Abstract
The “Cellular Oxygen METabolism” (COMET) system (Photonics Healthcare, Utrecht, The Netherlands) non-invasively measures mitochondrial oxygen tension (mitoPO2) in the skin. The effects of general anesthesia and major non-cardiac surgery on mitoPO2 remain unknown. In this pre-planned pilot substudy of the “Intraoperative blood pressure Management based on the individual blood PRessure profile: impact on postOperatiVE organ function” (IMPROVE) trial, we measured mitoPO2 from induction of general anesthesia until the end of surgery in 19 major non-cardiac surgery patients (10 assigned to personalized and 9 to routine intraoperative arterial pressure management). In the overall cohort, the median (25th to 75th percentile) preoperative awake mitoPO2 was 63 (53 to 82) mmHg and mitoPO2 after induction of general anesthesia was 42 (35 to 59) mmHg. The intraoperative average mitoPO2 was 39 (30 to 50) mmHg. Thirteen patients (68%) had intraoperative mitoPO2 values below 20 mmHg and the median percentage of surgical time with mitoPO2 < 20 mmHg was 17 (0 to 31)%. MitoPO2 was weakly correlated with mean arterial pressure (repeated measures correlation (rrm(n); rrm(984) = 0.26, 95% confidence interval 0.20 to 0.32; P < 0.001), but not meaningfully with heart rate (rrm(984) = -0.05, 95% confidence interval -0.11 to 0.01; P = 0.117). There was no important difference in intraoperative average mitoPO2 between patients assigned to personalized or to routine intraoperative arterial pressure management (P = 0.653). MitoPO2 under general anesthesia was about a quarter lower than preoperative awake mitoPO2, substantially fluctuated during major non-cardiac surgery, and transiently decreased below 20 mmHg in about two-thirds of the patients. Personalized – compared to routine – intraoperative arterial pressure management did not increase intraoperative mitoPO2. Whether intraoperative decreases in mitoPO2 are clinically meaningful warrants further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10877-024-01260-0.
Keywords: Anesthesia, Cardiovascular dynamics, Hemodynamic monitoring, Individualized, Microcirculation, Tissue perfusion
Introduction
Intraoperative hemodynamic management aims at optimizing arterial pressure and blood flow to ensure adequate organ perfusion and tissue oxygenation [1]. Tissue oxygenation is determined by oxygen delivery and cellular oxygen uptake [2]. However, it is not yet possible to routinely monitor cellular oxygen uptake during surgery [3].
About 90% of cellular oxygen is shifted to mitochondria for cellular respiration [4]. The “Cellular Oxygen METabolism” (COMET) system (Photonics Healthcare, Utrecht, The Netherlands) non-invasively measures mitochondrial oxygen tension (mitoPO2) in the skin using the protoporphyrin IX-triplet state lifetime technique [5, 6]. In short, mitochondrial oxygen content correlates inversely with fluorescence of protoporphyrin IX [7], which can be enriched in the skin by topical application of 5-aminolevulinic acid [8]. The COMET system measures the fluorescence of protoporphyrin IX to calculate mitoPO2. Monitoring mitoPO2 could potentially help clinicians guide intraoperative hemodynamic management. However, it is unknown if mitoPO2 is impaired during non-cardiac surgery with general anesthesia and if it is affected by intraoperative hemodynamic management.
In this pre-planned pilot substudy of the “Intraoperative blood pressure Management based on the individual blood PRessure profile: impact on postOperatiVE organ function" (IMPROVE) trial [9], we thus aimed to investigate the effects of general anesthesia and major non-cardiac surgery on mitoPO2. We further aimed to investigate if personalized – compared to routine – intraoperative arterial pressure management increases intraoperative mitoPO2 in patients having major non-cardiac surgery.
Materials and methods
Study design
This pre-planned pilot substudy of the IMPROVE trial [9] was conducted between July 2018 and April 2019 in the Department of Anesthesiology, Center of Anesthesiology and Intensive Care Medicine, University Medical Center Hamburg-Eppendorf (Hamburg, Germany). The IMPROVE trial including this pilot substudy was approved by the ethics committee (Ethikkommission der Ärztekammer Hamburg, Hamburg, Germany) on December 20, 2016 (registration number PV5413). All patients provided written informed consent. The IMPROVE trial tested the primary hypothesis that personalized intraoperative arterial pressure management maintaining preoperative baseline mean arterial pressure (MAP) from automated 24-h arterial pressure monitoring reduces the incidence of neurocognitive disorders (composite of delayed neurocognitive recovery and delirium) between postoperative days 3 and 7 compared to routine intraoperative arterial pressure management in patients having elective major non-cardiac surgery.
Patients
The IMPROVE trial included patients scheduled for elective major non-cardiac surgery with general anesthesia expected to last ≥ 90 min who were ≥ 50 years old and classified as American Society of Anesthesiologists physical status class II–IV. Patients who had a history of cerebrovascular events; had dementia; had kidney transplants; required dialysis; or were scheduled for emergency, cardiac, vascular, transplant, or neurosurgery were not included in the trial.
Basic anesthetic management
Epidural catheters – if clinically indicated – were inserted before induction of general anesthesia. General anesthesia was induced with sufentanil, propofol, and rocuronium or cisatracurium boluses according to routine care. After endotracheal intubation, the patients’ lungs were mechanically ventilated with a target end-tidal carbon dioxide of 35–40 mmHg. To maintain general anesthesia, patients received continuous remifentanil infusion or repeated boluses of sufentanil with inhaled sevoflurane or continuous propofol infusion. During surgery, arterial pressure was measured using a radial artery catheter.
Protocol
In the IMPROVE trial, patients were randomized in a 1:1 ratio to personalized or to routine intraoperative arterial pressure management. All patients had preoperative automated 24-h arterial pressure monitoring. Preoperative baseline MAP was individually defined as the mean of the mean daytime MAP and mean nighttime MAP measurements. In patients assigned to personalized intraoperative arterial pressure management, clinicians were asked to maintain intraoperative MAP above the preoperative baseline MAP. Specifically, clinicians were asked to maintain MAP within a range between the preoperative baseline MAP + 10 mmHg (with a minimum MAP target range of 65–75 mmHg and a maximum MAP target range of 100–110 mmHg). In patients assigned to routine intraoperative arterial pressure management, clinicians managed arterial pressure per institutional routine which generally is to maintain intraoperative MAP above 65 mmHg [10, 11]. Norepinephrine was used as the primary vasopressor per institutional routine. For the analysis, MAP values were recorded every five minutes during the induction of general anesthesia and surgery.
Measurement of mitoPO2
MitoPO2 was measured using the COMET system. On the evening before surgery, a 4 cm2 patch containing 8 mg 5-aminolevulinic acid (Alacare; Photonamic, Pinneberg, Germany) was applied to the clavipectoral triangle or above the sternum and protected from light with an additional patch. On the following day, before induction of general anesthesia, the patches were removed, the skin was cleaned, and the sensor of the COMET system was attached on the prepared skin area and shielded from surrounding light. We started measuring mitoPO2 with the COMET system before induction of general anesthesia and continued the measurements until the end of surgery. The COMET system provides updated mitoPO2 values every minute and additionally records the sensor temperature on the skin. We removed artefactual measurements before the analysis.
Endpoints
We calculated 5-min averages of mitoPO2 before induction of general anesthesia (preoperative awake mitoPO2), after induction of general anesthesia, at the beginning of surgery, and at the end of surgery. We further calculated average mitoPO2 during induction of general anesthesia and average mitoPO2 during surgery. Further, we report the lowest mitoPO2 and the lowest average mitoPO2 for 5 continuous minutes, during induction of general anesthesia and during surgery as well as the percentage of surgical time with mitoPO2 < 50 mmHg, < 40 mmHg, < 30 mmHg, and < 20 mmHg. All endpoints were calculated for the overall cohort and separately for patients assigned to personalized or to routine intraoperative arterial pressure management. Finally, we analyzed the correlation between mitoPO2 and MAP as well as the correlation between mitoPO2 and heart rate. On an exploratory basis, we compared mitoPO2 values between patients assigned to personalized and to routine intraoperative arterial pressure management.
Statistical analysis
Baseline, and clinical characteristics are reported separately for patients assigned to personalized and to routine intraoperative arterial pressure management. Categorical data are presented as absolute number (percentage), and continuous data are presented as median (25th to 75th percentile). We compared measurements between time points using two-tailed Wilcoxon signed-rank tests and measurements between patients assigned to personalized and to routine intraoperative arterial pressure management using two-tailed Mann–Whitney U-tests. The correlation between mitoPO2 and MAP as well as the correlation between mitoPO2 and heart rate were assessed using repeated measures correlation (rrm(n)) with 95%-confidence intervals (95% CI) [12, 13].
P-values are presented as descriptive summary measures, are not adjusted for multiple testing, and should not be interpreted as results of confirmatory hypothesis testing. Data were analyzed using GraphPad Prism V 10.1.1 (GraphPad Software, Boston, USA).
In the absence of previous studies investigating mitoPO2 in patients having non-cardiac surgery, no formal sample size calculation was performed for this pilot substudy. The observed mean difference in mitoPO2 before and after induction of general anesthesia of 20 mmHg (standard deviation 19 mmHg) in 19 patients corresponds to an effect size of −1.05 and results in a power (1-β error probability) of 98.8% with an α error probability of 0.05. The power calculation was performed using G*Power 3.1.9.7 (Kiel University, Germany) [14]. We included IMPROVE patients depending on the availability of study personnel and the COMET system.
Results
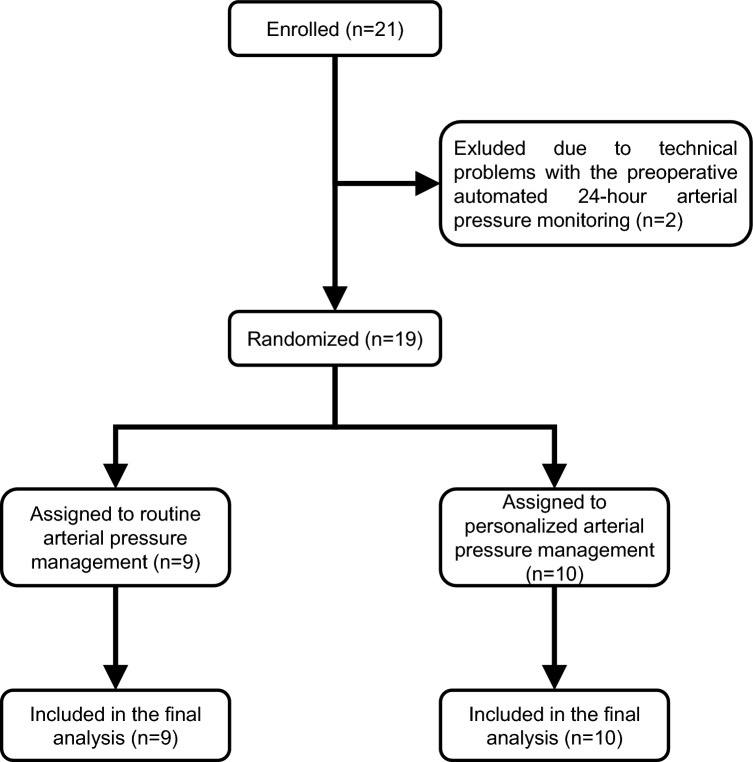
We included 21 patients in this pilot substudy, but excluded two who were excluded from the IMPROVE trial before randomization because of technical problems with the automated 24-h arterial pressure monitoring (Fig. 1; Table 1).
Fig. 1.
Flow chart illustrating enrolment, randomization, and reasons for exclusion
Table 1.
Patient characteristics
| Overall (n = 19) | Routine intraoperative arterial pressure management (n = 9) | Personalized intraoperative arterial pressure management (n = 10) | |
|---|---|---|---|
| Age, years | 64 (61 to 71) | 64 (60 to 72) | 65 (61 to 70) |
| Female, n | 9 (47%) | 5 (56%) | 4 (40%) |
| Height, cm | 172 (164 to 176) | 168 (165 to 172) | 174 (166 to 182) |
| Weight, kg | 77 (69 to 93) | 77 (70 to 90) | 77 (65 to 93) |
| Body mass index, kg/m2 | 25.7 (22.9 to 30.4) | 29.3 (24.0 to 31.0) | 24.2 (22.3 to 26.2) |
| American Society of Anesthesiologists physical status, II/III/IV | 7/11/1 | 3/6/0 | 4/5/1 |
| Arterial hypertension, n | 13 (68%) | 6 (67%) | 7 (70%) |
| Chronic obstructive pulmonary disease, n | 1 (5%) | 1 (11%) | 0 (0%) |
| Coronary artery disease, n | 3 (16%) | 2 (22%) | 1 (10%) |
| Diabetes mellitus, n | 2 (11%) | 1 (11%) | 1 (10%) |
| Type of surgery, n | |||
| General | 8 (42%) | 4 (44%) | 4 (40%) |
| Urology | 6 (32%) | 2 (22%) | 4 (40%) |
| Gynecology | 3 (16%) | 2 (22%) | 1 (10%) |
| Trauma/Orthopedic | 2 (11%) | 1 (11%) | 1 (10%) |
| Epidural catheter, n | 14 (74%) | 6 (67%) | 8 (80%) |
Data are presented as median (25th to 75th percentile) or absolute frequency (percentage) as appropriate
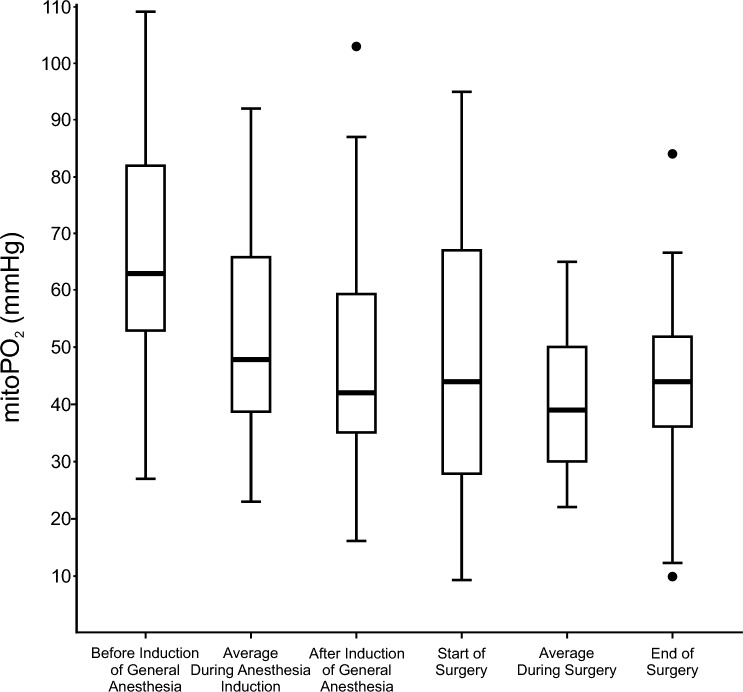
In the overall cohort, the median (25th to 75th percentile) preoperative awake mitoPO2 was 63 (53 to 82) mmHg before induction of general anesthesia (Fig. 2, Supplementary Table 1). After induction of general anesthesia, the median mitoPO2 was 42 (35 to 59) mmHg. The mitoPO2 after induction of general anesthesia was lower than the preoperative awake mitoPO2 in all but two patients (median difference −22 (−32 to −5) mmHg or −23 (−45 to −10) %; P < 0.001). During induction of general anesthesia, the median average mitoPO2 was 48 (38 to 66) mmHg, the median lowest mitoPO2 was 31 (17 to 46) mmHg, and the median lowest average mitoPO2 for 5 continuous minutes was 37 (22 to 50) mmHg.
Fig. 2.
Box plots showing mitochondrial oxygen tension (mitoPO2). The central box represents the values from the 25th to 75th percentile and its middle line represents the median. The vertical line extends from the minimum to the maximum value, excluding outliers. Outliers are defined as values that are smaller than the lower quartile minus 1.5 times or larger than the upper quartile plus 1.5 times the interquartile range
During surgery, the median average mitoPO2 was 39 (30 to 50) mmHg (Fig. 2; Table 2). The median mitoPO2 levels at the beginning of surgery (44 (28 to 64) mmHg) were similar to those at the end of surgery (44 (36 to 52) mmHg). However, in 13 patients (68%), the difference between mitoPO2 values at the beginning and the end of surgery exceeded ± 10 mmHg – increasing in 7 patients (37%) and decreasing in 6 patients (32%). During surgery, the median lowest mitoPO2 was 5 (4 to 7) mmHg and the median lowest average mitoPO2 for 5 continuous minutes was 9 (5 to 23) mmHg. In only two patients (11%) all intraoperative mitoPO2 was always higher than 50 mmHg. Thirteen patients (68%) had intraoperative mitoPO2 values below 20 mmHg. The median percentage of surgical time with mitoPO2 < 20 mmHg was 17 (0 to 31) %.
Table 2.
Intraoperative mitoPO2
| Overall (n = 19) | Routine intraoperative arterial pressure management (n = 9) | Personalized intraoperative arterial pressure management (n = 10) | P-value | |
|---|---|---|---|---|
| Average intraoperative mitoPO2, mmHg | 39 (30 to 50) | 46 (30 to 51) | 38 (31 to 44) | 0.653 |
| Lowest intraoperative mitoPO2, mmHg | 7 (5 to 20) | 14 (6 to 22) | 5 (4 to 7) | 0.072 |
| Lowest intraoperative 5-min average mitoPO2, mmHg | 9 (7 to 26) | 17 (8 to 24) | 9 (5 to 23) | 0.390 |
| Percentage of surgical time with mitoPO2 < 50 mmHg, % | 81 (48 to 90) | 85 (52 to 93) | 78 (52 to 87) | 0.772 |
| Percentage of surgical time with mitoPO2 < 40 mmHg, % | 43 (21 to 73) | 35 (20 to 73) | 46 (29 to 73) | 0.810 |
| Percentage of surgical time with mitoPO2 < 30 mmHg, % | 28 (7 to 55) | 23 (7 to 45) | 35 (10 to 56) | 0.596 |
| Percentage of surgical time with mitoPO2 < 20 mmHg, % | 17 (0 to 31) | 4 (0 to 31) | 17 (4 to 25) | 0.711 |
Data are presented as median (25th to 75th percentile). P-values reflect the difference between patients assigned to routine and personalized intraoperative arterial pressure management. mitoPO2 – mitochondrial oxygen tension
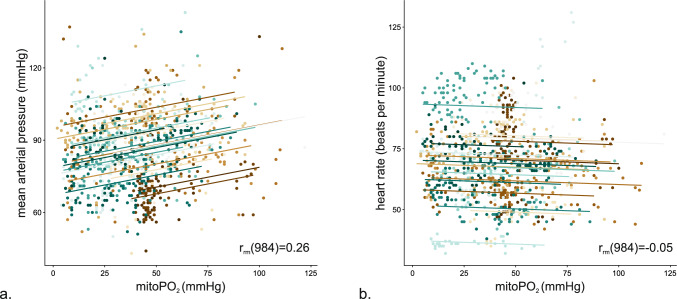
The correlation between mitoPO2 and MAP was weak (rrm(984) = 0.26, 95% CI 0.20 to 0.32; P < 0.001). There was no meaningful correlation between mitoPO2 and heart rate (rrm(984) = −0.05, 95% CI −0.11 to 0.01; P = 0.117) (Fig. 3).
Fig. 3.
Repeated measures correlation between mitochondrial oxygen tension (mitoPO2) and mean arterial pressure and between mitoPO2 and heart rate. Colored dots reflect measurement pairs from individual patients. Colored lines indicate the overall regression line with data points from individual patients
In patients assigned to personalized intraoperative arterial pressure management, the median intraoperative average MAP (92 (88 to 102) mmHg vs. 81 (71 to 90) mmHg; P = 0.008) and the median average norepinephrine infusion rate (0.22 (0.13 to 0.33) µg/kg/min vs. 0.06 (0.05 to 0.19) µg/kg/min; P = 0.015) were higher than in patients assigned to routine intraoperative arterial pressure management (Table 3). The cumulative amount of crystalloids and colloids patients were given was similar between the two groups (Table 3).
Table 3.
Intraoperative data
| Overall (n = 19) | Routine intraoperative arterial pressure management (n = 9) | Personalized intraoperative arterial pressure management (n = 10) | P-value | |
|---|---|---|---|---|
| Duration of surgery, min | 230 (130 to 330) | 170 (125 to 300) | 248 (181 to 348) | 0.289 |
| Crystalloids, ml | 2500 (2000 to 4500) | 2500 (1500 to 4000) | 3500 (2125 to 4500) | 0.389 |
| Colloids, ml | 50 (0 to 1500) | 0 (0 to 1500) | 500 (0 to 1375) | 0.653 |
| Norepinephrine infusion rate, µg/kg/min | 0.15 (0.07 to 0.24) | 0.06 (0.05 to 0.19) | 0.22 (0.13 to 0.33) | 0.014 |
| Blood loss, ml | 500 (315 to 1100) | 315 (175 to 725) | 800 (600 to 1100) | 0.749 |
| Preoperative baseline MAP, mmHg | 96 (91 to 99) | 96 (86 to 98) | 97 (93 to 103) | 0.390 |
| Intraoperative MAP, mmHg | 89 (81 to 94) | 81 (73 to 89) | 92 (89 to 101) | 0.178 |
| Intraoperative heart rate, beats/minute | 70 (63 to 80) | 77 (63 to 81) | 69 (64 to 74) | 0.490 |
Data are presented as median (25th to 75th percentile). P-values reflect the difference between patients assigned to routine and personalized intraoperative arterial pressure management. MAP – mean arterial pressure
During surgery, the median average mitoPO2 was 38 (31 to 44) mmHg in patients assigned to personalized and 46 (30 to 51) mmHg in patients assigned to routine intraoperative arterial pressure management (P = 0.653). There was also no clinically meaningful difference in the lowest intraoperative mitoPO2, the lowest average mitoPO2 for 5 continuous minutes, and the percentage of surgical time with mitoPO2 < 50 mmHg, < 40 mmHg, < 30 mmHg, and < 20 mmHg between patients assigned to personalized or to routine arterial pressure management (Table 2).
Discussion
In this pilot substudy of the IMPROVE trial, mitoPO2 under general anesthesia was about a quarter lower than preoperative awake mitoPO2 in patients having major non-cardiac surgery. During surgery, mitoPO2 substantially fluctuated and transiently decreased below 20 mmHg in about two-thirds of the patients. Personalized – compared to routine – intraoperative arterial pressure management did not increase intraoperative mitoPO2.
In our study, the mitoPO2 values before induction of general anesthesia were similar to those found in previous studies in young healthy volunteers [15, 16]. In line with these previous studies, we also found that mitoPO2 substantially varies among individuals. MitoPO2 under general anesthesia was about a quarter lower than preoperative awake mitoPO2 – whether this is the result of reduced oxygen delivery or altered oxygen utilization remains unknown. However, previous observational studies have shown that energy expenditure under general anesthesia is about one-quarter lower than preoperative awake resting energy expenditure in patients having non-cardiac surgery [17, 18].
The intraoperative average mitoPO2 values in our major abdominal surgery patients were similar to those reported in a study of 20 patients having neurosurgery [19]. However, in these neurosurgical patients, mitoPO2 decreased from around 60 mmHg to around 40 mmHg over the course of surgery. In our study, individual mitoPO2 substantially fluctuated and transiently decreased below 20 mmHg in about two-thirds of the patients. These mitoPO2 fluctuations occurred mostly independent of macrohemodynamic alterations and the overall correlation between mitoPO2 and MAP was weak. It will require investigation in larger cohorts, whether or not transient – short or prolonged – decreases in mitoPO2 are clinically important.
We also investigated the effect of two different intraoperative arterial pressure management strategies on mitoPO2. Personalized – compared to routine – intraoperative arterial pressure management resulted in higher MAPs, but there was no clinically important difference in intraoperative mitoPO2 between the two groups. Profound hypotension can lead to tissue hypoperfusion – and low mitoPO2 values. However, profound hypotension (with MAP values substantially below 65 mmHg) was rare in both groups. The few episodes of intraoperative hypotension we observed were short and not associated with low mitoPO2 values. Patients assigned to personalized intraoperative arterial pressure management were given more norepinephrine. Interestingly, these higher norepinephrine doses, that were required to maintain MAP in patients assigned to personalized intraoperative arterial pressure management also did not decrease mitoPO2.
MitoPO2 measurements in our study were feasible and safe. We did not observe any adverse events associated with mitoPO2 measurements. Monitoring mitoPO2 with the protoporphyrin IX triplet state lifetime method is an innovative and intriguing concept as it provides insight into the final pathway of oxygen delivery to the cells. Assuming that elective non-cardiac surgery patients do not have mitochondrial dysfunction or severe impairment of oxygen utilization, mitoPO2 could be a better surrogate for oxygen delivery and cellular oxygen uptake than estimation from systemic oxygen delivery or monitoring regional oxygen saturation with near-infrared spectroscopy – which measure oxygen content in the blood rather than in the tissue [20]. However, until now, monitoring mitoPO2 is only possible on the skin – which may not necessarily reflect mitoPO2 of vital organs. Changes in cutaneous mitoPO2 may thus not always mirror changes in visceral mitoPO2. Even though we carefully prepared the skin and carefully performed mitoPO2 measurements, it cannot be ruled out that there were constraining factors affecting the mitoPO2 measurements during surgery. Whether or not monitoring mitoPO2 in the skin provides clinically meaningful information and can help guide perioperative hemodynamic management in high-risk patients requires further investigation.
This was a pilot substudy of the monocentric IMPROVE trial. Due to the complexity of the mitoPO2 measurements, we only included a few patients. Our results thus need to be confirmed in larger studies. Naturally, the results are not generalizable to other settings, e.g., patients in the emergency room or the intensive care unit. We did not record the fraction of inspired oxygen, which may have an important effect on mitoPO2. Further, we only measured heart rate and arterial pressure as macrocirculatory variables and thus were not able to analyze the relationship between blood flow and mitoPO2.
MitoPO2 under general anesthesia was about a quarter lower than preoperative awake mitoPO2, substantially fluctuated during major non-cardiac surgery, and transiently decreased below 20 mmHg in about two-thirds of the patients. Personalized – compared to routine – intraoperative arterial pressure management did not increase intraoperative mitoPO2. Whether intraoperative decreases in mitoPO2 are clinically meaningful warrants further investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to thank Francesco Däke, Hanh HD Pham, Marie-Christin Rabe, Hanna Schlichting, and Sophia Skrovanek for their support with the study.
Author Contribution
Study design: MF, JN, and BS. Data acquisition: MF, CV, AB, and KB. Data analysis: MF, CV, AB and BS. Drafting the manuscript: MF and BS. All authors reviewed the manuscript critically and approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. The “Intraoperative blood pressure Management based on the individual blood PRessure profile: impact on postOperatiVE organ function" (IMPROVE) trial and this substudy were funded by the Else Kröner-Fresenius-Stiftung, Bad Homburg, Germany (2016_A200) and supported from institutional and/or departmental sources.
Data Availability
Data are available upon reasonable request.
Declarations
Conflict of interests
MF is a consultant for and has received honoraria for giving lectures from Edwards Lifesciences (Irvine, CA, USA). MF has received honoraria for consulting and giving lectures from CNSystems Medizintechnik (Graz, Austria). MF is an Editor for the Journal of Clinical Monitoring and Computing. BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from Edwards Lifesciences (Irvine, CA, USA). BS is a consultant for Philips North America (Cambridge, MA, USA) and has received honoraria for giving lectures from Philips Medizin Systeme Böblingen (Böblingen, Germany). BS has received institutional restricted research grants and honoraria for giving lectures from Baxter (Deerfield, IL, USA). BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from GE Healthcare (Chicago, IL, USA). BS has received institutional restricted research grants and honoraria for giving lectures from CNSystems Medizintechnik (Graz, Austria). BS is a consultant for Maquet Critical Care (Solna, Sweden). BS has received honoraria for giving lectures from Getinge (Gothenburg, Sweden). BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from Pulsion Medical Systems (Feldkirchen, Germany). BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from Vygon (Aachen, Germany). BS is a consultant for and has received institutional restricted research grants from Retia Medical (Valhalla, NY, USA). BS has received honoraria for giving lectures from Masimo (Neuchâtel, Switzerland). BS is a consultant for Dynocardia (Cambridge, MA, USA). BS has received institutional restricted research grants from Osypka Medical (Berlin, Germany). BS was a consultant for and has received institutional restricted research grants from Tensys Medical (San Diego, CA, USA). BS is an Editor of the British Journal of Anaesthesia. AB, CV, KB, and JYN have no conflicts of interest to declare.
Footnotes
Julia Y. Nicklas and Bernd Saugel contributed equally.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parker T, Brealey D, Dyson A, Singer M. Optimising organ perfusion in the high-risk surgical and critical care patient: a narrative review. Br J Anaesth. 2019;123(2):170–6. 10.1016/j.bja.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schober P, Schwarte LA. From system to organ to cell: oxygenation and perfusion measurement in anesthesia and critical care. J Clin Monit Comput. 2012;26(4):255–65. 10.1007/s10877-012-9350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flick M, Hilty MP, Duranteau J, Saugel B. The microcirculation in perioperative medicine: a narrative review. Br J Anaesth. 2024;132(1):25–34. 10.1016/j.bja.2023.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Snyder CM, Chandel NS. Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid Redox Signal. 2009;11(11):2673–83. 10.1089/ars.2009.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harms FA, Bodmer SI, Raat NJ, Stolker RJ, Mik EG. Validation of the protoporphyrin IX-triplet state lifetime technique for mitochondrial oxygen measurements in the skin. Opt Lett. 2012;37(13):2625–7. 10.1364/ol.37.002625. [DOI] [PubMed] [Google Scholar]
- 6.Ubbink R, Bettink MAW, Janse R, Harms FA, Johannes T, Münker FM, Mik EG. A monitor for cellular oxygen metabolism (COMET): monitoring tissue oxygenation at the mitochondrial level. J Clin Monit Comput. 2017;31(6):1143–50. 10.1007/s10877-016-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mik EG, Stap J, Sinaasappel M, Beek JF, Aten JA, van Leeuwen TG, Ince C. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 2006;3(11):939–45. 10.1038/nmeth940. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda H, Casas A, Batlle A. Aminolevulinic acid: from its unique biological function to its star role in photodynamic therapy. Int J Biochem Cell Biol. 2005;37(2):272–6. 10.1016/j.biocel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas JY, Bergholz A, Däke F, Pham HHD, Rabe M-C et al. Personalised blood pressure management during major noncardiac surgery and postoperative neurocognitive disorders a randomised trial. BJA Open. 2024;11:100294. 10.1016/j.bjao.2024.100294 [DOI] [PMC free article] [PubMed]
- 10.Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–74. 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Saugel B, Sessler DI. Perioperative Blood Pressure Management. Anesthesiology. 2021;134(2):250–61. 10.1097/ALN.0000000000003610. [DOI] [PubMed] [Google Scholar]
- 12.Bakdash JZ, Marusich LR. Repeated Measures Correlation Front Psychol. 2017;8:456. 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marusich L, Bakdash J (2021) rmcorrShiny: A web and standalone application for repeated measures correlation [version 1; peer review: 2 approved]. F1000Research 10 (697). 10.12688/f1000research.55027.1 [DOI] [PMC free article] [PubMed]
- 14.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 15.Baumbach P, Neu C, Derlien S, Bauer M, Nisser M, Buder A, Coldewey SM, (2019). A pilot study of exercise-induced changes in mitochondrial oxygen metabolism measured by a cellular oxygen metabolism monitor (PICOMET). Biochim Biophys Acta Mol Basis Dis. 1865(4): 749-758. 10.1016/j.bbadis.2018.12.003 [DOI] [PubMed]
- 16.Harms FA, Stolker RJ, Mik EG. Cutaneous respirometry as novel technique to monitor mitochondrial function: a feasibility study in healthy volunteers. PLoS ONE. 2016;11(7): e0159544. 10.1371/journal.pone.0159544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briesenick L, Schaade A, Bergholz A, Hoppe P, Kouz K, et al. Energy expenditure under general anesthesia: an observational study using indirect calorimetry in patients having noncardiac surgery. Anesth Analg. 2023. 10.1213/ane.0000000000006343. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsson J, Norén C, Hagel E, Kalman S, Bartha E. Peri-operative oxygen consumption revisited: An observational study in elderly patients undergoing major abdominal surgery. Eur J Anaesthesiol. 2021;38(1):4–12. 10.1097/eja.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 19.Harms FA, Streng L, Wefers Bettink MA, de Wijs CJ, Römers LH, et al. Monitoring of mitochondrial oxygen tension in the operating theatre: An observational study with the novel COMET® monitor. PLoS ONE. 2023;18(2): e0278561. 10.1371/journal.pone.0278561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber W, Zanner R, Schneider G, Schmid R, Lahmer T. Assessment of regional perfusion and organ function: less and non-invasive techniques. Front Med (Lausanne). 2019;6:50. 10.3389/fmed.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.