = 0.03] and birth length decreased by 0.24 cm (95% CI, −0.47 to −0.01, p = 0.04). Combined measures of (ln)cord plasma chlorpyrifos and diazinon (adjusted for relative potency) were also inversely associated with birth weight and length (p < 0.05). Birth weight averaged 186.3 g less (95% CI, −375.2 to −45.5) among newborns with the highest compared with lowest 26% of exposure levels (p = 0.01). Further, the associations between birth weight and length and cord plasma chlorpyrifos and diazinon were highly significant (p ≤ 0.007) among newborns born before the 2000–2001 U.S. Environmental Protection Agency’s regulatory actions to phase out residential use of these insecticides. Among newborns born after January 2001, exposure levels were substantially lower, and no association with fetal growth was apparent (p > 0.8). The propoxur metabolite 2-isopropoxyphenol in cord plasma was inversely associated with birth length, a finding of borderline significance (p = 0.05) after controlling for chlorpyrifos and diazinon. Results indicate that prenatal chlorpyrifos exposures have impaired fetal growth among this minority cohort and that diazinon exposures may have contributed to the effects. Findings support recent regulatory action to phase out residential uses of the insecticides.
Keywords: birth length, birth weight, insecticides, minority, prenatal, residential, urban, women
Pesticide use appears to be widespread among minority populations residing in New York City (Berkowitz et al. 2003; Surgan et al. 2002; Whyatt et al. 2002, 2003). A 1997 study of pesticide use in New York State found that the heaviest application (in gallons and pounds) of legally registered pesticides by licensed applicators occurred not in the agricultural counties but in the boroughs of Manhattan and Brooklyn in New York City (Thier et al. 1998). Chlorpyrifos was the insecticide most heavily applied in New York City, and one of the insecticides most heavily used by pest control operators for the New York City Housing Authority (Landrigan et al. 1999; Thier et al. 1998). A more recent survey of pest control measures used by residents of public housing in New York State conducted during 2000–2001 by the New York State Attorney General’s Office concluded that pest problems and pesticide use were related to housing density (Surgan et al. 2002). Specifically, 93% of the residents of public housing in New York City reported applying pesticides in their homes, and more than half said they did so once per week (Surgan et al. 2002). By contrast, only 41% of public housing residents in Syracuse, New York, a less densely populated area, applied pesticides, and more than half applied them once per year or less (Surgan et al. 2002). An ongoing prospective cohort study of mothers and newborns delivered at Mount Sinai Hospital has also documented considerable indoor pesticide exposure during pregnancy among minority women in New York City (Berkowitz et al. 2003). Similarly, our prospective cohort study being conducted by the Columbia Center for Children’s Environmental Health (CCCEH) has shown widespread pesticide use during pregnancy in minority communities in New York City (Whyatt et al. 2002, 2003). Specifically, of the 459 African-American and Dominican women interviewed, 85% reported using some form of pest control measures during pregnancy, and 35% reported using an exterminator. Most of the pesticide use was for cockroach control (Whyatt et al. 2002). All women had detectable levels of at least three insecticides (the organophosphates chlorpyrifos and diazinon and the carbamate propoxur) in personal air samples collected over 48 hr during the third trimester. The insecticides were detected in 45–74% of blood samples collected from the mothers and newborns at delivery; maternal and newborn levels were similar and highly correlated, indicating the pesticides had been transferred from the mother to fetus during pregnancy (Whyatt et al. 2003).
Collectively, these findings raise concern over the potential health effects of residential pesticide use to the developing fetus. Although little is known about the effects of residential pesticide exposure among human populations, experimental data in laboratory animals suggest that exposures to certain organophosphates (including chlorpyrifos and diazinon) during pregnancy or early life can impair fetal growth and neurocognitive development in the offspring [reviewed by Eskenazi et al. (1999)]. Reduction in birth weight was also seen experimentally in a two-generation reproductive study of propoxur in rats, but only at high exposure levels [U.S. Environmental Protection Agency (EPA) 1997]. Our prior data showed a statistically significant inverse association between chlorpyrifos levels in umbilical cord blood samples and birth weight and length among newborns in the CCCEH cohort (Perera et al. 2003). The present study extends these prior findings in the same cohort to evaluate the association between birth outcomes and levels of chlorpyrifos, diazinon, and propoxur measured in maternal personal air samples collected during pregnancy and in umbilical cord blood samples collected at delivery.
Women were enrolled into the cohort between 1998 and 2002 and the sample size is somewhat larger than that included in our previous report (Perera et al. 2003). The effects of the pesticides on birth outcomes were assessed in the total cohort, and then again after stratifying on year of delivery because our prior data showed that levels of all three insecticides in maternal personal air samples and umbilical cord blood samples had dropped substantially among cohort subjects by 2001, concurrent with the U.S. EPA regulatory action to phase out residential uses of diazinon and chlorpyrifos (Whyatt et al. 2003).
Materials and Methods
Study subjects.
The women in this report are part of an ongoing prospective cohort study of minority mothers and their newborns being conducted by the CCCEH. The study was initiated in 1997 to evaluate the effects of prenatal exposures to ambient and indoor pollutants on birth outcomes, neurocognitive development, and procarcinogenic damage among a cohort of mothers and newborns from minority communities in New York City. In 1998, the study began to gather information on prenatal pesticide use in response to growing concerns over the extent of residential insecticide use in New York City (Thier et al. 1998). To date, data on birth outcomes and insecticide levels in maternal personal air samples collected during pregnancy and/or in blood samples collected at delivery have been gathered on 314 mother–newborn pairs in the cohort. These 314 mothers and newborns are the subject of the present report. Bilingual research workers attended the prenatal clinics at Harlem and New York Presbyterian hospitals to explain the study and determine eligibility if a woman was interested in participating. Study protocols—including eligibility requirements, the numbers of subjects enrolled, as well as the comparability between those who agreed to participate and those who refused—have been described in detail previously (Perera et al. 2003; Whyatt et al. 2002, 2003). The study was restricted to women 18–35 years old who self-identified as African American or Dominican and had resided in Northern Manhattan (Central Harlem or Washington Heights/Inwood) or the South Bronx for ≥ 1 year before pregnancy. Women were excluded if they smoked cigarettes or used other tobacco products during pregnancy; used illicit drugs; had diabetes, hypertension, or known HIV; or had their first prenatal visit after the 20th week of pregnancy. The study was approved by the institutional review board of Columbia University, and informed consent was obtained from all study subjects.
Questionnaire data.
A 45-min questionnaire, administered to each woman in her home by a trained bilingual interviewer during the third trimester of pregnancy, collected information on demographics, home characteristics, lifetime residential history, history of active and passive smoking, occupational history, maternal education and income level, alcohol and drug use during pregnancy, and history of residential pesticide use. Information about pesticide use included whether or not any pest control measures were used by an exterminator or by others (the woman herself, other household members, or the building superintendent) during pregnancy and, if so, what types of measures were used (Perera et al. 2003; Whyatt et al. 2002, 2003).
Prenatal personal ambient air samples.
As described in detail previously (Perera et al. 2003; Whyatt et al. 2002, 2003), women in the cohort were asked to wear a small backpack during the third trimester of pregnancy holding a personal ambient air monitor during the daytime hours for 2 consecutive days and to place the monitor near the bed at night. The personal air sampling pumps operated continuously at 4 L/min over this period, collecting particles of ≤ 2.5 μm in diameter on a precleaned quartz microfiber filter and collecting semivolatile vapors and aerosols on a polyurethane foam cartridge backup. Analyses for pesticide levels were carried out at Southwest Research Institute as described previously (Perera et al. 2003; Whyatt et al. 2002, 2003). For quality control (QC), each personal monitoring was coded as to accuracy in flow rate, time, and completeness of documentation. A code of 0–1 indicated no or minor problems, 2 indicated greater concern, and 3 indicated unacceptable and not analyzed. Three samples received a code of 3 and are not included in results here. Eleven women had personal air monitoring results with a QC code of 2; we performed statistical analyses both including and excluding these subjects. Results were essentially identical and are therefore presented here for all women with a QC code of 0–2. The personal monitoring took place between February 1998 and May 2002.
Blood samples.
A sample of umbilical cord blood was collected as close to delivery as possible by syringing the blood into a heparinized syringe to avoid clotting. A sample of maternal blood was obtained within 2 days postpartum into heparinized Vacutainer tubes by the hospital staff. Blood processing and analysis have been described in detail previously (Whyatt et al. 2003). Analyses were undertaken at the Centers for Disease Control and Prevention (Whyatt et al. 2003). Methods for the laboratory assay, including QC, reproducibility, and limits of detection (LODs), have also been published (Barr et al. 2002). For chlorpyrifos and diazinon the parent compound was measured in plasma, and for propoxur the chemical-specific metabolite 2-isopropoxyphenol was measured (Whyatt et al. 2003). The deliveries took place between March 1998 and July 2002.
Measures of fetal growth.
As described previously (Perera et al. 2003), information was abstracted by the research workers from the mothers’ and infants’ medical records after delivery, including date of delivery, gestational age at birth, infant sex, birth weight, length, head circumference, infant malformations, Apgar scores, maternal height, prepregnancy weight, total weight gain, complications of pregnancy and delivery, and medications used during pregnancy.
Statistical analysis.
We conducted multiple regression analyses to measure the contribution of antenatal insecticide exposure to birth outcomes. Model building preceded formal hypothesis testing. Covariates were selected from variables known to be associated with insecticide exposure or fetal growth. To eliminate possible effects related to active smoking, subjects were excluded if the mother reported any smoking during pregnancy or if plasma cotinine levels in either maternal or cord blood samples collected at delivery exceeded 15 ng/mL (Perera et al. 2003). Covariates included in the final models were race/ethnicity, gestational age, parity, maternal prepregnancy weight, and net weight gain during pregnancy (maternal pregnancy weight gain minus the newborn’s weight), maternal self-reported environmental tobacco smoke in the home, sex of the newborn, and season of delivery. Models for head circumference also included whether or not the delivery was by cesarean section. The following variables were evaluated as possible markers for socioeconomic status but were not included in the final model because they were not associated with birth outcomes, did not improve the model fit, and did not substantially alter parameter estimates, confidence intervals (CIs), or significant levels on variables included in the final models: annual household income, maternal education, maternal marital status, material hardship during pregnancy, and degree of housing disrepair. Maternal alcohol consumption was not included because few women drank during pregnancy and because alcohol consumption was not a significant predictor of birth outcomes. Maternal exposure to polycyclic aromatic hydrocarbons (PAHs) during pregnancy (as measured by 48-hr personal monitoring) (Perera et al. 2003) was not included in the final models because it did not improve model fit. However, to ensure that exposure to PAHs was not a confounder, models including PAHs were evaluated: parameter estimates, CIs, and significance levels were not altered from those reported here, although sample size was reduced because the variable was missing for a number of women. Restricted analyses were also undertaken after removing the 12 infants born before 37 weeks’ gestation; main effects remained unchanged from those reported here for the whole cohort. Most of the deliveries were full term because women were not fully enrolled until environmental measures had been collected during the third trimester and blood samples (from the mother and/or newborn) had been obtained at delivery. Season of delivery was controlled as our prior data indicate that pesticide levels in personal air and/or blood samples varied significantly by season, with chlorpyrifos and 2-isopropoxyphenol levels being highest in the summer and diazinon levels being highest in the fall (Whyatt et al. 2003). To evaluate whether stratified race/ethnicity-specific analyses should be undertaken, interaction effects of pesticide levels and race/ethnicity on birth outcomes were assessed. None of the interaction terms was significant, and analyses are therefore presented for the whole cohort controlling for race/ethnicity.
As described previously (Perera et al. 2003; Whyatt et al. 2003), pesticide levels in maternal and umbilical cord plasma samples were highly correlated (r = 0.76 for chlorpyrifos, r = 0.68 for diazinon, and r = 0.53 for 2-isopropoxyphenol; p < 0.001, Spearman’s rank). Therefore, in cases where the umbilical cord blood sample was not collected, the mother’s values were used based on the following formulas derived from regression analyses:
 |
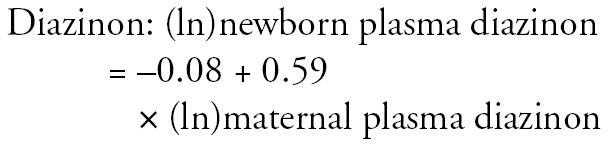 |
2-Isopropoxyphenol:
 |
For chlorpyrifos, levels in umbilical cord blood samples were available for 256 newborns and were imputed from maternal blood levels for 31 newborns; for diazinon, levels in umbilical cord blood samples were available for 257 newborns and were imputed from maternal blood levels for 45 newborns; and for 2-isopropoxyphenol, levels in umbilical cord blood samples were available for 257 newborns and were imputed from maternal blood levels for 45 newborns. Insecticide levels in both maternal personal air and blood samples were available for 259 of the 314 mother–newborn pairs (82%); measures in either personal air or blood were available for the remaining 55 pairs (18%). There was no difference in maternal self-reported pesticide use between mother–newborn pairs with insecticides measured in both maternal personal air and blood compared with pairs with measures in one or the other but not both (chi squared = 0.3, p = 0.6).
Pesticide levels in personal air and blood samples were log-transformed before statistical analyses to normalize positively skewed distributions. Values below the LOD were assigned a value of half the LOD. The log-transformed values were initially entered into parallel models as continuous variables. To evaluate the combined effects of chlorpyrifos and diazinon on birth outcomes, a methodology developed by the U.S. EPA for conducting cumulative risk assessment for organophosphates was used (U.S. EPA 2001b). Briefly, diazinon levels were put into chlorpyrifos equivalents based on the ratio of the chlorpyrifos and diazinon relative potency factors calculated by the U.S. EPA (U.S. EPA 2002a). The U.S. EPA has developed this methodology for cumulative risk assessment for organophosphates because the insecticides have a common mechanism of toxicity (acetylcholinesterase inhibition). The U.S. EPA used female rat brain acetylcholinesterase inhibition after oral administration as the end point for the toxicity weighting calculations; diazinon was calculated to be 6-fold less potent than chlorpyrifos (U.S. EPA 2002). Diazinon levels in chlorpyrifos equivalents were summed with chlorpyrifos levels using U.S. EPA methodology.
When the models indicated a significant association between a pesticide and one or more of the birth outcomes in a regression equation, the pesticide levels were also categorized into four exposure groups to evaluate the dose–response relationships. For pesticides in blood, 31% of chlorpyrifos levels, 48% of diazinon levels, and 50% of isopropoxyphenol levels were below the LOD. These samples were assigned to the lowest exposure group (group 1), and the remaining subjects were ranked into three additional exposure groups of equal number: Group 2 contained infants with the lowest third of detectable levels, group 3 contained infants with the middle third of detectable levels, and group 4 contained infants with the highest third of detectable levels. Categorization into exposure groups was done on all samples; however, size/group varied somewhat in the regression analyses because of missing values for other covariates. For chlorpyrifos, 32% of the newborns fell into group 1, 20% into group 2, 24% into group 3, and 25% into group 4; for the combined measured of chlorpyrifos and diazinon, 26% fell into group 1, 22% into group 2, 26% into group 3, and 26% into group 4. Dummy variables were used in the regression analyses to compare birth outcomes among newborns in exposure group 1 (those with nondetectable levels) with birth outcomes among newborns in exposure groups 2, 3, and 4. Stratified analyses were also conducted to evaluate the effects of the pesticide exposures on birth outcomes among newborns born before versus on or after 1 January 2001. Results are considered statistically significant at p < 0.05 (two-tailed).
Results
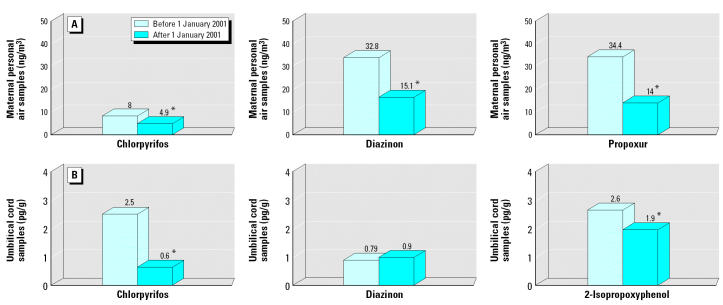
Demographics, birth outcomes, and exposure characteristics are presented in Table 1. Figure 1 shows levels of the insecticides in 48-hr personal air samples collected from the mother during pregnancy (Figure 1A) and in umbilical cord plasma samples collected from the newborn at delivery (Figure 1B) stratified by whether or not the infant was born before or after 1 January 2001. As discussed in detail previously (Whyatt et al. 2003), except for diazinon in blood samples the insecticide levels were substantially lower among infants born after 1 January 2001 (Figure 1; p ≤ 0.001, independent t-test). By contrast, pesticide use habits did not appear to change over the same period. Specifically, among women who gave birth before 1 January 2001, 85.6% reported using some form of pest control and 56.5% of users reported using one or more of the more highly toxic pest control methods (can sprays, pest bombs, or sprays by an exterminator); among women who gave birth after 1 January 2001, 84.0% reported using some form of pest control and 54.5% of users reported using one or more of the more highly toxic methods, a difference that was not significant (chi squared < 0.9, p > 0.7; data not shown).
Table 1.
Demographics, birth outcomes, and exposure characteristics of the populations (n = 314) (mean ± SD or percent).
| Characteristics | Values |
|---|---|
| Maternal age (years)a | 24.6 ± 4.9 |
| Ethnicity (%) | |
| African American | 42 |
| Dominican | 58 |
| Maternal education (%)a | |
| < High school degree | 34 |
| High school diploma or GED | 45 |
| > High school | 21 |
| Maternal environmental tobacco smoke | |
| % reporting smoker in home | 38 |
| Maternal alcohol consumptiona | |
| % reporting any drinking during pregnancy | 25 |
| % reporting regularb drinking | 2 |
| Maternal height (cm)a | 162.6 ± 7.9 |
| Maternal prepregnancy weight (kg) | 68.3 ± 17.7 |
| Maternal net weight gain during pregnancy (kg) | 12.8 ± 7.0 |
| Gestational age of newborn (weeks) | 39.3 ± 1.4 |
| Sex of the newborn (% female) | 53 |
| Newborn birth weight (g) | 3382.1 ± 485.8 |
| Newborn birth length (cm)a | 50.9 ± 2.6 |
| Newborn head circumference (cm)a | 34.1 ± 1.5 |
| Parity (% nulliparous) | 51 |
| Personal air pesticide levels (ng/m3)c | |
| Chlorpyrifos | 15.3 ± 31.8 |
| Diazinon | 117.2 ± 523.4 |
| Propoxur | 53.6 ± 113.2 |
| Umbilical cord blood pesticide levels (pg/g)d | |
| Chlorpyrifos | 4.0 ± 6.1 |
| Diazinon | 1.1 ± 1.3 |
| 2-Isopropoxyphenol | 3.1 ± 2.8 |
Missing data: maternal age, n = 1; education, n = 7; alcohol, n = 10; maternal height, n = 11; birth length, n = 5; head circumference, n = 16.
One alcohol drink or more per day during any trimester.
Pesticide levels in maternal air samples were available for chlorpyrifos, n = 271; for propoxur, n = 271; and for diazinon, n = 269.
Chlorpyrifos levels in umbilical cord blood samples were available for 256 infants and were imputed from the mothers’ values for 31 infants; diazinon levels in umbilical cord blood samples were available for 257 infants were imputed from the mothers’ values for 45 infants; and 2-isopropoxyphenol levels in umbilical cord blood samples were available for 257 infants and were imputed from the mothers’ values for 45 infants.
Figure 1. Geometric mean insecticide levels in (A) maternal personal air samples collected over 48 hr during the third trimester of pregnancy and (B) in umbilical cord blood samples at delivery stratified by whether the delivery took place before or after 1 January 2001. : *p ≤ 0.01 (independent t-test).
Table 2 provides results of the basic models of covariates used in the regression analyses. The covariates explained 24% of the variance in birth weight, 15% of the variance in birth length, and 32% of the variance in head circumference. There were no significant associations between the birth outcomes (weight, length, or head circumference) and maternal self-reported use of pest control methods during pregnancy (all p-values > 0.1 in the regression analyses; data not shown).
Table 2.
Regression models of covariates controlled in analyses of associations between insecticide levels and newborn birth outcomes.
| Birth weight (g; n = 314; R2 = 0.24, F = 9.6, p < 0.001)
|
Birth length (cm; n = 309; R2 = 0.15, F = 5.6, p < 0.001)
|
Head circumference (cm; n = 298; R2 = 0.32, F = 12.3, p < 0.001)
|
||||
|---|---|---|---|---|---|---|
| B ± SE | p-Value | B ± SE | p-Value | B ± SE | p-Value | |
| Constant | −2227.4 ± 733.9 | 0.003 | 27.5 ± 4.2 | < 0.001 | 16.3 ± 2.3 | < 0.001 |
| Gestational age (weeks) | 136.2 ± 18.5 | < 0.001 | 0.60 ± 0.1 | < 0.001 | 0.43 ± 0.06 | < 0.001 |
| Maternal prepregnancy weight (kg) | 4.8 ± 1.5 | 0.001 | 0.007 ± 0.008 | 0.39 | 0.015 ± 0.004 | 0.001 |
| Maternal net pregnancy weight gain (kg) | 7.8 ± 3.6 | 0.03 | 0.002 ± 0.02 | 0.92 | 0.027 ± 0.01 | 0.01 |
| Sex of newborna | −77.5 ± 49.5 | 0.12 | −0.68 ± 0.28 | 0.02 | −0.66 ± 0.15 | < 0.001 |
| Parityb | 41.4 ± 49.8 | 0.41 | 0.29 ± 0.29 | 0.31 | 0.23 ± 0.15 | 0.13 |
| Race/ethnicityc | −57.8 ± 53.0 | 0.28 | 0.22 ± 0.30 | 0.46 | −0.33 ± 0.16 | 0.04 |
| ETS in homed | −67.6 ± 51.4 | 0.19 | −0.52 ± 0.30 | 0.08 | −0.35 ± 0.16 | 0.02 |
| Season 1e | −120.0 ± 70.4 | 0.09 | −0.30 ± 0.40 | 0.46 | 0.03 ± 0.21 | 0.88 |
| Season 2f | −102.1 ± 70.9 | 0.15 | −0.67 ± 0.41 | 0.10 | 0.04 ± 0.21 | 0.87 |
| Season 3g | −185.4 ± 73.6 | 0.01 | −0.63 ± 0.42 | 0.14 | −0.4 ± 0.22 | 0.07 |
| Delivery by cesarean sectionh | NA | NA | 0.17 ± 0.11 | 0.14 | ||
Abbreviations: ETS, environmental tobacco smoke; NA, not applicable.
0 = male; 1 = female.
0 = nulliparous; 1 = at least one prior live birth.
0 = Dominican; 1 = African American.
0 = no; 1 = yes.
0 = summer; 1 = winter;
0 = summer; 1 = spring;
0 = summer; 1 = fall;
0 = no; 1 = yes.
Table 3 shows results of the regression analyses of the association between birth outcomes and the organophosphate insecticides chlorpyrifos and diazinon in a) personal air samples collected from the mother over 48 hr during the third trimester of pregnancy and b) blood samples collected from the newborn at delivery. The effect size (B) represents the change in birth weight (grams), length (centimeters), and head circumference (centimeters) for each log unit increase in insecticide levels. None of the associations between the birth outcomes and the organophosphate levels in maternal personal air samples was statistically significant (all p-values > 0.1). The associations between birth outcomes and the organophosphate insecticide levels in maternal personal air samples remained nonsignificant when analyses stratified on year of delivery (before vs. after 1 January 2001; data not shown).
Table 3.
Change in birth outcomes for each log unit increase in organophosphate insecticide levels in 48-hr personal air samples collected from mothers during the third trimester of pregnancy (ng/m3) and in umbilical cord plasma samples collected from newborns at delivery (pg/g).a
| Birth weight (g)
|
Birth length (cm)
|
Head circumference (cm)
|
||||
|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Maternal personal air samples | ||||||
| Chlorpyrifos | −17.7 (−64.2 to 28.9) | 0.46 | −0.02 (−0.28 to 0.25) | 0.91 | −0.04 (−0.18 to 0.10) | 0.59 |
| Diazinon | 13.8 (−23.2 to 50.8) | 0.46 | 0.07 (−0.14 to 0.28) | 0.52 | −0.03 (−0.14 to 0.09) | 0.67 |
| Sum chlorpyrifos and diazinonb | −5.1 (−50.7 to 40.4) | 0.82 | −0.01 (−0.27 to 0.25) | 0.92 | −0.03 (−0.17 to 0.11) | 0.71 |
| Cord plasma samples | ||||||
| Chlorpyrifos | −42.6 (−81.8 to −3.8) | 0.03 | −0.24 (−0.47 to −0.01) | 0.04 | −0.01 (−0.13 to 0.11) | 0.86 |
| Diazinon | −44.2 (−119.5 to 31.0) | 0.25 | −0.32 (−0.75 to 0.11) | 0.15 | −0.07 (−0.30 to 0.16) | 0.53 |
| Sum chlorpyrifos and diazinonb | −49.1 (−91.3 to −6.9) | 0.02 | −0.27 (−0.52 to −0.02) | 0.03 | −0.02 (−0.15 to 0.11) | 0.73 |
Each (ln)insecticide level was entered as the independent variable into a parallel multiple linear regression model; model covariates were gestational age of the newborn (weeks), maternal prepregnancy weight and net weight gain during pregnancy (kilograms), newborn sex (0 = male; 1 = female), parity (0 = nulliparous; 1 = at least one prior live birth), race/ethnicity (0 = Dominican; 1 = African American), environmental tobacco smoke in the home (0 = no; 1 = yes), and season of delivery (dummy variable 1: 0 = summer; 1 = winter; dummy variable 2: 0 = summer; 1 = spring; dummy variable 3: 0 = summer; 1 = fall); models for head circumference included whether or not the delivery was by cesarean section (0 = no, 1 = yes).
Sum of chlorpyrifos and diazinon in chlorpyrifos equivalents adjusted for relative potency.
We found a significant inverse association between birth weight and length and levels of the organophosphates in umbilical cord plasma. Specifically, birth weight decreased by 42.6 g (95% CI, −81.8 to −3.8, p = 0.03), and birth length decreased by 0.24 cm (95% CI, −0.47 to −0.01, p = 0.04) for each log unit increase in cord plasma chlorpyrifos levels. Birth weight and length also decreased with increasing levels of cord plasma (ln)diazinon and the effect size was similar to that seen for chlorpyrifos, but the standard error was larger and the results were not significant. However, when chlorpyrifos and diazinon levels were summed (after adjusting for relative potency), birth weight decreased significantly by 49.1 g (95% CI, −91.3 to −6.9, p = 0.02) and birth length by 0.27 cm (95% CI, −0.52 to −0.02, p = 0.03) for each log unit increase in the sum of the insecticides in chlorpyrifos equivalents.
Table 4 shows results of the regression analyses of birth weight and length among infants stratified into four exposure groups based on increasing levels of chlorpyrifos and diazinon in cord plasma. The effects were principally seen among newborns with the highest exposures (group 4). Birth weight averaged 150.1 g less (95% CI, −287.7 to −12.5) among newborns in group 4 compared with those in group 1 for chlorpyrifos exposures (p = 0.03), and birth length averaged 0.75 cm less (95% CI, −1.6 − 0.06, p = 0.07). Birth weight averaged 186.3 g less (95% CI, −327.2 to −45.4) among newborns with group 4 compared with group 1 combined exposures to chlorpyrifos and diazinon (in chlorpyrifos equivalents, adjusted for relative potency; p = 0.01), and birth length averaged 0.8 cm less (95% CI, −1.6 to 0.02, p = 0.056). By contrast, there were no significant differences in birth outcomes among newborns in the second and third exposure groups compared with those in the first exposure group.
Table 4.
Differences in birth weight (g) and birth length (cm) by cord plasma organophosphate exposure groups.a
| Chlorpyrifos
|
Chlorpyrifos and diazinonb |
|||
|---|---|---|---|---|
| B ± SE | p-Value | B ± SE | p-Value | |
| Birth weight | ||||
| Group 1 vs. group 2 | 39.2 (−107.3 to 185.7) | 0.60 | −78.5 (−225.5 to 68.5) | 0.29 |
| Group 1 vs. group 3 | −50.9 (−188.2 to 86.3) | 0.47 | −33.1 (−173.7 to 107.4) | 0.64 |
| Group 1 vs. group 4 | −150.1 (−287.7 to −12.5) | 0.03 | −186.3 (−327.2 to −45.4) | 0.01 |
| Birth length | ||||
| Group 1 vs. group 2 | 0.17 (−0.70 to 1.0) | 0.71 | −0.06 (−0.93 to 0.81) | 0.89 |
| Group 1 vs. group 3 | −0.21 (−1.0 to 0.61) | 0.61 | −0.005 (−0.84 to 0.82) | 0.99 |
| Group 1 vs. group 4 | −0.75 (−1.6 to 0.06) | 0.07 | −0.80 (−1.6 to 0.02) | 0.056 |
Newborns were categorized into four exposure groups based on cord plasma organophosphate levels (see “Materials and Methods”). Dummy variables were used in the regression analyses to compare birth outcomes among newborns in exposure group 1 with birth outcomes among newborns in exposure groups 2, 3, and 4. Covariates included in the regression models were gestational age of the newborn (weeks), maternal prepregnancy weight and weight gain during pregnancy (kilograms), newborn sex (0 = male; 1 = female), parity (0 = nulliparous; 1 = at least one prior live birth), ethnicity (0 = Dominican; 1 = African American), environmental tobacco smoke in the home (0 = no; 1 = yes) and season of delivery (dummy variable 1: 0 = summer; 1 = winter; dummy variable 2: 0 = summer; 1 = spring; dummy variable 3: 0 = summer; 1 = fall).
Sum of chlorpyrifos and diazinon in chlorpyrifos equivalents adjusted for relative potency.
Table 5 shows the association between the umbilical cord plasma organophosphate levels and birth weight and length among newborns born before versus after 1 January 2001. A difference in the magnitude of the effects by year of delivery was seen. Specifically, among newborns born before 1 January 2001, an inverse and highly significant association was seen between (ln)chlorpyrifos levels and both birth weight (B = −67.3 g/unit, p = 0.008) and birth length (B = −0.43 cm/unit, p = 0.004). By contrast, among newborns born after 1 January 2001, the relationships were no longer inverse nor significant (p > 0.5), although the 95% CIs were wider because of reduced sample size. Similarly, among newborns born before 1 January 2001, a highly significant inverse association was seen between the (ln)combined levels of chlorpyrifos and diazinon (in chlorpyrifos equivalents) and both birth weight (B = −72.5 g/unit, p = 0.007) and length (B = −0.46 cm/unit, p = 0.004). Among newborns born after 2001, the magnitude of the effect was much less and no longer significant (p > 0.8; Table 5). Among newborns born before 1 January 2001, 34% had combined group 4 exposure levels of chlorpyrifos and diazinon, whereas among newborns born after 1 January 2001, only one (1.5%) had combined group 4 exposure levels—a difference that was highly significant (chi squared = 50, p < 0.001).
Table 5.
Regression analysesa of birth weight and length and organophosphate levels in umbilical cord plasma samples for infants born before and after 1 January 2001.
| Birth weight (g)
|
Birth length (cm)
|
|||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Born before 1 January 2001 (n = 237) | ||||
| Chlorpyrifos | −67.3 (−116.6 to −17.8) | 0.008 | −0.43 (−0.73 to −0.14) | 0.004 |
| Sum chlorpyrifos and diazinonb | −72.5 (−125.0 to −20.0) | 0.007 | −0.46 (−0.77 to −0.14) | 0.004 |
| Born after 1 January 2001 (n = 77) | ||||
| Chlorpyrifos | 30.7 (−108.6 to 169.9) | 0.66 | 0.07 (−0.65 to 0.79) | 0.85 |
| Sum chlorpyrifos and diazinonb | 0.6 (−144.7 to 145.9) | 0.99 | −0.07 (−0.82 to 0.67) | 0.84 |
Each (ln)insecticide level was entered as the independent variable into a parallel multiple linear regression model. Model covariates were gestational age of the newborn (weeks), maternal prepregnancy weight and weight gain during pregnancy (kilograms), newborn sex (0 = male; 1 = female), parity (0 = nulliparous; 1 = at least one prior live birth), ethnicity (0 = Dominican; 1 = African American), environmental tobacco smoke in the home (0 = no; 1 = yes), and season of delivery (dummy variable 1: 0 = summer; 1 = winter; dummy variable 2: 0 = summer; 1 = spring; dummy variable 3: 0 = summer; 1 = fall); models for head circumference included whether or not the delivery was by cesarean section (0 = no, 1 = yes).
Sum of chlorpyrifos and diazinon in chlorpyrifos equivalents adjusted for relative potency.
Results of the regression analyses of the associations between birth weight and length and the carbamate insecticide propoxur in maternal personal air samples, as well as the propoxur metabolite 2-isopropoxyphenol in umbilical cord blood samples, are presented in Table 6. Birth length decreased by 0.51 cm (95% CI, −0.98 to −0.05) for each log unit increase in cord plasma 2-isopropoxyphenol levels (p = 0.03). None of the other associations was statistically significant. After stratifying infants into four exposure groups based on increasing levels of 2-isopropoxyphenol in cord plasma, a decrease in birth length was seen among newborns in both group 3 and group 4 compared with group 1; however, the differences were not significant. Specifically, compared with newborns in group 1, birth length was 0.54 cm less (95% CI, −1.3 to −0.27) among newborns in group 3 (p = 0.19) and 0.68 cm less (95% CI, −1.5 to −0.14) among newborns in group 4 (p = 0.1; data not shown). Table 6 also shows the association between (ln)2-isopropoxyphenol and birth length stratified by year of delivery. Among newborns born before 2001, the association between (ln)2-isopropoxyphenol and birth length was statistically significant (B = −0.73 cm/unit, p = 0.01). Among newborns born after 1 January 2001, the association remained inverse but the magnitude of the effect was less and no longer significant (B = −0.30 cm/unit, p = 0.56). No association was seen between infant head circumference and levels of propoxur or its metabolite in maternal personal air and cord blood samples (data not shown).
Table 6.
Change in birth outcomes for each log unit increase in propoxur in maternal personal air samples (ng/m3) and 2-isopropoxyphenol in cord plasma samples (pg/g).
| Birth weight (g)
|
Birth length (cm)
|
|||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Propoxur in maternal personal air (total sample) | 19.5 (−29.6 to 68.5) | 0.44 | 0.19 (−0.09 to 0.47) | 0.17 |
| 2-Isopropoxyphenol in cord plasma (total sample) | −65.6 (−146.7 to 15.4) | 0.11 | −0.51 (−0.98 to −0.05) | 0.03 |
| Stratified by year of delivery | ||||
| Born before 1 January 2001 | −75.8 (−171.1 to 19.5) | 0.12 | −0.73 (−1.3 to −0.17) | 0.01 |
| Born after 1 January 2001 | −107.3 (−298.7 to 84.2) | 0.27 | −0.30 (−1.3 to 0.71) | 0.56 |
Each (ln)insecticide level was entered as the independent variable into a parallel multiple linear regression model. Model covariates were gestational age of the newborn (weeks), maternal prepregnancy weight and net weight gain during pregnancy (kg), newborn sex (0 = male; 1 = female), parity (0 = nulliparous; 1 = at least one prior live birth), ethnicity (0 = Dominican; 1 = African American), environmental tobacco smoke in the home (0 = no; 1 = yes) and season of delivery (dummy variable 1: 0 = summer; 1 = winter; dummy variable 2: 0 = summer; 1 = spring; dummy variable 3: 0 = summer; 1 = fall); models for head circumference included whether or not the delivery was by cesarean section ((0 = no, 1 = yes).
A weak but significant correlation was seen between 2-isopropoxyphenol and the sum of chlorpyrifos and diazinon in cord plasma (r = 0.25 among newborns born before 1 January 2001, p < 0.001, Spearman’s rank). Therefore, regression analyses were also undertaken controlling for all three insecticides simultaneously to ensure that the main effects reported above were not caused by confounding by the other insecticide. These analyses were restricted to newborns born before 2001. After controlling for cord plasma 2-isopropoxyphenol (in addition to the other potential confounders), the associations between birth weight and length and cord plasma (ln)chlorpyrifos as well as the sum of cord plasma (ln)chlorpyrifos and diazinon (in chlorpyrifos equivalents adjusted for relative potency) remained significant (p ≤ 0.02), and the effect size remained similar to that seen without 2-isopropoxyphenol in the model. Among newborns born before 1 January 2001, birth weight averaged 196.5 g less (95% CI, −369.9 to −23.1, p = 0.03) among those with group 4 compared with those in group 1 for combined cord plasma chlorpyrifos and diazinon exposures (controlling for 2-isopropoxyphenol and the other potential confounders). However, after controlling for chlorpyrifos and diazinon, the association between 2-isopropoxyphenol and birth length remained inverse but the effect size was reduced (B = −0.57 cm/unit compared with B = −0.73 cm/unit) and the association dropped to borderline significance (p = 0.05).
Discussion
These results confirm our earlier findings of an inverse association between chlorpyrifos levels in umbilical cord plasma and birth weight and length (Perera et al. 2003). Further, a dose–response relationship was additionally seen in the present study. Specifically, the association between cord plasma chlorpyrifos and reduced birth weight and length was found principally among newborns with the highest 25% of exposure levels. By 2001, after exposures had been reduced because of U.S. EPA regulatory action, almost none of the newborns had these higher exposure levels, and the association between cord plasma chlorpyrifos levels and birth weight and length was no longer inverse or significant.
Results from the present study also suggest the possibility that prenatal diazinon exposures may have contributed to fetal growth deficits. Although the associations seen here between cord plasma diazinon levels and birth weight and length were not significant, both decreased with increasing diazinon levels, and the effect size was similar to that seen for chlorpyrifos. In addition, when we used the U.S. EPA methodology for cumulative risk assessment for organophosphates to look at the combined effect of chlorpyrifos and diazinon, the magnitude of the effect on birth weight was greater than that for chlorpyrifos alone. These findings are consistent with experimental evidence in laboratory animals, which has shown a link between both chlorpyrifos and diazinon exposures during pregnancy and reduced fetal growth (Eskenazi et al. 1999; Smegal 1999; U.S. EPA 2000b). Further, combined exposures to diazinon and chlorpyrifos were common among the present cohort. Both were detected simultaneously in 100% of the maternal personal air samples and in more than one-third of cord blood samples. A significant correlation was seen between the two insecticides in personal air (r = 0.3, p < 0.001) and cord blood (r = 0.57, p < 0.001, Spearman’s rank).
Nonetheless, caution is needed when evaluating these results, in part because the U.S. EPA toxicity weighting factors used here to sum chlorpyrifos and diazinon levels are based on brain acetylcholinesterase inhibition, whereas the mechanisms for the potential fetal growth effects associated with the insecticides are not known. Chlorpyrifos has been shown experimentally to inhibit acetylcholinesterase, down-regulate muscarinic receptors, inhibit the adenylate cyclase signaling cascade, decrease brain DNA and RNA synthesis, and suppress neurite outgrowth (Dam et al. 1998; Eskenazi et al. 1999; Johnson et al. 1998; Slotkin 1999; Song et al. 1997). Experimental evidence has also linked chlorpyrifos and diazinon exposures during gestation or the early postnatal period to adverse neurodevelopmental sequelae in the offspring (reviewed in Eskenazi et al. 1999; Landrigan et al. 1999).
Additionally, in the present study we found the association between chlorpyrifos and diazinon exposures and fetal growth only when the biomarkers were used as a dosimeter of prenatal exposure and not when environmental measures (maternal 48-hr personal air levels during the third trimester) were used. There are several possible explanations for this discrepancy. Biomarkers can be useful in understanding the role of environmental contaminants during fetal development in part because they are integrating dosimeters. This may be particularly important for insecticides because exposure can come from multiple sources (diet, residential, and workplace use) and multiple routes (ingestion, inhalation, and dermal absorption). Both chlorpyrifos and diazinon, for example, are registered for use on numerous food crops (Smegal 1999; U.S. EPA 2000b), and diet may be a significant source of exposure to these insecticides (Curl et al. 2003; Fenske et al. 2002). A recent aggregate-exposures study of four pesticides, including chlorpyrifos and diazinon, among 102 children from Minnesota concluded that ingestion was by far the dominant route of exposure (Clayton et al. 2003). Another study found chlorpyrifos residues in 38% of the food samples collected over 4 days from 75 individuals (MacIntosh et al. 2001), although dietary intakes were estimated to account for only approximately 13% of aggregate exposures (Pang et al. 2002). Dermal absorption and nonintentional ingestion may also be significant sources of exposure to residues of the insecticides on surfaces in the home after residential use (Camann et al. 1995; Gordon et al. 1999; Gurunathan et al. 1998; Whitmore et al. 1994). Finally, the biomarkers may provide better dosimeters of the dose to the target tissue than measures of maternal prenatal exposure because they reflect not only the amount of insecticides absorbed by the mother but also the amount of the absorbed dose that has been transferred to the developing fetus.
However, limitations in the biomarkers need to be recognized. We measured the biomarkers at a single time point only (delivery), and it is not clear to what extent these measurements reflect exposures over critical windows during pregnancy. In cases of chronic exposure, a biomarker measured at a single time point can provide a representative dosimeter even if the toxicant has a short half-life. Cotinine, for example, has a half-life of 15–40 hr in plasma but is well validated as a biomarker of cigarette smoke exposure, including during pregnancy (Kemmeren et al. 1994; Pojer et al. 1984; Woodward et al. 1991). However, this may not be the case for short-term biomarkers if exposures are sporadic. Previous studies with chlorpyrifos, which is similar to other semivolatile pesticides including diazinon, indicate that after residential use, air levels peak in a two-phase process: an aerosolized particle phase with residue concentration on surface areas peaking after 36 hr and a gas phase that begins 12 hr after application and continues for at least 2 weeks, with residue concentrations on surface areas peaking after 72 hr at levels similar to those in the initial particle phase and then declining rapidly (Gurunathan et al. 1998). Our prior data indicate that women in the cohort often used insecticides repeatedly during pregnancy (Whyatt et al. 2002). How long the insecticides persist in the indoor environment is not known, although it is probably longer than in the outdoor environment because of less degradation by microorganisms, hydrolysis, and ultraviolet light. Once absorbed, the insecticides appear to be rapidly eliminated with biologic half-lives on the order of hours to days in adults (Barr et al. 2002). Data are lacking on the half-life of the insecticides in the fetus, and it is possible that it is longer than in the adult because of reduced clearance mechanisms, as has been documented for other toxicants including nicotine (Lambers and Clark 1996; National Research Council 1993). In addition, other factors may be operating to modulate fetal insecticide levels either during pregnancy or at delivery.
Our findings support the recent U.S. EPA regulatory action to phase out residential uses of these insecticides. In June 2000, the U.S. EPA entered into an agreement with the registrant to begin phasing out residential uses of chlorpyrifos and to terminate all retail sales for indoor use by December 2001 (U.S. EPA 2000a). In January 2001, the U.S. EPA entered into an agreement with the registrant to begin phasing out residential uses of diazinon and to terminate all retail sales for indoor use by December 2002 (U.S. EPA 2001a). Before this regulatory action, the U.S. EPA estimated that approximately 75% of U.S. diazinon use and 50% of U.S. chlorpyrifos use were for residential pest control (U.S. EPA 2000a, 2001a). Our results indicate that these regulatory actions have been successful at substantially reducing use and exposures among minority residents in New York City (Carlton et al. 2004; Whyatt et al. 2003). In a survey that we conducted during June and July 2002 of pesticide products sold in stores in minority communities of New York City, only 4 of 135 stores surveyed sold products containing chlorpyrifos, although 40% still had products containing diazinon (Carlton et al. 2004). In a follow-up 1 year later, chlorpyrifos was found in only one store and diazinon was found in 18%, although it was still available in 80% of the supermarkets (Carlton et al. 2004). Our data also show that levels of these insecticides in personal air and blood samples collected from our cohort mothers and newborns have been decreasing substantially between 1998 and 2002 (Whyatt et al. 2003). Results from the present study additionally suggest that the U.S. EPA regulatory action may well have improved birth outcomes among minority residents in New York City.
Because these are the first reports of an association between umbilical cord plasma chlorpyrifos and diazinon levels and reduced birth weight and length, they require confirmation, especially in light of the uncertainties over the toxicokinetics of the insecticides in the fetus and the time frame of exposure represented by the biomarker. If the fetal growth effects seen here do, in fact, prove causal, there may be continued cause for concern, particularly for pregnant farm workers. Although the U.S. EPA regulatory action ended all residential and some agriculture uses of chlorpyrifos and diazinon, both are still used in agriculture, including on multiple food crops (Smegal 1999; U.S. EPA 2000b). Further, the growth effects seen in the present study are of a similar magnitude to those observed with maternal smoking during pregnancy (U.S. Public Health Service 1990) and appear to have occurred at very low exposure levels. All of the estimated inhalation exposures of the mothers to chlorpyrifos over the 48 hr of the personal monitoring were well below the U.S. EPA reference dose, and cord blood levels ranged from 0.4 to 63 pg/g. Estimated inhalation exposure to diazinon for a few women exceeded the U.S. EPA reference dose (Whyatt et al. 2002, 2003), but most were below it and the blood levels ranged from 0.5 to 13 pg/g. Given the potential implication of these results to public health, additional research should be undertaken to confirm or refute these findings and to evaluate continued risks, if any, associated with ongoing uses of the insecticides. It should be noted that the newborns in this cohort are being followed and associations between prenatal insecticide exposure and the child’s neurocognitive development are being assessed.
References
- Barr DB, Barr JR, Maggio VL, Whitehead RD, Sadowski MA, Whyatt RM, et al. A multi-analytic method for the quantification of contemporary pesticides in human serum and plasma using high resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:99–111. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camann DE, Harding HJ, Clothier JM, Kuchibhatla RV, Bond AE. 1995. Dermal and in-home exposure of the farm family to agricultural pesticides. In: Measurement of Toxic and Related Air Pollutants (Tuerst RG, Jauanty RKM, eds). Pittsburgh, PA:Air and Waste Management Association, 548–554.
- Carlton EJ, Moats HL, Feinburg M, Shepard P, Garfinkel R, Whyatt R, et al. Pesticide sales in low-income, minority neighborhoods. J Commmunity Health. 2004;29:231–244. doi: 10.1023/b:johe.0000022029.88626.f4. [DOI] [PubMed] [Google Scholar]
- Clayton CA, Pellizzari ED, Whitmore RW, Quakenboss JJ, Adgate J, Sefton K. Distribution, associations, and partial aggregate exposure of pesticides and polynuclear aromatic hydrocarbons in the Minnesota Children’s Pesticide Expsoure Study (MNCPES) J Expo Anal Environ Epidemiol. 2003;13:100–111. doi: 10.1038/sj.jea.7500261. [DOI] [PubMed] [Google Scholar]
- Curl CL, Fenske RA, Elgethun Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ Health Perspect. 2003;111:377–382. doi: 10.1289/ehp.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Brain Res Dev Brain Res. 1998;108:39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107:409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Kendan G, Lu C, Fisher-Anderson, Curl CL. Assessment of organophosphate pesticide exposure in the diets of preschool children in Washington State. J Expo Anal Environ Epidemiol. 2002;12:21–28. doi: 10.1038/sj.jea.7500197. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Callahan PJ, Nishioka MG, Brinkman MC, O’Rourke MK, Lebowitz MD, et al. Residential environmental measurements in the national human exposure assessment survey (NHEXAS) pilot study in Arizona: preliminary results for pesticides and VOCs. J Expo Anal Environ Epidemiol. 1999;9:456–470. doi: 10.1038/sj.jea.7500042. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Robson M, Freeman N, Buckley B, Roy A, Meyer A, et al. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ Health Perspect. 1998;106:9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Seidler FJ, Slotkin TA. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chloropyrifos. Brain Res Bull. 1998;45:143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- Kemmeren JM, Van Poppel G, Verhoef P, Jarvis MJ. Plasma cotinine: stability in smokers and validation of self-reported smoke exposure in nonsmokers. Environ Res. 1994;66:235–243. doi: 10.1006/enrs.1994.1059. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Wetmur JG, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107:431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh DL, Kabiru CW, Echols SL, Ryan PB. Dietary exposure to chlorpyrifos and levels of 3,5,6-trichloro-2-pyridinol in urine. J Expo Anal Environ Epidemiol. 2001;11:279–285. doi: 10.1038/sj.jea.7500167. [DOI] [PubMed] [Google Scholar]
- National Research Council 1993. Pesticides in the Diets of Infants and Children. Washington, DC:National Academy Press. [PubMed]
- Pang Y, MacIntosh DL, Camann DE, Ryan PB. Analysis of aggregate exposure to chlorpyrifos in the NHEXAS-Maryland investigation. Environ Health Perspect. 2002;110:235–240. doi: 10.1289/ehp.02110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pojer R, Whitfield JB, Poulos V, Eckhard IF, Richmond R, Hensley WJ. Carboxyhemoglobin, cotinine, and thiocyanate assay compared for distinguishing smokers from non-smokers. Clin Chem. 1984;30:1377–1380. [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smegal DC. 1999. Chlorpyrifos: HED Preliminary Risk Assessment for the Reregistration Eligibility Decision (RED) Document. Washington, DC:U.S. Environmental Protection Agency.
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Surgan MH, Congdon T, Primi C, Lamster S, Louis-Jacques J. 2002. Pest control in public housing, schools and parks: urban children at risk. LAW 180-4 PESP 202-7643. Albany, NY:New York State Department of Law, Environmental Protection Bureau.
- Thier A, Enck J, Klossner C. 1998. Plagued by Pesticides: An Analysis of New York State and New York City’s 1997 Pesticide Use and Sales Data. New York:New York Public Interest Research Group.
- U.S. EPA 1997. Reregistration Eligibility Decision Propoxur. EPA 738-97-009. Washington, DC:U.S. Environmental Protection Agency.
- U.S. EPA 2000a. Chlorpyrifos. Revised Risk Assessment and Agreement with Registrants. Washington, DC:U.S. Environmental Protection Agency, Office of Pesticide Programs.
- U.S. EPA 2000b. Diazinon. Revised HED Preliminary Human Health Risk Assessment for the Reregistration Eligibility Decision (RED) D262343, PC Code: 057801. List A, Case No. 0238. Washington, DC:U.S. Environmental Protection Agency, Office of Pesticide Programs.
- U.S. EPA 2001a. Diazinon Revised Risk Assessment and Agreement with Registrants. Washington, DC:U.S. Environmental Protection Agency, Office of Pesticide Programs.
- U.S. EPA 2001b. Organophosphate Pesticides: Preliminary OP Cumulative Risk Assessment. Washington, DC:U.S. Environmental Protection Agency, Office of Pesticide Programs. Available: http://www.epa.gov/pesticides/cumulative/pra-op [accessed 30 July 2003].
- U.S. EPA 2002. Organophosphate Pesticides: Preliminary OP Cumulative Risk Assessment, Relative Potency Factors (4/17/2002). Washington, DC:U.S. Environmental Protection Agency, Office of Pesticide Programs. Available: http://www.epa.gov/pesticides/cumulative/pra-op [accessed 30 July 2003].
- U.S. Public Health Service, Office of the Surgeon General 1990. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Washington, DC:U.S. Public Health Service, Office on Smoking and Health.
- Whitmore R, Immerman FW, Camann DE, Bond AE, Lewis RG, Schaum JL. Non-occupational exposures to pesticides for residents of two U.S. cities. Arch Environ Contam Toxicol. 1994;26:47–59. doi: 10.1007/BF00212793. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, et al. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110:507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M, Tunstall-Pedoe H, Smith WC, Tavendale R. Smoking characteristics and inhalation biochemistry in the Scottish population. J Clin Epidemiol. 1991;44:1405–1410. doi: 10.1016/0895-4356(91)90101-e. [DOI] [PubMed] [Google Scholar]