Abstract
Microfluidic devices, including lab-on-a-chip devices, have many advantages over conventional laboratory techniques. Although common in some applications, there are several barriers to wider adoption, including the high initial cost to fabricate a design and the labor-intensive process of development. This work seeks to address those barriers by combining 3D printing and computer aided design tools. Building on existing open-source software from electronic design automation (EDA), we present a design, verification, and manufacturing toolchain for 3D design for microfluidic devices. The process starts with a list of components and connections then, automatically lays out a microfluidic device using a library of components, simulates the device, and produces a 3D CAD file that is used for DLP 3D printing process. The automated design and fabrication process was demonstrated by automatically designing and fabricating a calcium quantification assay. This toolchain automatically generated a microfluidic chip that meters each reagent with an error of less than 2.24% as verified by Xyce simulation of the chip. Physical chips were printed and found to perform with errors less than 9.2% on average compared to the assay performed by hand. The demonstration showed the ability of the toolchain to automatically generate a functional microfluidic chip for use with real assays using previously developed EDA tools.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-15976-9.
Subject terms: Lab-on-a-chip, Microfluidics, Software
Introduction
Microfluidics is becoming a powerful tool for healthcare diagnostics and biological studies. It is utilized in many applications, such as virus and pathogen detection1,2proteomics3genomics4,5synthetic biology6,7and biological disease research8,9. Microfluidic approaches have many advantages over traditional diagnostic and wet chemistry methods, including small sample and reagent volumes, increased throughput, reduced contamination, improved repeatability when automated, and high sensitivity and specificity10,11. A microfluidic device uses small diameter channels, typically between 1 and 500 μm, to perform functions on a small volume of a sample fluid (pL – mL). It is typical to fabricate microfluidic devices using photolithography techniques similar to those used in microelectronics and micro-electromechanical system (MEMS) manufacturing. Specifically, the process of soft lithography is often used—a mold is created through lithography or other techniques upon which an elastomer, usually polydimethylsiloxane (PDMS), is cast12. As in microelectronics, however, developing new microfluidic devices is time-consuming and expensive, often requiring teams of expert designers and a high upfront cost to begin prototyping and manufacturing. However, soft lithography is not well suited for scaling up to high-volume production. PDMS has slow curing times and liquid handling requirements, making the transition from prototype to mass production challenging. Further, complex microfluidic designs are difficult to fabricate with limited ability to utilize multiple layers, as the assembly of which complicates manufacturing due to the alignment of multiple soft, elastic, thin layers13. Using more established mass production methods is often not cost-effective due to the wide range of disparate devices and applications, leading to lower manufacturing quantities and high inventory costs since many different devices need to be stored. Furthermore, the fabrication of enclosed channels requires precision polymer or glass alignment and bonding process, leading to additional costs and complications. These factors have proven to be a barrier to the wider adoption of microfluidic technology and advancement in design complexity14,15.
Recently, to resolve some of these challenges, researchers have utilized 3D printing for rapid prototyping and small and mid-scale production of microfluidic devices, driven at least partially by the limitations of current manufacturing methods. Several 3D printing techniques have been demonstrated to be viable for the fabrication of microfluidic devices, including MultiJet printing (MJP)16–18fused deposition modeling (FDM)19–21direct ink writing (DIW) or bioprinting22and stereolithography (SLA)23 using digital light processing (DLP)24–27liquid crystal display (LCD)28–30 and two-photon polymerization31,32. In recent years, 3D printing techniques have become a viable manufacturing method for microfluidics, but in this work we focus on DLP stereolithography based methods as this process has been demonstrated to be able to fabricate high resolution microfluidic devices with many of the most crucial components such as a microvalves33,34with some as small as 15 μm x 15 µm24micromixers17micro-heating elements26 and interface inputs and outputs35. Additionally, LCD based stereolithography has also been used to demonstrate features such as microvalves, an ELISA-on-a-chip circuit, and organ-on-a-chip devices29 with a low cost printer, but not with the same resolution as DLP. DLP Stereolithography is a process that uses a micromirror array to generate and image that can be used to cure a photopolymer in a layer-by-layer process. Within stereolithography there are different implementations that has trade-offs with cost, resolution and print area which is discussed by Amini et al.23 More general reviews of the progress of 3D printing for microfluidic applications are available36,37.
The ease with which components can be repeatably fabricated with 3D printing techniques greatly reduces the effort necessary to increase the complexity of devices. For example, Gong et al. created a dilution mixer38 containing just 16 valves to create 8 pumps, 2 regions of interest, and 33 input and output interface connections—already an intricate system. In this design and many other 3D printed microfluidic systems27,39,40the components in this chip are often duplicates or similar enough that parametric design files can be developed to reduce the complexity of the development of the device design. The adoption of such systems makes components easier to reuse, increases the repeatability of designs, and reduces the overall design effort41; however, there are limits to this approach. As device designs become more complex, revisions to designs can require substantial routing changes and component repositioning that current computer-aided design (CAD) software, such as SolidWorks or AutoCAD is not currently designed to handle. All this complexity requires rapidly increasing amounts of time from a microfluidic engineer.
The field of design automation has sought to improve the labor-intensive design process required to design and validate microfluidic chips. Microfluidic design automation (MFDA) tools are largely inspired by similar tools used in microelectronics, where mature tools have been developed for massively complex designs—a field referred to as electronic design automation (EDA). These design tools are responsible for automating integrated circuit design to produce a set of masks and manufacturing instructions for fabrication. Some of the most notable projects in MFDA have included Cloud Columba423DµF43and Flui3d44 for developing the physical design of the microfluidic chip, such as component placement and routing connections. Specifically, Cloud Columba is a design synthesis tool capable of generating layouts for photolithography microfluidic devices, while 3DµF and Flui3d are interactive layer-based CAD tools with design rule checking. The major advantage these software packages provide is an easier and more efficient interface for microfluidic chip design compared to the general CAD tools of SolidWorks and AutoCAD. It should be noted that several other design synthesis tools have been demonstrated for microfluidics45–48; however, these tools are generally proof of concept and do not have the source code available or are limited by interoperability.
Apart from design synthesis, other important efforts have focused on implementing simulations that can be used to validate the functionality of the device. Simulation in microfluidics generally takes one of two forms: finite element analysis (FEA) or computational fluid dynamics (CFD), mostly used for detailed components analysis, and modified nodal analysis (MNA), which can better handle system-level simulation. MNA is more commonly used since it more efficiently captures the simulation of the entire microfluidic circuit during runtime. This approach usually leads to the microfluidic system being implemented in a SPICE circuit that utilizes electrical and microfluidic analogs to solve for pressures and flow rates of the microfluidic devices49. Due to the limitation of the accuracy of simple MNA methods, other approaches have adopted hybrid methods using computational fluid dynamics (CFD)50 or by using droplet simulators that handle fluid channels51. It can be seen from Takken et al.50 that even the inclusion of modified nodal analysis can greatly improve the simulation speed compared to CFD methods.
While several tools have been shown to improve a microfluidic designer’s ability to develop new assays, there is limited ability to translate outputs between most tools, and many have tackled relatively small problems where semi-automation can be used effectively52. To address the fragmentation of design automation software, several MFDA groups have created toolchains, but they are quite limited as they are still centered around one or two layered devices generally created using molded PDMS. Only recently have these toolchains been expanded to the design of multilayered devices using 3D printing44. To address many of these issues with MFDA, the focus of this work is to link automated design processes creating continuous-flow microfluidic chips with automated manufacturing by 3D printing, leading to a versatile design platform for a variety of assays with the ability to build digital-like devices using reconfigurable53 quickly and easily.
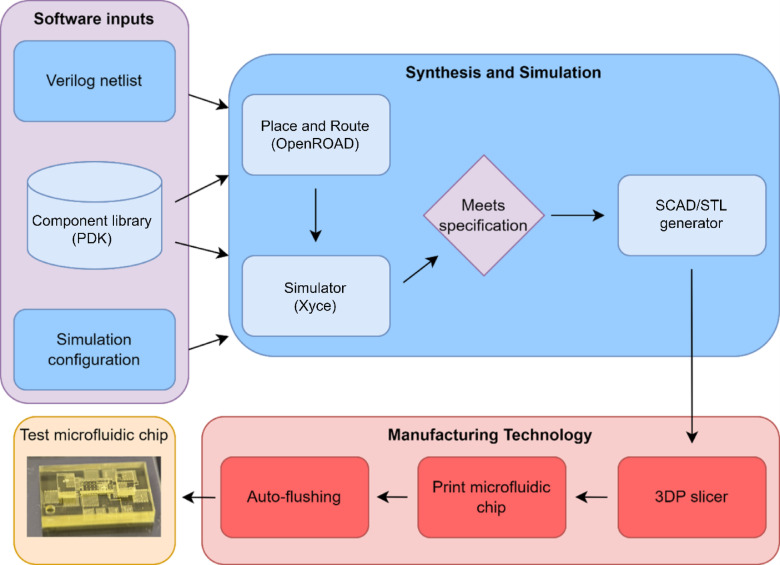
This paper presents OpenMFDA, an open-source platform for MFDA, and a design automation tool to generate microfluidic physical designs, simulation and verification with a process for automating manufacturing of microfluidic devices. The software toolchain capitalizes on existing open-source electronic design automation (EDA) tools and links them to DLP 3D printing techniques for a process that can rapidly generate and subsequently manufacture microfluidic devices. As a design environment, OpenMFDA combines the capabilities of tools from OpenROAD54which are used for physical design synthesis, and Xyce55a SPICE based simulator, to perform physical design and then simulate microfluidic chips, respectively. The toolchain additionally incorporates 3D printing and a postprocessing system to automate the manufacturing process. Specifically, the OpenMFDA process links steps such as placement and routing, system-level simulation and validation, and manufacturing preprocessing and postprocessing steps into a complete end-to-end process. Figure 1 illustrates the proposed software flow that links with a 3D printing workflow. The process begins with a component netlist, a textual representation of the schematic of physical components, and ends with a 3D printed device using DLP printing. While many other design automation workflows exist, the OpenMFDA process has some unique benefits that we highlight when compared to some of the most notable MFDA efforts briefly summarized in Table 1. One of the major contributions of OpenMFDA relative to these other flows is that it incorporates physical design synthesis using 3D geometries, has the ability to extract simulation level schematics, and aligns design dimensions with DLP 3D printing grids for accurate manufacturing. The other important aspect of the toolchain is the implementation of a component library, which represents the available microfluidic components, that is customizable and expandable. The source code is available allowing users to expand the library by incorporating their microfluidic component geometries and behaviors.
Fig. 1.
Software flow of OpenMFDA, which uses the physical design components of OpenROAD to generate the placement and routing. A python script generates the Xyce netlist file for simulation that can then be run in a Xyce simulator to simulate the circuit behavior. After a design simulation shows that the device meets the desired specifications, an OpenSCAD file is generated and then exported to an STL file that can be read by a 3D printing system. For manufacturing, the file can be sliced, 3D printed, and the printed device postprocessed. All of the processes of OpenMFDA are performed using open-source software and all component library files are text files shared on Github.
Table 1.
Comparison of prominent MFDA tools and engineering tools with respect to different design automation features. “Mechanical design” evaluated the ability of the software to perform drafting functions, as well as its simulation capabilities. We note FEA means “finite element analysis” and MNA refers to “modified nodal analysis.” “design automation” tasks were evaluated by support of complete automated generation of layout of the device of the steps listed. The “component library and code base” section evaluated the ability of users to implement their own geometries into the software, demonstration of support for design rules in implementation, and if the code base is open-source. The last rows compare the ability of the software to link with up with 3D printing processes by support of automated slicing where useable and compatible model exports for 3D printing.
| Feature | SolidWorks, AutoCAD | COMSOL | Columba45 | 3DµF46 | Flui3d47 | MMFT 54 | OpenMFDA |
|---|---|---|---|---|---|---|---|
| Mechanical Design | |||||||
| 2D Geometric design | ✔ | ✔ | ✘ | ✔ | ✔ | ✔ | ✘ |
| 3D Geometric design | ✔ | ✔ | ✘ | ✘ | ✔ | ✘ | ✘ |
| FEA Simulation | ✔ | ✔ | ✘ | ✘ | ✘ | ✔ | ✘ |
| MNA Simulation | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ | ✔ |
| Design Automation | |||||||
| 2D automated component placement | ✘ | ✘ | ✔ | ✘ | ✘ | ✘ | ✔ |
| 2D automated routing | ✘ | ✘ | ✔ | ✘ | ✘ | ✘ | ✔ |
| 3D routing | ✘ | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ |
| Component library and base code for design automation | |||||||
| Extendable component library | ✘ | ✘ | ✘ | ✔ | ✔ | ✘ | ✔ |
| Parametric component library | ✘ | ✘ | ✘ | ✔ | ✘ | ✘ | ✔ |
| Support for design rules | ✘ | ✘ | ✔ | ✘ | ✔ | ✘ | ✘ |
| Open-source | ✘ | ✘ | ✘ | ✔ | ✔ | ✔ | ✔ |
| Auxiliary 3D printing functions | |||||||
| 3D printing preparation | ✘ | ✘ | ✘ | ✘ | ✔ | ✘ | ✔ |
| Compatible 3D model export | ✔ | ✔ | ✘ | ✘ | ✘ | ✔ | ✔ |
In this paper, we demonstrate the utility of OpenMFDA, by using the tool to generate a microfluidic chip that implements a calcium quantification assay. This assay combines a sample with two reagents in a specific ratio and then observes a color change to measure the calcium concentration. This chip has previously been demonstrated, allowing the comparison of the automatically generated chip with the previous custom designed devices56.
Methods
The OpenMFDA process is shown schematically in Fig. 1. Our current version of the OpenMFDA toolchain requires the user to specify what types of components are needed in the final device and how they are connected. We anticipate that future versions will automate that process based on basic assay or functional features. Thus, the OpenMFDA process starts with a user generating a Verilog-AMS file specifying the components and port locations needed from a component library and the connections between components (each separately named), which is referred to as a netlist. The netlist is essentially a code-based schematic representation of the device. The OpenMFDA software then submits the requirements file to OpenRoad which performs a place and route operation and then passes the layout schematic to Xyce that then simulates the generated structure. If the structure meets requirements, an OpenSCAD file is generated of the device to enable a DLP 3D printing process. The device is printed and flushed, a post-processing step required with DLP printers to remove resin from the channels. The device is then ready to use.
In the following sections, we describe the details of the creation of a microfluidic component library, implementation of placement and routing, simulation of the automatically generated design, fabrication of the design chip, and validation experiments for the overall process. We also include specific instructions used for the software toolchain with respect to the calcium quantification chip used to demonstrate the toolchain in the supplemental documentation, which can be found at our Github repository.
Component library
A component library was created representing some of the basic microfluidic components needed for typical continuous flow microfluidic devices (devices where pressure sources outside the chip drive fluids through the chip continually). To start the base library, we developed standard and parametric components such as inlets and outlets, channels, serpentine channels, chambers, membrane valves of several implementations, and several mixers which can be found in our GitHub repository https://github.com/utah-MFDA. In an approach analogous to EDA, the component library has been packaged as a process development kit (PDK) for producing microfluidic devices on a specific DLP 3D printer. The PDK for OpenMFDA is a set of building blocks to which each block is a component within the library with each component formatted to be used with DLP printing. Each component included in the library has an associated set of files that contain information needed to implement the layout. The library exchange format (LEF) files57which contains an abstract “black-box” representation of the important geometric blocks for layout of the standard components in the library, which includes component size, obstructed areas, connection locations for routing, and which is packaged with a SCAD file that contains the detailed geometric information to be used in rendering the STL file when 3D printing. To verify the chip meets the required fluid physics needs, in this case, metering each reagent appropriately, a simulation file for each component containing equations for fluidic resistance and the mixing relationships that occurs between inputs and outputs is provided in analog equations encoded in the Verilog-AMS files. These combined library files are used as inputs to the OpenMFDA software, which uses them to design and verify the microfluidic chip. When combined with 3D printing and system-level simulations, these library components can be combined into new devices for new applications and designs can be quickly iterated through and optimized. At present, the design of the PDK will need to be tuned for each printer as the capability of each printer varies as well as the optical and curing properties of the resin used. For this work, the primary parameters for the 3D printer that were used to configure the library structures were pixel size, layer height and minimum positive and negative feature size. Additional definitions within the library include defining the core area, which is determined by the number of pixels in the XY dimension for the printer, in this case 2550 × 1600 pixels, with routing rules tuned based on the printer capabilities discussed in the fabrication section. Details for developing components can be found in the PDK in our Github repository, https://github.com/utah-MFDA/h.r.3.3_pdk, with additionally details for adding additional PDKs in the docs directory at https://github.com/utah-MFDA/openmfda_flow.
Placement and routing
To perform the placement and routing step, we use OpenROAD58an open-source tool for placement and routing of application-specific integrated circuits (ASIC). Several configuration files were needed to adapt OpenROAD for microfluidic component placement and routing. First, we adapted the configuration files that are standard for electronic design automation, including: a technology (TLEF) file that contains layout information for the chip footprint such as routing sizes to be used and what vertical connections between routing layers are allowed, and a library exchange format (LEF) file that contains all of the standard component geometries exported from the component library. The standard component LEF files are pulled from the library with any parametric component files to be dynamically generated and included in a design-specific LEF library at runtime. The last configuration files required are Makefile configurations that refence other configuration files that contain layout area information for their respective steps, the location of the I/O connections, bounding area for components, and routing grid which will guide the of the routing algorithm such that routing paths generated will be preferentially snapped to. Note that the I/O configuration was arranged to be used with a standard interface chip compatible with the flushing system and standard chip operating tools available in our labs. This set of configuration files for OpenROAD was developed to mimic a process design kit (PDK) in EDA that considers the specifications of the 3D printer in terms of feature size, total available area, number of layers that can fit in the maximum thickness, etc.
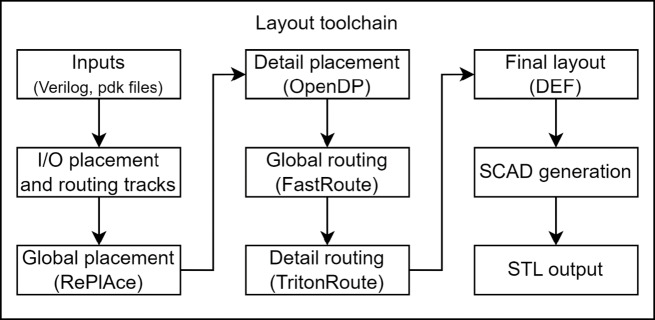
To implement the process and develop a specific microfluidic chip design a user would need to define a digital Verilog file, (which will be called a “Verilog file” and not to be confused with the analog versions, Verilog-A or Verilog-AMS), that would contain the netlist and define additional configuration files. These files are used for design specific definitions which include the I/O locations, defining the 3D printer technology used to develop the design for, which informs OpenMFDA of the design constraints and components available from the component library that will be used to develop the physical design. To run the entire flow, “make,” a program that will be used to facilitate running the different software modules for each step, is run with the name of the target platform and name of the design as inputs to the program that calls each of the different modules automatically in sequence in OpenROAD (Fig. 2). The final output of the OpenROAD flow is a design exchange format (DEF) file. The DEF file contains specific physical design information about the placement locations of the components, the routing channel segments, and I/O port locations, and would need to be combined with the LEF and SCAD files to fully render the design. Features and component dimensions are defined in terms of discrete pixels in the design files in recognition of the discrete aspects of the 3D printing process, as partial pixel sizes are difficult to fabricate without custom printer instructions and to eliminate ambiguity and variability later on during manufacturing. From OpenROAD we used placement and routing algorithms RePlace and OpenDP for the global and detail placement, respectively, and FastRoute and TritonRoute for global routing and detailed routing, respectively. To use the final output from OpenROAD we developed and utilized a custom software package in Python to translate the output DEF from OpenROAD into a 3D model readable by OpenSCAD using the SolidPython library that was used to generate the stereolithography file STL.
Fig. 2.
Flow chart of the physical design software toolchain showing the sequence of each step of the most significant modules used from OpenROAD utilized for the OpenMFDA process as well as external scripts to go from netlist (Verilog) to a 3D renderable STL output.
Simulation
Each “successful” design was simulated as part of the toolchain to verify that the generated design would meet the physical requirements of the assay or system. To implement system-level verification through simulation, we utilize a modified nodal analysis approach, where microfluidic components are represented by their electrical analogs. We utilized an open-source electric circuit solver, Xyce, to calculate the fluidic resistances, pressure, and flow rates using Verilog-AMS extensibility for non-electrical disciplines to represent the fluidic values. Each component would be developed as individual blocks that were coded into Verilog-AMS and added to the component library with its characteristics equations that related geometric parameters to physical metrics like pressure, flow rate, and chemical concentration within the analog blocks for each Verilog-AMS module. After completion of the placement and routing step, Xyce would be called in the OpenMFDA workflow to simulate the design by generating a Xyce netlist circuit file (cir) referencing the compiled library of the Verilog-AMS code for all the components. The approach was used to analyze the pressure and flow rate of the circuit due to the inclusion of components as well as the parasitic pressure losses that would occur with additional routing channels after completion of the placement and routing steps. The fluidic resistances of the different components are derived from the Hagen-Poiseuille relationship. Capacitive effects were ignored due to the high stiffness of the bulk material and the lack of valve or membrane components in the simulated devices. Chemical concentration calculations are performed using a virtual port to carry the chemical concentration information through the flow simulations. Separate simulations would have to be used for each different input chemical due to the lack of vector support in Xyce. However, due to the speed of these simulations, running multiple simulations has little impact on the final total runtime to verify the function of the microfluidic chip. Outputs from Xyce were used to compare the various designs generated and select an optimal design for further processing.
Fabrication and Post-Processing
To fabricate our microfluidic devices, we use a custom DLP 3D printer equipped with a 365 nm LED light engine and photosensitive resin (97% PEGDA, 2% NPS, 1% Irgacure), which we had develop previously59with a pixel pitch of 7.6 μm and layer thickness of 10 μm. Flushing was done with a custom flushing system that selectively applies controlled pressure differences across the terminals of the device in order to displace unpolymerized resin through an interface chip, and Chip-To-Chip interconnects35. Fine-tuning of pressure settings used for flushing a variety of microfluidic structures ensured high success rates and increased efficiency of the post-processing routine.
Experimental chip quantification and verification
We chose a Ca2+ arsenazo assay to demonstrate the full OpenMFDA process. The demonstration chip was a version of a previously published device designed to measure Ca2+ concentration in urine56which enables comparison between the automatically generated chip and the previously published chip. The assay requires the mixing of a urine (Ca2+) sample with water (diluent) and then a reagent (arsenazo) in specific ratios to produce a final color that correlates with the concentration of Ca2+ in the sample. The chip is expected to perform the assay using a uniform pressure into all inlets (e.g. a household water pressure) to make the assay passive and as simple as possible from an operating standpoint. For the proposed chip, the ratio of resistance through which the fluids pass before mixing determines the ratio of each liquid in the final channel where the color of the mixture is observed. To implement this assay using OpenMFDA, these resistances were provided in the Verilog netlist file, as specified serpentines for each input branch of the design, and the OpenMFDA process modified the serpentine channel placement to generate the appropriate resistances, laid out the channels, connected them together, simulated the channel network, and then ultimately printed the design. The printed chip was then tested for function.
The fabricated output of the OpenMFDA process was tested using a standard microfluidic test apparatus in our lab. The experimental setup included a chip specific interface chip that with only three input channels connected by tubing to vials containing the regents for the assay and one output vial to store the output solution. The interface chip was mounted to the assay chip mating the I/Os that are based on Gong et al.35. The input reagents were calcium hydroxide (0–10 mg/dL) as the sample simulant, arsenazo III (200 µM, A92775-1G, Sigma-Aldrich) as the calcium indicator, and deionized water as the dilution buffer. A pump supplying 2 PSI of pressure was connected to a vial of each input fluid to pump into each input connector of the interface chip. The reagents were metered and mixed by the chip into a vial, the contents of which were later loaded into a well plate for optical absorption testing. The optical properties of the resulting sample were measured using a plate reader (Synergy 2, BioTek, USA).
Results
MFDA component library and integrated microfluidic design automation
The OpenMFDA software toolchain was shown to be able to automatically design, simulate, and fabricate a microfluidic chip that operated as expected using entirely open source software, including existing EDA tools. This software and hardware process covers all steps starting from a Verilog-based netlist, which simply is a textual schematic representation of the component and connections, to produce a fully functioning continuous flow-based microfluidic chip. The OpenMFDA software was shown to successfully read in the netlist and other configuration files, fetch information from the component library and the platform configuration files, create a model of chip, generate a device based on the netlist with the specified components and routing constraints, and send that information to OpenROAD for placement and routing followed by generation of the connections between components. The final layout could then be used to generate a simulation input, which was then run by Xyce to determine the expected operation of the chip. If the chip had a low enough error, the design was used to create a CAD file of the design which could be directly integrated with a DLP 3D printing process based on an STL output. The details of this process follow.
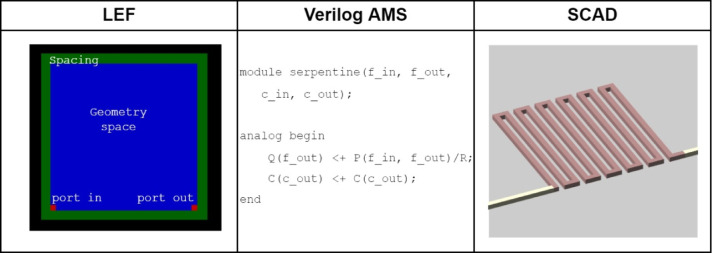
A component library utilizing a suite of three files for each component in standard file formats: library exchange format (LEF), Verilog-AMS, and SCAD, was successfully implemented for a range of components. The files were the inputs of the component blocks to the software toolchain. In Fig. 3, an example of each file type is presented. In the library, we provided a set of parametric and standard components including various serpentine lengths, mixers, reservoirs, and valves of a few different sizes. The combination of these files allows for the component library to implement true 3D microfluidic components during placement and routing. We additionally show support for parametric components by using the module functionality of SCAD and Verilog-AMS to build generic components that can be resized during the process. We were able to implement parametric structures (reconfigurable or customizable structures) based on the LEF file standard, that allows for calling the component and resizing it during the placement and routing implementation to generate the new components in the library. In Fig. 4, we show the output of two devices using this parametric definition in our Verilog netlist allowing resizing of components and automatically generating matching SCAD files. Figure 4a shows a call of a serpentine channel 300 pixels wide with 12 turns and, Fig. 4b with a modified call in the netlist generating only 8 turns. Figure 4c and d demonstrate a similar call for a 50 pixel diameter valve and 100 pixel diameter valve structures similar to valves developed by Noriega et al.24.
Fig. 3.
Representation of the information contained by each file type used as OpenMFDA inputs, a 300px serpentine is used as an example. The LEF file is illustrated based on the geometry space (obstruction), total size with spacing, and port locations which are represented by the different squares. The Verilog-A code is reduced to contain only the essential definitions that include the module header, and analog block that is used in relating the port pressure drops and chemical mixing changes. Lastly, the SCAD file was rendered using OpenSCAD from text with added routing channels.
Fig. 4.
Various parametric cell components, first comparing the call of a serpentine channel with 300 pixel long segment length, 50 pixel short vertical lengths, and (a) 12 turns and then (b) with 8 turns. A parametric valve is additionally compared here with a (c) 100 pixel diameter valve and (d) 50 pixel radius valve.
We successfully demonstrated the ability of the OpenMFDA toolchain, to generate a functional continuous-flow-based microfluidic chip that implemented the mixing of 3 liquid components in a specific ratio to generate an output fluid whose color represented the quantity of Ca2+ in the sample. The chip design used the component library to generate a microfluidic chip compatible with our manufacturing process to meet the flow ratio requirements. The chip successfully implemented the assay to quantify the amount of calcium in the sample based on the absorbance of the Ca2+-arsenazo III complex at 650 nm56,60. The chip could meter each of the different solutions based on the resistances of different inputs when the same pressure was applied at each input with a ratio of 2:25:225 of the sample simulant, arsenazo III, and dilution buffer, respectively. The schematic of the microfluidic chip to be developed by OpenMFDA is shown in Fig. 5. The Verilog AMS and LEF files were modified to meet the requirements of the assay and the software toolchain proceeded from that point.
Fig. 5.
Schematic of the smart toilet device showing the different components for each branch and final target concentration at the final output. Each input would be metered by increasing the resistance by an increase of length with larger serpentine channels.
Placement and routing with openroad
We demonstrated that by using the OpenROAD modules and custom Solid Python scripts, we can successfully layout the requested components from the library in an appropriate arrangement and generate a SCAD file that can be used to generate a 3D rendering of the placed components. The generated SCAD file could be converted to an STL file that is compatible with the slicer and manufacturing infrastructure (Fig. 5) of our DLP 3D printer.
OpenROAD was able to successfully implement, layout, and connect the library components in the Verilog netlist (and related library files that include information about the target printer). The OpenROAD process worked as shown in Fig. 2. The placement program was able to successfully find a location within the device that is not violating any rules, such as component overlap, while using an optimization cost function to guide the process to better solutions. Layout was followed by a routing step which similarly determined the path for the connecting channels between each of the devices as declared in the netlist.
The result of these efforts for the calcium assay chip, as shown in Fig. 6a shows the top view of the placement and routing solution as abstract blocks, and detailed render in Fig. 6b, using OpenROAD, which generated component placement solutions for mixed-sized microfluidic components and the connecting channels61,62. Additionally, these results demonstrate the ability to generate a 3D microfluidic design using OpenROAD by utilizing the layered representations in EDA for component geometries and routing. The vertical space of the chip was defined using the metal layers as routing channel layers that take up the Z-direction of the microfluidic device. To represent the 3D space for the respective component, the LEF file uses obstruction definition layers to prevent routing connections (the next step) through the component and pins are declared as the interface locations for the component ports. The corresponding CAD file was then scaled such that the LEF file could map onto the CAD file, in our case SCAD, so that the outputs of OpenROAD could be used to generate the final STL file to be sliced. Figure 6c and d show the use of the vertical direction by some components and routing at each connection. Additionally, OpenROAD guided the routing channels around taller 3D geometries by treating each 3D component as an obstruction in the plane for routing, as shown in the example device. Of the several modules used to implement the place and route process, we found the variables for the global placer, RePlAce, to be most consequential to the layout and additional routing lengths. Ideally, the objective for a placement and routing system would be to minimize the routing lengths of the device. In our case, the mixing chip function depends on each input flow rate, which is determined by the channel length of each routing path. Due to the nature of parallel resistances and the multiplicative scaling of tolerances, it was found to be very sensitive to parasitic resistances. We found that the function of the device can be significantly affected by the added routing lengths. Since there is no way to avoid adding routing length between components in this approach, we were required to assume some length would be added to the components when developing the netlist for the calcium device. After some optimization, we found that assuming an additional 5 mm in routing length added to the design between each component and a length of 8.5 mm of length to be used for the dilution input, we could generate a flow with proper metering of each chemical. We are working to address this problem in future versions of the OpenMFDA software so that these compensation steps can be automatic. Additionally, the algorithm parameters for the coarse placer, RePlAce for the OpenROAD modules can be adjusted to generate designs that perform the target assay so that the resistance ratios are more consistent with the intended design. Adjustment of these placement algorithm parameters can be treated as an iterative process to enable optimization. These algorithm parameters include initial wirelength coefficient, initial density coefficient, target density, number of bins per side, and max and min phi. We iterated with these parameters until a design could meter each output to the desired concentration below a relative error of less than 10%.
Fig. 6.
Calcium quantification chip design from tool chain rendering the placement and routing solution in OpenSCAD (a), top view with feature details (b), isometric view (c) and side view (d). Red channels are sample input channels, green is indicator input channels, blue is the dilution channel, purple are the mixer components, and yellow is the output channel and component.
Simulation with Verilog-AMS and Xyce
We used Verilog-AMS and an open source SPICE simulator, Xyce, to evaluate the designs generated by the placement and routing module. We were able to encode the fluidic resistance behavior on a by component basis to solve for the pressure and flow rate throughout the entire circuit. Additionally, we demonstrated that we could couple the chemical concentration with the fluid flow in the Verilog-AMS models to better evaluate the chip function as an additional virtual node. From the simulation results, the output chemical concentration was used to validate the final mixing ratio specifications of the calcium quantification chip and respective errors from the design produced from placement and routing step. The relative error in the chemical concentration of the proposed placement and routing solution to the specified design was used to compare the different chip designs produced in the place and route step and enabled an optimization process. Example results of a simulation showing the flow rate at each output node are shown in Table 2. And chemical concentration results are shown in Table 3 for the calcium quantification chip. As noted previously, we used an average added length when designing the original resistance of the components to compensate for the additional routing between components on the chip when the placement and routing step is performed; this resulted in a high error of the pre-routing estimates of the output concentrations. As mentioned previously, assuming an added routing length per component was crucial for generating chips that could function below the tolerable error threshold, as inputs with lower relative contributions to the total volume, on the order of 2 magnitudes, were significantly affected by the variable connections occurring at the other inputs.
Table 2.
Simulated flow rates for the calcium quantification chip for each input.
| Input | Simulated flow rate |
|---|---|
| Dilution buffer | 88.2 µl/min |
| Arsenazo III | 28.6 µl/min |
| Sample (Ca(OH)2) | 0.87 µl/min |
Table 3.
Predicted mixing concentrations and contributions for the calcium quantification chip from the Xyce simulation for our chosen design. Outlet contributions are of percent relative volume the total flow at the output of the system.
| Chemical | Outlet contribution percent |
Expected contribution percent |
Outlet Mixing error | Pre-routing error | Outlet Concentration | Expected Concentration |
|---|---|---|---|---|---|---|
| Dilution Buffer | 90.6% | 89.2% | 0.17% | 11.5% | N/A | N/A |
| Arsenazo III | 9.78% | 10.72% | 1.21% | 93.0% | 391 µM | 396 µM |
| Sample (Ca(OH)2) | 0.086% | 0.08% | 8.08% | 90.3% | 1.51 µM | 1.40 µM |
Fabrication and post processing
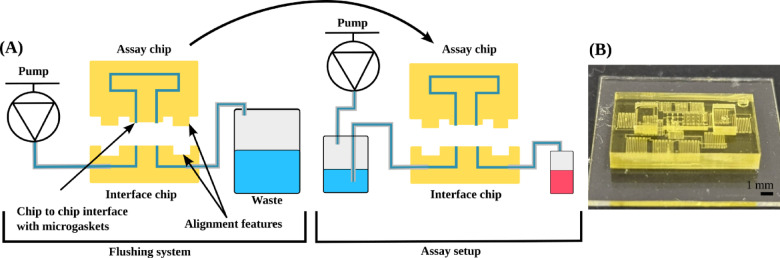
After selecting an automatically generated design from the place and route process based on the simulation results, an STL file of the design was generated. A custom slicer was then used to generate the images to be used on the DLP 3D printer. The microfluidic chip executing the assay was designed in conjunction with a custom interface chip that would be connected to a flushing system to remove the resin remaining in the channels after the printing process. The chip would then be ready to be mounted to a similar interface chip run the assay (Fig. 7a). This interface chip was designed to be a standard geometry to connect the printed device to perform the assay, which we would use for connecting to an auto flushing system that flushed all unpolymerized resin from interior negative space features of the device. A similar interface chip was then used to connect the microfluidic assay chip to a pump array to run the assay. The chip that executed the microfluidic assay additionally contained gasket features to seal with the interface chip (Fig. 7b). Additionally, the design process added spacing around each component and connection to prevent unintended connections, which can occur if the walls between channels are too small or weak, and to facilitate the flushing process. To reliably produce microfluidic chips, it was found that a minimum pitch of 30 pixels (228 μm) was appropriate for printing of 14 pixel (106 μm) wide channels, though larger routing pitch sizes could be used if routing congestion is not an issue. Additional minimum spacing was also provided on the outer edges, which was about 0.5 mm (approximately 60 pixels). These design rules were built into the library configurations, so designs when developed by the automated process would be more likely to be successful.
Fig. 7.
(a) Diagram of flushing system with important features called out to be transferred to the assay setup post flushing, (b) image of a fully flushed smart toilet chip mounted to a glass slide.
Chip demonstration and quantification
After printing and flushing the microfluidic chips, we used the produced chips to quantify the calcium concentration of samples. We were able to produce a linear calibration curve similar to that previously reported56. The results compared to hand pipetted controls of the assay are shown in Fig. 8, and individual point data table S1. Using the passive metering microfluidic chip, we replicated the readings and demonstrated that the chips produced using our MFDA approach yielded an acceptable result with each testing point, having less than 9.2% average error. Each test was run with a unique 3D printed chip, and as a result, each data point represents a value from a different chip (table S2). On average, the experimental errors observed for all chips were 5.0%.
Fig. 8.
Best fit curves for manual pipetting test and measured chip outputs (n = 3).
Discussion
In this work, we introduce OpenMFDA as a tool to support several areas in the microfluidic chip design process that enable automated chip design and fabrication. OpenMFDA is demonstrated as a complete and fully automated process for microfluidic design automation, from design to a usable test chip. The toolchain presents an integrated process for creating physical designs, verifying the designs, and manufacturing the device, as demonstrated through our calcium quantification chip case study.
The OpenMFDA toolchain leverages existing EDA tools such as OpenROAD54 and Xyce55 to automate the design and simulation of microfluidic devices. Since microfluidic devices are relatively simple compared to electronic designs, these tools are very capable of handling the current and future scale for typical microfluidic devices. The OpenMFDA process integrates layout with 3D geometry rendering using OpenSCAD enabling a microfluidic designer to take advantage of truly 3D devices and manufacturing. It should be noted, however, there is at least one downside to using EDA tools. Due to the scale and requirements of IC designs, which can include billions of transistors, assumptions in the software can reflect the scale and domain requirements of electronics in the automation algorithms, partially to simplify each process, trading speed for quality, which can occasionally produce non-optimal chips on the scales applicable to microfluidics. Given the open-source nature of this process and since microfluidics are much simpler than typical EDA problems, future work can utilize these tools to develop domain-specific algorithms that can focus more on the quality of the designs rather than just doing it quickly.
One of the other major contributions of this process is the support of 3D designs with the inclusion of verified components developed around 3D printing. We have developed a process design kit (PDK) like environment, where classes of components can be validated to work within a particular 3D printer. One of the issues with 3D printing in microfluidics is that it usually requires very specific domain knowledge of the process to be utilized effectively. For a microfluidic engineer to fabricate structures such as microvalves, one must understand some of the nuanced aspects of the 3D printing process. We alleviate this issue by implementing our component library in a PDK format by constraining the process so that the engineers who are experts on the process can design the component with layer and pixel alignment built into the process. The availability of the library allows other engineers to easily import the component by a simple inclusion on a netlist while preserving the necessary fabrication implementation details. Future work would be to further implement the PDKs to contain libraries with components verified for a distinct resin and 3D printer pairing so that resins with particular properties, such as high-resolution control, can contain additional subsets of components within the library. Building support for additional printers, however, creates issues when translating dimensional information between pixel resolution and layer height capability. Finally, for a lab to utilize the entire process flow effectively, 3D printing knowledge is still required to maintain high-quality manufacturing output.
It has been noted that one of the issues with microfluidics is the lack of standardization, processes and software that support the design and validation process63. As part of OpenMFDA we have provided a parametric component library that provides basic geometries and common components with the idea being that much of the details of drafting and implementation will be wrapped into a simple interface. The component library includes the parametric microfluidic components, allowing users to develop a range of devices quickly and easily from already demonstrated components. Furthermore, users can generate new components, add them to the library, and create new microfluidic components and devices with little effort, reducing the need for continuously developing and validating new chip designs. The focus on the library shifts the perspective of the design from drafting the component level details to selecting components on a schematic or netlist level. This shift is shown in our example Verilog netlists that list components with connecting wires specified between them in the parentheses following. While we have provided a very primitive library of components for our system, we expect the library to grow beyond our demonstrations. We provide access to the source code of the existing library with tools and instructions to rebuild it to promote modification and extensibility. Additionally, we encourage users to participate and continually share new libraries and components.
Currently, interfacing with the tool requires defining most inputs to the software as text files. Tasks such as adding new components not in the library require the user to interpret and manually define and verify each of three separate files (LEF file, Verilog-AMS file, and SCAD file), which is challenging for many microfluidic engineers. The difficulty of adding new components is partially alleviated through parametric development of the base library, in which new components can be added as derivatives. Access to the current source code can facilitate the development of new components as existing components serve as templates. The formats used in this flow are well-defined standard files in EDA, which should ease some of the transition. The development of parametric components within the library does help support custom sizing but cannot guarantee the functionality of sizing outside of the standard set of components. Additionally, most users will have the ability to export geometries from other CAD software, assuming that the geometry can be exported into a STL format, as OpenSCAD does have the ability to import STL files as model geometry. This feature can reduce the difficulty of generating the CAD files through the current programming like interface. In the future, we expect to simplify the development of new components with a separate file generation tool with simpler user interfaces and library development tools.
Conclusion
Microfluidics is a powerful tool in healthcare, but can be limited in its development by the current design tools, as components and whole chips need to be designed and verified manually by highly trained engineers. In an effort to develop an automated method for the design of microfluidic chips usable by less skilled operators, we have demonstrated an open-source approach using several mature tools adapted from EDA for microfluidic design. We present a portable, extendable, and operating-system agnostic toolchain that can quickly generate and share complex microfluidic designs. Our approach enables microfluidic engineers to design for state-of-the-art manufacturing, as shown with stereolithography 3D printing, and import their own component designs in a way that can be easily transferred to other microfluidic developers. Because we simply encode the designs in textual language, users and developers can easily upload designs and components to repositories such as Metafluidics64AutoPAD65and GitHub and utilize version control much like software. Many open-source tools in EDA have lowered the barrier of entry for engineers, especially when developing complex chips, and we envision a similar role for this MFDA toolchain. The application of the OpenMFDA toolchain to chips generated on a 3D printer further democratizes the ability to design and produce microfluidic chips to the point where the need for a microfluidics expert could be reduced in the design process.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Science Foundation grant number 2140148. 3D printer development at Brigham Young University was supported by the National Institutes of Health grant number R15GM123405.
Author contributions
B.G., S.T., D.W., A.S. contributed to software implementation and toolchain flow design. B.G. designed the 3D printed smart toilet chip and ran verification experiments. S.Z. designed and implemented the automated flushing system. This project was supervised by P.G., G.P.N., B.K.G. All authors contributed to the preparation of the manuscript.
Data availability
All data generated or analyzed has been provided in this published article and supplementary. Additionally, the designs and instructions for using the toolchain are provided on GitHub [https://github.com/utah-MFDA].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foudeh, A. M., Didar, T. F., Veres, T. & Tabrizian, M. Microfluidic designs and techniques using Lab-on-a-Chip devices for pathogen detection for Point-of-Care diagnostics. Lab. Chip. 12 (18), 3249–3266 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Nasseri, B. et al. Point-of-Care microfluidic devices for pathogen detection. Biosens. Bioelectron.117, 112–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitorino, R., Guedes, S., da Costa, J. P. & Kašička, V. Microfluidics for peptidomics, proteomics, and cell analysis. Nanomaterials11 (5), 1118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basha, I. H. K., Ho, E. T. W., Yousuff, C. M. & Hamid, N. H. B. Towards multiplex molecular Diagnosis—A review of microfluidic genomics technologies. Micromachines8 (9), 266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim, S. et al. others. High-Throughput automated microfluidic sample Preparation for accurate microbial genomics. Nat. Commun.8 (1), 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gach, P. C., Iwai, K., Kim, P. W., Hillson, N. J. & Singh, A. K. Droplet microfluidics for synthetic biology. Lab. Chip. 17 (20), 3388–3400 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Huang, H. & Densmore, D. Integration of microfluidics into the synthetic biology design flow. Lab. Chip. 14 (18), 3459–3474 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Bhatia, S. N. & Ingber, D. E. Microfluidic Organs-on-Chips. Nat. Biotechnol.32 (8), 760–772 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Schuster, B. et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun.11 (1), 5271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, R., Liu, Y., Mou, L., Deng, J. & Jiang, X. Microfluidics-Based biomaterials and biodevices. Adv. Mater.31 (45), 1805033 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Roy, S. et al. Recent developments towards portable Point-of-Care diagnostic devices for pathogen detection. Sens. Diagn.1 (1), 87–105 (2022). [Google Scholar]
- 12.Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng.3 (1), 335–373 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Halldorsson, S., Lucumi, E., Gómez-Sjöberg, R. & Fleming, R. M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron.63, 218–231 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharjee, N., Urrios, A., Kang, S. & Folch, A. The upcoming 3D-Printing revolution in microfluidics. Lab. Chip. 16 (10), 1720–1742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen, A. V., Beauchamp, M. J., Nordin, G. P. & Woolley A. T. 3D printed microfluidics. Annu. Rev. Anal. Chem.13 (1), 45–65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sochol, R. et al. others. 3D printed microfluidic circuitry via Multijet-Based additive manufacturing. Lab. Chip. 16 (4), 668–678 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enders, A., Siller, I. G., Urmann, K., Hoffmann, M. R. & Bahnemann J. 3D printed microfluidic Mixers—a comparative study on mixing unit performances. Small15 (2), 1804326 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Childs, E. H., Latchman, A. V., Lamont, A. C., Hubbard, J. D. & Sochol, R. D. Additive assembly for PolyJet-Based Multi-Material 3D printed microfluidics. J. Microelectromechanical Syst.29 (5), 1094–1096 (2020). [Google Scholar]
- 19.Nelson, M. D., Ramkumar, N., Gale, B. K. & Flexible Transparent, Sub-100 Μm microfluidic channels with fused deposition modeling 3D-Printed thermoplastic polyurethane. J. Micromechanics Microengineering. 29 (9), 095010 (2019). [Google Scholar]
- 20.Quero, R. F., da Silveira, G. D., da Silva, J. A. F. & de Jesus, D. P. Understanding and improving FDM 3D printing to fabricate High-Resolution and optically transparent microfluidic devices. Lab. Chip. 21 (19), 3715–3729 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Romanov, V. et al. FDM 3D printing of High-Pressure, Heat-Resistant, transparent microfluidic devices. Anal. Chem.90 (17), 10450–10456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su, R. et al. C. 3D printed Self-Supporting elastomeric structures for multifunctional microfluidics. Sci. Adv.6 (41), eabc9846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amini, A., Guijt, R. M., Themelis, T., De Vos, J. & Eeltink, S. Recent developments in digital light processing 3D-Printing techniques for microfluidic analytical devices. J. Chromatogr. A. 1692, 463842 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Sanchez Noriega, J. L. et al. others. Spatially and optically tailored 3D printing for highly miniaturized and integrated microfluidics. Nat. Commun.12 (1), 5509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo, A. P. et al. High-Precision stereolithography of biomicrofluidic devices. Adv. Mater. Technol.4 (6), 1800395 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez, D. et al. 3D Printing-Enabled uniform temperature distributions in microfluidic devices. Lab. Chip. 22 (22), 4393–4408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warr, C. A. et al. 3d-Printed microfluidic droplet generator with hydrophilic and hydrophobic polymers. Micromachines12 (1), 91 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Z., Martin, N., Hini, D., Mills, B. & Kim, K. Rapid fabrication of multilayer microfluidic devices using the liquid crystal Display-Based stereolithography 3D printing system. 3D Print. Addit. Manuf.4 (3), 156–164 (2017). [Google Scholar]
- 29.Shafique, H. et al. High-Resolution Low-Cost LCD 3D printing for microfluidics and Organ-on-a-Chip devices. Lab. Chip. 24 (10), 2774–2790 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Leong, K. M. et al. Democratizing access to microfluidics: rapid prototyping of open microchannels with Low-Cost LCD 3D printers. ACS Omega. 9 (45), 45537–45544 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luitz, M. et al. Fabrication of embedded microfluidic chips with single micron resolution using Two-Photon lithography. Adv. Mater. Technol.8 (22), 2300667 (2023). [Google Scholar]
- 32.Perrucci, F. et al. others. Optimization of a suspended two photon polymerized microfluidic filtration system. Microelectron. Eng.195, 95–100 (2018). [Google Scholar]
- 33.Lee, Y. S., Bhattacharjee, N. & Folch, A. 3D-Printed Quake-Style microvalves and micropumps. Lab. Chip. 18 (8), 1207–1214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadianyazdi, A., Miller, I. J. & Folch, A. Tunable resins with PDMS-like elastic modulus for stereolithographic 3D-Printing of multimaterial microfluidic actuators. Lab. Chip. 23 (18), 4019–4032 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong, H., Woolley, A. T. & Nordin, G. P. 3D printed high density, reversible, Chip-to-Chip microfluidic interconnects. Lab. Chip. 18 (4), 639–647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, R., Wang, F. & McAlpine, M. C. 3D printed microfluidics: advances in strategies, integration, and applications. Lab. Chip. 23 (5), 1279–1299 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez, G., Roppolo, I., Pirri, C. F. & Chiappone, A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit. Manuf.55, 102867 (2022). [Google Scholar]
- 38.Gong, H., Woolley, A. T. & Nordin, G. P. 3D printed selectable Dilution mixer pumps. Biomicrofluidics13 (1), 014106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinnen, H., Viglione, M., Munro, T. R., Woolley, A. T. & Nordin, G. P. 3D-Printed microfluidic One-Way valves and pumps. Micromachines14 (7), 1286 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbard, J. D. et al. Fully 3D-Printed soft robots with integrated fluidic circuitry. Sci. Adv.7 (29), eabe5257 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaugh, C. E. A New Approach for 3D Printed Microfluidic Device Design Based on Pre-Defined Components. PhD Thesis, Brigham Young University, (2022).
- 42.Tseng, T. M., Li, M., Zhang, Y., Ho, T. Y. & Schlichtmann, U. Cloud Columba: Accessible Design Automation Platform for Production and Inspiration. In 2019 IEEE/ACM International Conference on Computer-Aided Design (ICCAD); IEEE, ; pp 1–6. (2019).
- 43.Sanka, R., Lippai, J., Samarasekera, D., Nemsick, S. & Densmore D. 3dµf-Interactive design environment for continuous flow microfluidic devices. Sci. Rep.9 (1), 9166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y., Li, M., Tseng, T. M. & Schlichtmann, U. Open-Source interactive design platform for 3D-Printed microfluidic devices. Commun. Eng.3 (1), 71 (2024). [Google Scholar]
- 45.Yao, H., Wang, Q., Ru, Y., Cai, Y. & Ho, T. Y. Integrated Flow-Control codesign methodology for Flow-Based microfluidic biochips. IEEE Des. Test.32 (6), 60–68 (2015). [Google Scholar]
- 46.Grimmer, A., Wang, Q., Yao, H., Ho, T. Y. & Wille, R. Close-to-Optimal Placement and Routing for Continuous-Flow Microfluidic Biochips. In 2017 22nd Asia and South Pacific Design Automation Conference (ASP-DAC); IEEE, ; pp 530–535. (2017).
- 47.Minhass, W. H. et al. Scheduling and fluid routing for Flow-Based microfluidic Laboratories-on-a-Chip. IEEE Trans. Comput. -Aided Des. Integr. Circuits Syst.37 (3), 615–628 (2017). [Google Scholar]
- 48.Wang, Q. et al. Physical Co-Design of flow and control layers for flow-Based microfluidic biochips. IEEE Trans. Comput. -Aided Des. Integr. Circuits Syst.37 (6), 1157–1170 (2017). [Google Scholar]
- 49.Oh, K. W., Lee, K., Ahn, B. & Furlani, E. P. Design of Pressure-Driven microfluidic networks using electric circuit analogy. Lab. Chip. 12 (3), 515–545 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Takken, M. & Wille, R. Accelerated computational fluid dynamics simulations of microfluidic devices by exploiting higher levels of abstraction. Micromachines15 (1), 129 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fink, G., Costamoling, F., Wille, R. M. M. F. T. & Droplet Simulator Efficient simulation of Droplet-Based microfluidic devices. Softw. Impacts. 10.1016/j.simpa.2022.100440 (2022). [Google Scholar]
- 52.McDaniel, J., Grover, W. H. & Brisk, P. The Case for Semi-Automated Design of Microfluidic Very Large Scale Integration (mVLSI) Chips. In Design, Automation & Test in Europe Conference & Exhibition (DATE), ; IEEE, 2017; pp 1793–1798. (2017).
- 53.Ibrahim, M., Sridhar, A., Chakrabarty, K. & Schlichtmann, U. Synthesis of reconfigurable Flow-Based biochips for scalable Single-Cell screening. IEEE Trans. Comput. -Aided Des. Integr. Circuits Syst.38 (12), 2255–2270 (2018). [Google Scholar]
- 54.Ajayi, T., Blaauw, D. & Openroad Toward a Self-Driving, Open-Source Digital Layout Implementation Tool Chain. In Proceedings of Government Microcircuit Applications and Critical Technology Conference; (2019).
- 55.Verley, J., Keiter, E. R., Thornquist, H. K. & Xyce Open Source Simulation for Large-Scale Circuits.; Sandia National Lab.(SNL-NM), Albuquerque, NM (United States), (2018).
- 56.Tazin, N. et al. Automated Passive Serial Dilution Microfluidic Chip for Calcium Quantification Based on the Arsenazo III Method. Sens. Diagn. published on the web. (2022).
- 57.LEF/DEF 5.8 Language Reference. (2017). https://coriolis.lip6.fr/doc/lefdef/lefdefref/lefdefref.pdf
- 58.Temple, S., Neto, W. L., Austin, M., Tang, X. & Gaillardon, P. E. Lsoracle: Using Mixed Logic Synthesis in an Open Source Asic Design Flow. In Workshop on Open-Source EDA Technology; (2021).
- 59.Gong, H., Bickham, B. P., Woolley, A. T. & Nordin, G. P. Custom 3D printer and resin for 18 µm x 20 µm microfluidic flow channels. Lab. Chip. 17 (17), 2899–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boonyasit, Y. et al. Passive micromixer integration with a microfluidic chip for calcium assay based on the Arsenazo III method. BioChip J.5, 1–7 (2011). [Google Scholar]
- 61.Cheng, C. K., Kahng, A. B., Kang, I., Wang, L. & Replace Advancing solution quality and routability validation in global placement. IEEE Trans. Comput. -Aided Des. Integr. Circuits Syst.38 (9), 1717–1730 (2018). [Google Scholar]
- 62.Lu, J. et al. ePlace: Electrostatics-Based placement using fast fourier transform and nesterov’s method. ACM Trans. Des. Autom. Electron. Syst. TODAES. 20 (2), 1–34 (2015). [Google Scholar]
- 63.Gurkan, U. A. et al. others. Next generation microfluidics: fulfilling the promise of Lab-on-a-Chip technologies. Lab. Chip. 24 (7), 1867–1874 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong, D. S. et al. Open-Source, Community-Driven microfluidics with metafluidics. Nat. Biotechnol.35 (6), 523–529 (2017). [DOI] [PubMed] [Google Scholar]
- 65.DeChiara, N. S., Wilson, D. J. & Mace, C. R. An open software platform for the automated design of Paper-Based microfluidic devices. Sci. Rep.7 (1), 16224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed has been provided in this published article and supplementary. Additionally, the designs and instructions for using the toolchain are provided on GitHub [https://github.com/utah-MFDA].