Abstract
Particular attention has been paid to the nutritional potential of edible insects as well as the health benefits associated with their bioactive compounds. This paper focused on an in-depth review compiling the most recent information on health benefits of insect bioactive metabolites as well as their purification and identification, in addition to consumer attitudes towards edible insects. It was found that, insect bioactive metabolites, including marcocarpal, grandinol, trolline, pancratistatin, narciclasin, ungeremin, cantharidin, cordycepin, roseoflavin, lecithin, reblastatin, chitin, chitosan and desmosterol deemed to have biological activities, such as tumor suppression, anticancer, antihypertensive, anti-inflammatory, antioxidant, immunomodulator, neuroprotective, glycemic and lipid regulation, blood pressure reduction, regulation of intestinal bacterial flora and cardiovascular protection among others. Furthermore, proper sample preparation and extraction is the first step in the purification of bioactive metabolites from edible insects. After concentration, bioactive metabolites are purified using chromatographic and separation techniques including High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Thin-Layer Chromatography (TLC), Size-Exclusion Chromatography (SEC). Finally, their nutritional potential, health benefits, environmentally friendly, great taste, traditions, taboo, safety concerns, unpleasant past experiences, allergies, and unnaturalness are among the main factors influencing attitudes towards insect consumption.
Subject terms: Biochemistry, Biotechnology
Introduction
With a rapidly growing world population1 and the goal of promoting healthier as well as sustainable food systems2, there is a growing demand for alternative proteins3. This situation is exacerbated by the scarcity of essential arable land4, environmental pressures linked to the uncertainties of climate change5,6. Edible insects are thus seen as a formidable alternative to address the issues of global food insecurity7 for their nutritional potential8,9, taste10, economic benefits11,12, environmental benefits13, as well as their potential health benefits14.
In many parts of the world, entomotherapy is used as medicine and is an important alternative to modern therapy through their bioactive metabolites including pancratistatin, narciclasin, ungeremin, cantharidin, cordycepin, roseoflavin, lecithin, reblastatin, chitin, and chitosan15,16. These bioactive compounds present important physiological effects on living organisms through their physiological properties encompassing anti-obesity, antihypertensive, antithrombotic, antioxidant, hypocholesterolemic, antimicrobial, opioid, cytomodulatory, anti-inflammatory, cardioprotective, immunomodulatory, antiangiogenic, and immunomodulatory activities14,17.
Given their diverse functions, high bioavailability and efficacy even at low concentrations, bioactive compounds attract a great deal of attention, although some bioactive compounds are naturally present in isolation, many are hidden within the intact structure18. Even though effort is being made, consumer attitudes and willingness to consume insects remain a major challenge in many societies19, due to traditions, superstitions and taboos as well as familiarity with insect20, their appearance and great taste8,21.
Considering the attention paid to insects as food and feed, this review compiled the most recent information focusing on health benefits of insect bioactive metabolites as well as their purification and identification, and finally a particular attention was paid to sensory attributes and consumer attitudes towards edible insects.
Potential health benefits of insect bioactive metabolites
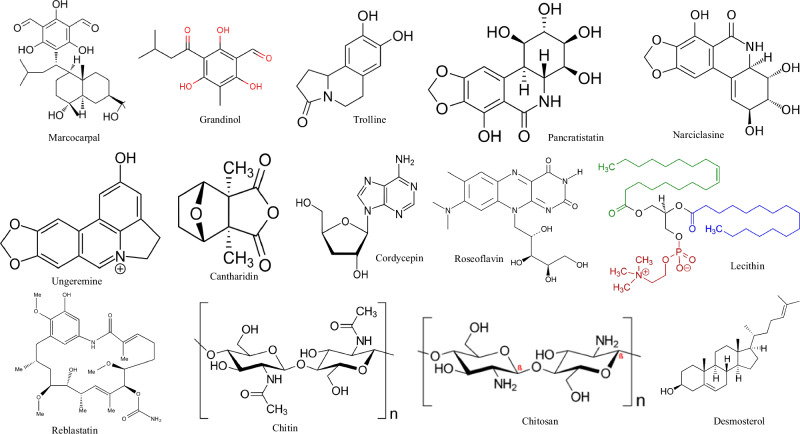
Insects are characterized by several bioactive metabolites, including marcocarpal, grandinol, trolline, pancratistatin, narciclasine, ungeremine, cantharidin, cordycepin, roseoflavin, lecithin, reblastatin, chitin, chitosan and desmosterol (Fig. 1), which confer a variety of beneficial biological activities to human health, including tumor suppression, anti-cancer, anti-hypertensive, anti-inflammatory, antioxidant, immunomodulatory, neuroprotective, blood sugar and lipid regulation, blood pressure reduction, regulation of intestinal bacterial flora and cardiovascular protection (Table 1). While Fig. 2 illustrates the biological activities of bioactive insect metabolites and their mechanisms of activity, detailed information associating insect species to their bioactive metabolites is depicted in Table 1.
Fig. 1.
Health benefits of insect bioactive metabolites and their potential mechanisms.
Table 1.
Potential health benefits of bioactive metabolites found in edible insects
| Scientific name | Metabolite | Biological activity and study description | Reference |
|---|---|---|---|
| Blattodea | |||
| Macrotermes natalensis | Actinomycin D | Antibacterial, antitumor (In vitro). Assessment using two different bioassays. | Benndorf et al.22 |
| Rubterolone A–F | Farnesyl-protein transferase inhibitor (In vitro). Assessment using two different bioassays. | Benndorf et al.22 | |
| Barceloneic acid A | Benndorf et al.22 | ||
| Periplaneta americana | Periplatins A–D | Antiproliferative (In vitro). Significant cytotoxic activities in HepG2 and MCF-7 cells with IC50 values in the ranges 6.41-23.91 μM and 6.67-39.07 μM was observed. Isolated from the 70% ethanol extract of the whole body. | Luo et al.23 |
| Polyphaga plancyi | Eupolyphagin |
Antiproliferative. Two series of novel 2-substituted-4-amino-6-halogenquinolines 8a-l and 13a-H were designed, synthesized and evaluated for their antiproliferative activity against H-460, HT-29, HepG2 and SGC-7901 cancer cell lines in vitro. IC50 values of 0.03 μM, 0.55 μM, 0.33 μM and 1.24 μM. |
Jiang et al.112 |
| Polyphaga plancyi | Plancyamides A; and B, plancypyrazine A; plancyols A and B | Antiproliferative activity evaluated toward extracellular matrix in animal model (rat renal proximal tubular cells), human cancer cells (K562, A549, and Huh7), EV71, ROCK2, JAK3, DDR1, and coagulation. | Zhu et al.113 |
| Odontotermes formosanus | 5-Hydroxyramulosin and biatriosporin M | Antifungal and antibacterial. The phylogenetic diversity of fungi isolated from O. formosanus was investigated by dilution-plate method, combined with morphological characteristics and 5.8S rDNA sequencing. The antimicrobial activities of all endophytic fungi extracts were tested by using the filter paper method against E. coli (ATCC 8739), B. subtilis (ATCC 6633), S. aureus (ATCC 6538), and C. albicans (ATCC 10231). Medium inhibitory activities against B. subtilis and S. aureus, with the IZD range of 8.32-9.13 mm was observed. | Xu et al.64 |
| 1-(2,5-Dihydroxyphenyl)-3-hydroxybutan- 1-one | Xu et al.64 | ||
| Roseoflavin | Xu et al.64 | ||
| Roseoflavin | Zhou et al.65 | ||
| 8-methylamino-8-demethyl-d-riboflavin | Zhou et al.65 | ||
| Periplaneta americana | Isocoumarins periplatins A–D | Cytotoxic activities against human liver (HepG2) and breast cancer (MCF-7) cells with IC50 values in the ranges 6.41-23.91 μM and 6.67-39.07 μM. | Luo et al.23 |
| Polyphaga plancyi | Plancyamide A; Plancypyrazine A; Plancypyrazine B; Plancyol A | Antiproliferative activity evaluated toward extracellular matrix in rat renal proximal tubular cells, human cancer cells (K562, A549, and Huh7), EV71, ROCK2, JAK3, DDR1, and coagulation. | Zhu et al.113 |
| Macrotermes natalensis | Natalamycin A | Bioassay-guided fractionation based on antifungal activity led to the isolation of natalamycin A, Geldanamycin and Reblastatin (In vitro). | Kim et al.114 |
| Geldanamycin | |||
| Reblastatin | |||
| Termisoflavones A–C | Improved cisplatin-induced kidney cell damage to 80% of the control value at a cisplatin dose of 25 μM (In vitro). | Kang et al.115 | |
| Isoflavanoids | |||
| Dentigerumycins B–D | Cisplatin-induced cytotoxity. The structures of the complex nonribosomal peptide synthetase-polyketide synthase (NRPS/PKS) hybrid bioactive compounds were determined by 1D- and 2D-NMR spectroscopy, high-resolution mass spectrometry, and circular dichroism (CD) spectroscopy. | Wyche et al.116 | |
| Macrotermycin A–C | Bioassay-guided metabolomic analyses. Macrotermycins A and C had antibacterial activity against human-pathogenic S. aureus and a selective antifungal activity against a fungal parasite of the termite fungal garden. | Beemelmanns et al.31 | |
| Banegasine, Cyclo-NMe-L-3,5-dichlorotyrosine-Dhb | Antifungal (In vitro). Antifungal activity assessed using two different bioassays. | Benndorf et al.22 | |
| Rubrominin A–B | |||
| Microtermolide A–B | Farnesyl-protein transferase inhibitor. Microtermolides A and B were isolated from a Streptomyces sp. strain associated with fungus-growing termites. 1D- and 2D-NMR spectroscopy and high-resolution mass spectrometry were used to determine the structures of A and B. | Carr et al.117 | |
| Drimenol-type sesquiterpenes | Antibacterial (In vitro). Formation of two structurally related monocyclic sesquiterpenes (nectrianolines) was catalyzed by heterologously expressed enzymes potentially involved in terpene biosynthesis. | Kreuzenbeck et al.63 | |
| Natalenamides A–C | Inhibitory effects on 3-isobutyl-1-methylxanthine (IBMX)-induced melanin production (In vitro). | Lee et al.32 | |
| Macrotermes spp. | Efomycin K, Efomycin L, Efomycin M, Efomycin G and Elaiophylin | Antifungal. Phylogenetic analysis of gene cluster domains was used to provide a biosynthetic rational for these new derivatives. | Klassen et al.72 |
| Efomycin M | Inhibited selectin-mediated leukocyte rolling In vivo inflammatory skin models using transplanted human skin biopsies. | von Bonin et al.118 | |
| Roseoflavin, 8-methylamino-8-demethyl-; D-riboflavin; Natalamycin; Termisoflavones A-C | Antibacterial and antifungal (In vitro). | Zhou et al.65 | |
| Coleoptera | |||
| Blaps japanensis | Blapsols A–D | Structures determined by means of spectroscopic and X-ray crystallographic methods. Chiral HPLC was used to separate (-)- and (+)-enantiomers of compounds 1-4, which were isolated from Blaps japanensis as racemic mixtures. Effects towards COX-2 with IC50 values in the range of 1.3-17.8 μM were observed. Multiple assays including anti-tumor, anti-inflammatory, and renal protection activities were determined using in vitro biological evaluations. | Seabrooks & Hu17 |
| Yan et al.42 | |||
| Catharsius molossus | Molossusamides A–C | Antibacterial and anti-inflammatory (In vitro). Cytotoxicity, MDCK cell based anti-influenza, EV71 inhibition and cyclooxygenase inhibitory assays were used to evaluate the biological activities of all the compounds. | Lu et al.67 |
| Cantharis vesicatoria | Cantharidin and norcantharidin | Caprine luteal cell steroidogenesis inhibitor. Steroidogenic effects of cantharidin and norcantharidin (0.1, 1.0, and 10 μg ml-1) were assessed from luteal cells isolated from corpora lutea of native Taiwan goats maintained in vitro and treated for 4 and 24 h. | Twu et al.119 |
| Copris tripartitus | Tripartin | Histone H3 lysine 9 demethylase KDM4 inhibitor (In vitro). | Kim et al.120 |
| Coprisamides A–B | Quinone reductase inducer (In vitro). | Kim et al.121; Um et al.122 | |
| Coprisidin A | Na + /K+ ATPase inhibitor (In vitro). | ||
| Coprisidin B | NAD(P)H:quinone oxidoreductase 1 inducer (In vitro). | ||
| Tripartilactam | Its structure was elucidated by the combination of NMR, MS, UV, and IR spectroscopy and multistep chemical derivatization. Tripartilactam was evaluated as a Na + /K+ ATPase inhibitor (IC50 = 16.6 μg/mL) in vitro. | Park et al.123 | |
| Dendroctonus frontalis | Mycangimycin, Frontalamide A, and Frontalamide B | Antimalarial (In vitro). Genome analyses and genetic manipulation of the producing organism led to the identification of the frontalamide biosynthetic gene cluster and several biosynthetic intermediates. | Blodgett et al.124 |
| Holotrichia diomphalia | Tricin, palmitinic acid; eicosane | Antifungal (In vitro). Chemical compositions of the fatty oils were obtained by two different methods and determined by GC/MS. | Dong et al.73 |
| Hycleus lunata | Cantharidin | Antitumor in mice model. Cantharidin treatment induced abnormal mitochondrial characteristics, with a decrease in mitochondrial glutathione, succinate dehydrogenase activity, mitochondrial membrane potential, and induced apoptosis and necrosis in DL cells. | Prasad & Verma125 |
| Cantharidin and norcantharidin | SoNar, a highly responsive NAD + /NADH sensor, allows high-throughput Metabolic screening of anti-tumor agents in vitro and in vivo. | Zhao et al.126 | |
| Palasonin and Palasoninimide | Whole specimens of Hycleus lunata or body components were hydrolysed with 50-300 µl 6 N hydrochloric acid at 120 °C for 4 h. Each Hycleus extract was injected in a capillary glass chromatograph. Protein phosphatase 2 A inhibitors. | Dettner et al.127 | |
| Cantharidin | Antiproliferative; immunomodulatory | Lang & Lang128 | |
| Mylabris phalerata | Cantharidin and norcantharidin | Caprine luteal cell steroidogenesis inhibitor. Steroidogenic effects of cantharidin and norcantharidin (0.1, 1.0, and 10 μg ml-1) were assessed from luteal cells isolated from corpora lutea of native Taiwan goats maintained in vitro and treated for 4 and 24 h. | Twu et al.119 |
| Onthophagus lenzii | Lenzimycins A–B | Selective isolation of bacterial strains associated with the dung beetle, O. lenzii. PTH23 and lenzimycins A and B (1-2) inhibited the growth of Bacillus sp. CCARM 9248 and of some human pathogenic bacteria, including E. faecium and certain strains of E. faecalis. | An et al.68 |
| Tenebrio molitor | Defatted larvae | Diet enriched with defatted larvae of the mealworm Tenebrio molitor (TM) endowed with ACE inhibitory activity was studied in both spontaneously hypertensive rats (SHR) and in the age-matched normotensive Wistar Kyoto strain fed for 4 weeks with standard laboratory rodent chow supplemented with or without TM or captopril. In SHR, the TM diet led to a significant reduction in blood pressure, heart rate and coronary perfusion pressure, as well as an increase in red blood cell glutathione/glutathione disulphide ratio. Rat brain slices of SHR were more resistant to oxidative stress with lower levels of inflammatory cytokines. | Pessina et al.30 |
| Peptides CSR, APVAH, PAALST, AAGAPP AR and APYF | Fractionation and identification of dipeptidyl peptidase IV (DPP-IV) and α-glucosidase inhibitory peptides was carried out. Peptides from 500 to 1600 Da showed the highest level of DPP-IV inhibition with IC50 value of 0.91 mg ml−1 and peptides below 500 Da showed the highest level of α-glucosidase inhibition with IC50 value of 2.58 mg ml−1. | Rivero-Pino et al.129 | |
| Tenebrio molitor Larvae | Peptides NYVADGLG, AAAPVAVAK, YDDGSYKPH | Antioxidant and anti-inflammatory activities of peptide fractions from hydrolysates obtained by in vitro gastrointestinal digestion. Peptide fraction from the T. molitor protein preparation revealed the highest Fe2+ chelating ability with EC50 value 2.21 μg mL−1 and the highest reducing power (0.198). ACE, pancreatic lipase, and α-glucosidase inhibitory in vitro were reported. | Zielinska et al.130,131 |
| Diptera | |||
| Hermetia illucens | Pyrone derivatives | Compounds from C. multifidum, a fungus with moderate antimicrobial activity isolated from H. illucens gut microbiota. Extract from C. multifidum resulted in a moderate activity against a strain of methicillin-resistant S. aureus (MRSA). Bioguided isolation of the extract showed the characterization of six α-pyrone derivatives (1-6) and one diketopiperazine (7). Among these compounds, 5,6-dihydro-4-methoxy-6-(1-oxopentyl)-2H-pyran-2-one (4) showed the greatest activity with IC50 = 11.4 ± 0.7 μg/mL and MIC = 62.5 μg/mL against MRSA. | Correa et al.69 |
| Diketopiperazine | |||
| Photoinduced, melanins and ommochromes | Antibacterial, antifungal, antioxidant, and anti-inflammatory. Isolated pigments were analyzed by HPLC and represented a mixture of several ommochromes of the ommatin series. | Dontsov et al.44; Richter et al.34 | |
| Defensins-DLP2 and DLP4 | Peptides-DLP2 and DLP4 showed potent antimicrobial activity against Gram-positive bacteria. The survival of mice challenged with MRSA were 80-100% at the doses of 3-7.5 mg/kg DLP2 or DLP4. The bacterial translocation burden over 95% in spleen and kidneys and serum pro-inflammatory cytokines levels were reduced by DLP2 and DLP4, and promoted anti-inflammatory cytokines levels; as well as improved lung and spleen injury. | Li et al.47 | |
| Bioactive peptides | Antioxidant activity. Proteomics-based analysis was used to characterize and quantify functional proteins and bioactive peptides produced from the enzymatic digestion of H. illucens fed with food wastes. The 60S ribosomal protein L5 (RpL5) in BSF interacted with a variety of ribosomal proteins and played a key role in the glycolytic process (AT14039p). Higher antioxidant activity was observed in peptide sequences such as GYGFGGGAGCLSMDTGAHLNR, VVPSANRAMVGIVAGGGRIDKPILK, AGLQFPVGR, GFKDQIQDVFK, and GFKDQIQDVFK. | Lu et al.43 | |
| Musca domestica | Protein-enriched fraction (PEF) | Antiviral, immunomodulatory, and free radical scavenging activities of PEF isolated from the larvae of the housefly was assessed. Infection of avian influenza virus H9N2 and had a virucidal effect against the multicapsid nucleopolyhedrovirus of the alfalfa looper, A. californica Speyer inhibited in vitro. Excellent scavenging activity for 1,1-diphenyl-2-picrylhydrazyl and superoxide anion radicals were similar to those of ascorbic acid. | Ai et al.48 |
| Hemiptera | |||
| Aspongopus chinensis | (±)-Aspongamide A | Inhibition of Smad3 phosphorylation in transforming growth factor-β1 (TGF-β1) induced rat renal proximal tubular cells and suppressed extracellular matrix expression in mesangial cells under diabetic conditions. | Yan et al.132; Shi et al.133 |
| Aspongopusamides A–D | Anti-inflammatory and therapeutic agents against renal protection in high-glucose-induced mesangial cells and COX-2 inhibition. | Shi et al.133 | |
| Kerria lacca | Shellolic acid A | Isolated from Shellac. HR-ESI-MS, UV, IR, 1D and 2D NMR methods were used to elucidate the structures. The Mosher’s method, circular dichroism (CD) and optical rotation analyses were used for absolute configurations. Cytotoxic and anti-bacterial activities of the isolates were evaluated. Inhibitory activity against B. subtilis with the MIC value of 0.1 mg/mL. | Lu et al.53 |
| Hymenoptera | |||
| Apis mellifera | Lupeol, lupenone; Lupeol acetate | This paper proposed a new accelerated solvent extraction method to obtain low-polarity actives with a high cytotoxic profile from red propolis. To identify the chemical compounds, the extracts were analyzed by gas chromatography-mass spectrometry. To profile the cytotoxicity of the bioactives obtained, the 3-(4,5-dimethyl-2-thiazole)-2,5-diphenyl-2-H-tetrazolium bromide colorimetric assay was performed on different tumor cell lines (HCT116 and PC3). The extract obtained at 70 °C and a 10-minute extraction cycle showed the highest cytotoxic activity against the cell lines tested. | de Carvalho et al.134 |
| Apis mellifera pupae | Polypeptide components (BPP-21 and BPP-22) | Immunomodulatory activity in vivo and in vitro. Protein hydrolysis using alkaline protease yielded novel bee chrysalis polypeptides (BPP). A diethylaminoethylsepharose fast-flow column and a Sephadex G-25 column were used to isolate and purify two purified polypeptide components (BPP-21 and BPP-22). Identification was made using HPLC size exclusion and amino acid composition analyses. BPP-22 significantly increased the delayed-type hypersensitivity reaction, cytokine levels (interleukin (IL)-2 and interferon (IFN)-γ), immunoglobulin (Ig) levels (IgA, IgG and IgM) and common blood indices in immunosuppressed mice treated with cyclophosphamide. BPP-22 potentially promoted cytokine secretion (IL-2, tumor necrosis factor-α and IFN-γ) and nitric oxide production by increasing homologous mRNA expression, and could exert immunomodulatory activity by increasing phosphorylation of ERK and p38, and modulating expression of intranuclear transcription factors (EIK-1, MEF-2 and CREB) in the MAPK signaling pathway. | Chen et al.50 |
| Polyrhachis dives | (±)-Polyrhadopamine A, ( ± )-Polyrhadopamine B, ( ± )-Polyrhadopamine C, trolline, ( ± )-Polyrhadopamine C; β-carboline-3-carboxamide 5-(3-indolylmethyl)- nicotinsaureamide | Thirteen non-peptidic nitrogen compounds were isolated from Polyrhachis dives. Multiple assays, including renal protection, T and B cell proliferation, TNF-α, COX-1, COX-2 and Jak3 kinase inhibition were used to determine their biological potential. Several of these non-peptidic nitrogen compounds have shown immunosuppressive, anti-inflammatory and renoprotective properties. | Tang et al.35,132 |
| Polybia paulista | Polybioside | From the venom of the social wasp P. paulista was used to isolate the polybioside (1) with a structure attributed to 3,4,5-trihydroxy-6-(hydroxymethyl) tetrahydro-2H-pyran-2-yl 3-(1H-imidazol-4-yl) propanimidate by NMR and MS protocols. Application of the polybioside in the rat brain, followed by detection of c-Fos protein expression in certain brain regions, indicated that the compound is neuroactive in a number of brain areas, and induces convulsions in rats, even when applied peripherally. | Saidemberg et al.51 |
| Tetraponera rufonigra | Tetraponerins | A general stereocontrolled methodology was applied to access all natural tetraponerins from (+)-T1 to (+)-T8. Their anticancer activity against four different human cell lines, notably the MCF-7 breast carcinoma cell line, was observed through the cytotoxic activity of (+)-T7. | Bosque et al.52 |
| Solenopsis invicta | Solenopsin A | The SVR angiogenesis assay was used to isolate solenopsine, an alkaloid component of the fire ant (Solenopsis invicta), which inhibited phosphatidylinositol-3-kinase signaling and angiogenesis. | Arbiser et al.135 |
| Lepidoptera | |||
| Bombyx mori | Hexapeptide | Ultramicroprocessed silkworm chrysalis protein alkalase hydrolysate was used to purify a novel immunomodulatory hexapeptide using sephadex gel filtration chromatography and reverse-phase high-performance liquid chromatography. The purified peptide had a molecular mass of 656.17 Da and an amino acid sequence of Pro-Asn-Pro-Asn-Thr-Asn (PNPNTN). Splenocyte proliferation was 87.35% in the presence of 100 μg/ml of purified peptide. | Li et al.49 |
| Bombyx mori pupa | Peptides SQSPA, QPGR, NSPR, QPPT, KHV and GNPWM. | α-glucosidase in silico SGID, simulated gastrointestinal digestion (pepsin, trypsin, pancreatin, depending on the authors). | Zhang et al.136 |
| Bombyx mori | Alkaloids and flavonoids | The anti-diabetic effects of the α-glucosidase inhibitor 1-deoxynojirimycin (DNJ) isolated from B. mori were evaluated in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an established animal model of human type 2 diabetes mellitus, and Long-Evans Tokushima Otsuka (LETO) rats were used as control. DNJ treatment resulted into significant anti-diabetic effects in LETO rats, with significant improvements in fasting blood glucose and glucose tolerance and, in particular, an increase in insulin sensitivity. | Kong et al.137; Liu et al.138 |
| Byasa polyeuctes | Papilistatin | A novel inhibitor of cancer cell growth, papilistatin was isolated by bioassay-guided separation of an extract from the wings of a Taiwanese butterfly, Byasa polyeuctes termessa. Analysis of 1D and 2D NMR spectra and by HRMS was used to determine its structure. Papilistatin showed cancer cell growth inhibition with GI50’s of 0.093-3.5 μg/mL against a panel of six human and murine P388 cancer leukemia cell lines, as well as antibacterial activity. | Pettit et al.71 |
| Orthoptera | |||
| Brachystola magna | Pancratistatin; Narciclasine; Ungeremine | Anticancer and antiproliferative in vitro. | Seabrooks & Hu17 |
| Pettit et al.139 | |||
| Gryllodes sigillatus | Peptides IIAPPER, KVEGDLK, LAPSTIK, VAPEEHPV, YKPRP, PHGAP and VGPPQ | α-Amylase, a-glucosidase, DPP-IV, ACE inhibition, lipase inhibition and anti-inflammatory. | Hall et al.140; Zielinska et al.130,131 |
| Schistocerca gregaria | Desmosterol, (3β, 5α) cholesta-8, 14, 24-trien-3-ol, 4, 4-dimethyl, (3β, 20 R) cholesta-5, 24-dien-3, 20-diol | Antimicrobial | Cheseto et al.55 |
| α-Glucosidase (AIGVGAIER, GKDAVIV, FDPFPK and YETGNGIK) | ACE and lipase inhibition, antioxidant and anti-inflammatory. | Zielinska et al.130,131 | |
Fig. 2.
Chemical structures of selected bioactive metabolites found in edible insects.
Anti-cancer and tumor suppressive effects have been observed for bioactive metabolites such as actinomycin-D, isocoumarins periplatins A-D, (R)-(+)-palasonin, palasonimide, cantharimide, palasonin, cantharidin, norcantharidin, pederin, pancratistatin, narciclasin and ungeremin found in insect species including Macrotermes natalensis22, Periplaneta americana23, Hycleus oculatus24, Hycleus lunata25, Paederus sp26 and Brachystola magna17 by inducing apoptosis and inhibiting cancer cell growth, in addition to inhibiting tumor metastasis and affecting cancer cell energy metabolism.
Hypertension is one of the main risk factors for cardiovascular disease, affecting millions of people every year. Angiotensin-converting enzyme (ACE) plays a key role in the regulation of blood pressure, and its efficacy in the treatment of hypertension has been proven27. Protein hydrolysates from insect species belonging to the orders Coleoptera, Diptera, Hymenoptera, Lepidoptera and Orthoptera have demonstrated ACE inhibitory activity. In other research, specific ACE inhibitory peptides from Bombyx mori, Tenebrio molitor, Spodoptera littoralis and Oecophylla smaragdina have been identified27,28. It has been reported that protein synthesis can release certain amino acids with important physiological regulatory functions including inhibiting severe hypertension and lowering blood pressure as a result of combination of lysine and methionine, and histidine29.
Pessina and collaborators30 reported anti-hypertensive effects of defatted Tenebrio molitor exhibiting strong ACE inhibitory activity with a dose-devendent reduction of svstolic blood pressure in hypertensive rats. Additionally, biactive metabolites such as macrotermycin A-D, natalenamides A-C, blapsols A-D, molossusamides A-C, photoinduced, melanins and ommochromes, aspongopusamides A-D, polyrhadopamines A-E, troline found in Macrotermes natalensis31,32, Blaps japanensis17,33, Hermetia illucens34, Polyrhachis dives35 were highlighted to have anti-inflammatory properties through effective inhibition of LOX and COX-2 activity, regulation of expression of inflammation- and immunity-related factors to improve the inflammation pathology. By boosting probiotic production and reducing pro-inflammatory cytokines and plasma lipids, immune response and function in humans, particularly in the gastrointestinal tract, would be linked to insect chitin content.
Chitin is the second most abundant biopolymer found in the exoskeletons of arthropods including insects36. It has some potential antinutritional properties, particularly when consumed in large quantities. The antinutritional aspects of chitin includes digestibility issues, inhibition of nutrient absorption and interference with protein digestion. Moreover, Chitin is largely indigestible to humans due to a lack of the enzymes needed to break it down, as its structure is resistant to digestive enzymes such as amylase, protease and lipase37. Additionally, chitin can bind to certain nutrients, potentially reducing their bioavailability. Furthermore, due to its rigid, fibrous nature, large quantities of chitin can lead to reduced protein absorption or incomplete digestion by interfering with the digestion of proteins and other macromolecules in the stomach38.
Glycosaminoglycan, a polysaccharide found in Gryllus bimaculatus, showed a significant anti-inflammatory effect against chronic arthritis in mice by inhibiting C-reactive protein (CRP) through suppression of a number of inflammatory biomarkers in vitro39. Furthermore, in combination with indomethacin, glycosaminoglycan was more effective than either agent alone in suppressing paw edema39. Furthermore, in rats fed a high-fat diet, glycosaminoglycan reduced CRP levels, abdominal and epididymal fat mass and various serobiochemical parameters (phospholipids, aspartate transaminase (AST), alanine transaminase (ALT), total cholesterol and glucose)40. Another study in diabetic mice revealed that glycosaminoglycan supplementation reduced blood glucose and LDL cholesterol levels, and increased the activity of antioxidant enzymes, notably catalase, superoxide dismutase and glutathione peroxidase41.These findings indicate that the glycosaminoglycan present in Gryllus bimaculatus may help reduce the risk of cardiovascular disease.
The A-D blapsols contained in Blaps japanensis demonstrated antioxidant properties42. Similarly, higher antioxidant activity was found in peptide43, photoinduced, melanins and ommochromes44 by analyzing DPPH and hydroxyl radical scavenging activities. Moreover, carminic acid found in Dactylopius coccus also exhibited antioxidant activity17. Insect hydrolysates and peptide fractions have demonstrated antioxidant properties, by contributing to reduce inflammation and oxidative stress by lowering the level of free radicals present in the body45,46. Di Mattia and collaborators37 reported that water-soluble extracts found in grasshoppers, silkworms and crickets have an antioxidant capacity around five times greater than that of fresh orange juice in vitro, due to their higher protein/peptide content.
Cantharidin17 and defensins-DLP2 and DLP447 found in Hycleus lunata exhibited immunomodulatory effects by promoting the expression of immune-related factors, enhancing natural killer cell activity as well as stimulating and activating the innate immune system. Furthermore, protein-enriched fraction from Musca domestica also showed immunomodulatory effects48. Moreover, immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated present in Bombyx mori pupae protein has potential therapeutic value as an immunomodulatory bioactive metabolite49. Purified polypeptide components (BPP-21 and BPP-22) found in Apis mellifera pupae revealed immunomodulatory activity in vivo and in vitro by increasing the phosphorylation of ERK and p38, and modulating the expression of intranuclear transcription factors (EIK-1, MEF-2 and CREB) in the MAPK signaling pathway50.
Pessina and collaborators30 observed neuroprotective effects in defatted Tenebrio molitor larvae. Additionally, bioactive compounds such as Coprismycin A-B and Collismycin A contained in Paederus sp have shown neuroprotective effects26. Moreover, polybioside51 and tetraponerins52 present respectively in Polybia paulista and Tetraponera rufonigra revealed neuroprotective effects.
Anti-microbial effects have been observed in several insect bioactive metabolites, such as Shellolic acid A found in Kerria lacca53, macrocarpal and grandinol found in Amauronematus amplus, Arge sp, Dineura pullior, Nematus brevivalvis, Nematus pravus, Nematus viridescens, Nematus viridis, Perga affinis, Pristiphora alpestris, Trichiosoma scalesii54, Desmosterol, (3β, 5α) cholesta-8, 14, 24-trien-3-ol, 4, 4-dimethyl, (3β, 20 R) cholesta-5, 24-dien-3, 20-diol found in Schistocerca gregaria55.
The antimicrobial effect of Tenebrio molitor and Zophobas morio has been proven in reducing E. coli and Salmonella infections in broilers56 due to their chitin content, which is a polymer of b-1, 4N-acetylglucosamine and is the primary component of the insect exoskeleton57,58. The latter and its degraded products, such as chitosan, exert antimicrobial, antioxidant, anti-inflammatory, anticancer and immunomodulatory activity59. Moreover, Nino et al.60 and Torres-Castillo et al.61 reported potential bioactivity of insect phenolics including tricin, luteolin, apigenin, orientin, iso-orientin, vitexin, iso-vitexin, kaempferol, quercetin, isorhamnetin, myricetin, ferulic acid, sinapic acid, gallic acid, 4-hydroxybenzoic acid, syringic acid, p-coumaric acid, caffeic acid, ferulic acid, sinapic acid, linked to chronic diseases such as antioxidant, anti-inflammatory, and anticancer, among others. Chitooligosaccharides, depolymerized products of chitin and chitosan, taken orally for eight weeks significantly reduced the level of the pro-inflammatory cytokine TNF-α and interleukin (IL)-1β in elderly people62.
Antibacterial activity was observed for actinomycin-D, macrotermycin A-D and pseudoxyallemycin-B present in Macrotermes natalensis22,31,63, 1-(2,5-Dihydroxyphenyl)-3-hydroxybutan-1-one, Roseoflavin and 8-methylamino-8-demethyl-d-riboflavin found in Odontotermes formosanus64,65, roseoflavin, 8-methylamino-8-demethyl-D-riboflavin, natalamycin and termisoflavones A-C present in Macrotermes spp65,66, molossusamides A-C found in Catharsius molossus67, lenzimycins A-B found in Onthophagus lenzii68, α-pyrone, diketopiperazine, pyrone derivatives, diketopiperazine, photoinduced, melanins and ommochromes found in Hermetia illucens69,70, and papilistatin found in Byasa polyeuctes71.
Bioactive metabolites found in insects such as 5-Hydroxyramulosin and biatriosporin-M found in Odontotermes formosanus64, natalamycin-A, geldanamycin, reblastatin, banegasin, cyclo-NMe-L-3,5-dichlorotyrosine-Dhb and rubrominin A-B found in Macrotermes natalensis22, efomycin K, efomycin L, efomycin M, efomycin G, elaiophylin, roseoflavin, 8-methylamino-8-demethyl, D-riboflavin, natalamycin, termisoflavones A-C present in Macrotermes spp65,72, tricin, palmitinic acid and eicosane found in Holotrichia diomphalia73 have shown antifungal effects.
Insect-based feeding is associated with the production of short-chain fatty acids (SCFAs), in terms of increasing the abundance and diversity of beneficial bacteria in the gut. One study showed that chitin is broken down into propionate and butyrate SCFAs by the gut microbiota58, followed by a reduction in blood cholesterol and triglyceride levels in chickens fed insect meal, with an increase in energy58. An increase in white blood cells, haemoglobin and red blood cells, followed by improved immune function was observed in fish supplemented with chitin and chitosan74. A reduction in triglyceride and cholesterol levels and an increase in blood calcium levels were observed in chickens supplemented with H. illucens larvae. This is explained by the fact that chitin’s positive charge enables it to bind negatively charged free fatty acids and bile acids75.
There are a variety of hypoglycemic bioactive metabolites in insects and their products, including proteins, peptides, polysaccharides, unsaturated fatty acids, alkaloids, and flavonoids76. Silkworm hydrolysate and fibroin are said to be ideal blood sugar regulators53. In addition, silkworm larvae, honey and chrysalises contain a large number of polysaccharides with hypoglycemic effects77. Removing the acetyl group, chitin is transformed into soluble chitosan. The oligosaccharides obtained by enzymolysis or acid hydrolysis of chitosan also have hypoglycemic effects in humans78. Insect fat is rich in unsaturated fatty acids79, including linoleic acid, which can improve glucose tolerance, with effects on insulin and reduces the incidence of cardiovascular and retinal complications in diabetic patients80.
Studies on trace elements show that magnesium, zinc, calcium, iron, copper, chromium, nickel, selenium among others are linked to human blood sugar metabolism with hypoglycemic effects81. Additionally, edible insects contain high levels of linolenic acid, which can prevent the synthesis of fatty acids and glycyrrhizin and accelerate the β-oxidation of fatty acids. Linolenic acid functions to reduce triacylglycerides, prolong clotting time and combat thrombosis, and is widely present in lepidopterous larvae, such as Clanis bilineata tsingtauica Mell, Tenebrio molitor, Zophobas atratus82. Moreover, chitin and chitosan present in Tenebrio molitor larvae can reduce blood pressure, blood lipids, and promote cholesterol metabolism78,83.
Moreover, Teixeira et al. 84 reported 177 peptides with predicted bioactivities and 61 peptides with bioactivity assessed In vitro and 3 peptides with bioactivity assessed In vivo from Gryllodes sigillatu, Gryllus assimilis, Schistocerca gregaria, Alphitobius diaperinus, Tenebrio molitor,Polyphylla adspersa, Apis mellifera, Oecophylla smaragdina, Bombyx mori, Spodoptera littoralis, Hermetia illucens, and Musca domestica.
Purification and identification of bioactive metabolites found in edible insects
The purification of bioactive metabolites from edible insects
Several key techniques and methodologies are being used to isolate, identify and purify bioactive metabolites present in the tissues of edible insects. These bioactive metabolites are of growing interest due to their potential health benefits, including antimicrobial, antioxidant, anti-inflammatory and anticancer properties. The general process for purifying bioactive metabolites from edible insects is described below.
Sample preparation and extraction
Proper sample preparation and extraction is the first step in the purification of bioactive metabolites from edible insects. The insect species selected can vary according to the bioactive compounds sought85. Depending on the solubility of the target metabolites, bioactive compounds can be extracted using a variety of solvents, including methanol and ethanol for extracting polar compounds like polyphenols and peptides86, hexane for lipid-soluble compounds such as fatty acids and sterols87, water for hydrophilic bioactive compounds, especially antioxidants88, and acetone is also used for both lipid and protein extractions89. Once extraction is complete, the resulting solution is usually concentrated using techniques such as rotary evaporation to remove the solvent. In addition, filtration is performed to remove insoluble solids, leaving a clear extract ready for further purification.
Purification Techniques
Once extraction is complete, techniques such as rotary evaporation are used to remove the solvent and concentrate the solution. In addition, insoluble solids are removed by filtration, leaving a clear extract ready for further purification. After concentration, bioactive metabolites are purified using chromatographic and separation techniques including High-Performance Liquid Chromatography (HPLC), one of the most common methods for separating and purifying bioactive metabolites from insect extracts90, Gas Chromatography (GC) which is particularly particularly useful for purifying volatile compounds, such as fatty acids and terpenoids91, Thin-Layer Chromatography (TLC), this one can be used as a preliminary purification step for lipophilic compounds such as sterols and antioxidants, even though not as advanced as HPLC; Size-Exclusion Chromatography (SEC): SEC is beneficial technique for separating compounds based on their molecular size. Very useful when purifying large molecules like proteins or polysaccharides from insect exoskeletons92, and Ion-Exchange Chromatography which is a method particularly used for isolating charged compounds, such as bioactive peptides93.
Characterization of purified bioactive metabolites in edible insects
After purification, isolated metabolites are characterized to confirm their identity as well as their bioactivity using several techniques including mass spectrometry (MS): a powerful tool for identifying the molecular weight and structure of bioactive metabolites94, nuclear magnetic resonance (NMR): often used for detailed structural characterization of purified metabolites, particularly to identify complex molecules such as fatty acids and peptides; and UV-Vis spectrophotometry, which is frequently used to identify and quantify light-absorbing bioactive compounds, including polyphenols and flavonoids95.
Identification of bioactive metabolites in edible insects
The identification of bioactive metabolites in edible insects has garnered much attention due their potential health benefits, such as antimicrobial, antioxidant, anti-inflammatory and even anticancer properties, due to their wealth of bioactive compounds, including peptides, lipids, polyphenols, vitamins, minerals and chitin derivatives96. Insects are rich in proteins which can be hydrolyzed to release bioactive peptides with potential health-promoting properties including antimicrobial, antihypertensive by inhibiting angiotensin-converting enzyme (ACE), and antioxidant effects97.
Moreover, edible insects are caracerized by a variety of lipids, including essential fatty acids important for human health. Insects such as crickets, mealworms and grasshoppers contain polyunsaturated fatty acids (PUFAs), notably omega-3 and omega-6 fatty acids98. Furthermore, many edible insects are rich in polyphenolic compounds, particularly phenolic acids and flavonoids, known for their antioxidant in cells and tissues, free radical scavenging activity and potentially anti-cancer properties61.
Additionally, edible insects contain essential vitamins and minerals that support various bodily functions including metabolism, immune function, wound healing, bone health, red blood cell production, and maintaining a healthy nervous system8. Other bioactive metabolites such as sterols and triterpenoids are found in the lipids of insects and are known to contribute cardiovascular health by lowering cholesterol, reduce inflammation, and exhibit anticancer properties99. In addition to chitin, other polysaccharides such as glucans found in the hemolymph of insects have been studied for their potential bioactivity including anticancer and immunomodulatory properties by stimulating the immune system and improving resistance to infections98.
Consumer attitudes toward edible insects
Consumer attitudes toward edible insects have been a subject of interest and debate in recent years19. As the world grapples with the challenges of sustainable food production and environmental concerns, edible insects have emerged as a potential solution to address these issues14. However, the acceptance and adoption of edible insects as a mainstream food source largely depends on consumer attitudes and perceptions21.
One of the primary factors influencing consumer attitudes toward edible insects is cultural and societal norms100. In many Western countries, insects are not traditionally part of the culinary landscape and are often associated with disgust or considered as pests101. This deeply ingrained cultural bias leads to a significant barrier to acceptance. However, in other cultures, such as parts of Asia, Africa, and Latin America, insects have long been consumed and are even considered delicacies102. Cultural exposure and familiarity with edible insects play a crucial role in shaping consumer attitudes and acceptance103.
Many people are concerned about the safety of consuming insects, particularly regarding potential allergenic reactions or contamination104. However, numerous studies have shown that edible insects are safe for human consumption when sourced from reliable and regulated suppliers105. In fact, insects are often rich in protein, vitamins, and minerals, making them a nutritious and sustainable food option9. Education and awareness campaigns highlighting the nutritional benefits and safety standards associated with edible insects can help reshape consumer attitudes.
Traditional livestock production, such as cattle farming, is resource-intensive and contributes to greenhouse gas emissions and deforestation. In contrast, insects require minimal resources, emit fewer greenhouse gases, and can be reared on organic waste, making them an environmentally friendly alternative106. Consumers who are conscious of these environmental issues may be more open to incorporating insects into their diet as a sustainable choice5,107,108.
The way edible insects are marketed and presented to consumers can significantly impact their perception and willingness to try them109. Manufacturers and retailers should focus on creating appealing and visually appealing products that align with consumers’ taste preferences and dietary habits110. Clever marketing strategies that emphasize the novelty, sustainability, and health benefits of edible insects can help overcome initial resistance and spark curiosity among consumers111.
Furthermore, taste preferences are often developed through exposure and personal experiences. Offering opportunities for consumers to sample and taste insect-based products in a non-threatening and controlled environment can help overcome the initial resistance and foster positive experiences110. Social influences, such as peer recommendations and endorsements from influential figures, can also sway consumer attitudes and drive acceptance. Overcoming cultural biases, addressing safety concerns, and raising awareness about the nutritional and environmental benefits of edible insects are crucial steps in reshaping consumer attitudes. By actively engaging consumers, providing appealing product options, and dispelling misconceptions, edible insects have the potential to become a viable and sustainable food source in the future.
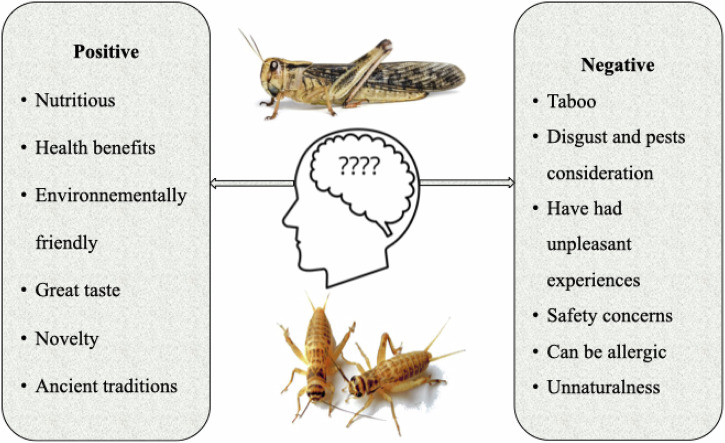
It can be concluded that some of the main positive factors influencing attitudes towards insects include nutritional potential, health benefits, environmentally friendly, great taste, and traditions; on the other hand, the main factors underlining negative attitudes towards insects are, among others, taboo, safety concerns, unpleasant past experiences, allergies and unnaturalness as summarized in Fig. 3.
Fig. 3.
Attitudes towards edible insects as food.
Conclusion and future perspectives
It can be generally concluded that insect bioactive metabolites, including marcocarpal, grandinol, trolline, pancratistatin, narciclasin, ungeremin, cantharidin, cordycepin, roseoflavin, lecithin, reblastatin, chitin, chitosan and desmosterol play a crucial role in conferring several beneficial biological activities, such as tumor suppression, anticancer, antihypertensive, anti-inflammatory, antioxidant, immunomodulator, neuroprotective, glycemic and lipid regulation, blood pressure reduction, regulation of intestinal bacterial flora and cardiovascular protection among others. However, proper sample preparation and extraction is the first step in the purification of bioactive metabolites from edible insects. After concentration, bioactive metabolites are purified using chromatographic and separation techniques including High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Thin-Layer Chromatography (TLC), Size-Exclusion Chromatography (SEC). It is noteworthy that nutritional potential, health benefits, environmentally friendly, great taste, traditions, taboo, safety concerns, unpleasant past experiences, allergies, and unnaturalness are among the main factors influencing attitudes towards insects.
Given the immense insect biodiversity, more in-depth investigations should focus on undiscovered bioactive metabolites, for more information on their potential as a sustainable therapeutic source. Particular attention should be paid to increasingly describing the therapeutic benefits and modes of action of insect bioactive metabolites. Additionally, as many human experiments as possible to explore the biological activities of these bioactive metabolites should also be carried out. Studies focusing on cross-reactivity of edible insects, as well as novelty, smart marketing, and good education can further influence attitudes towards insect consumption.
Acknowledgements
We would like to thank JKUAT, UEA and the RUFORUM for their support.
Author contributions
J.I., S.N., K.K. and J.K. contributed to the research design, wrote and revised the manuscript; J.I. processed data, conceptualization and formal analysis; J.I., S.N., K.K. and J.N. data curation and investigation; J.I. drafted the manuscript; all authors reviewed the manuscript. All authors contributed to this work and approved the final text of the manuscript.
Data Availability
The datasets generated or analyzed in the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Desa, U. N. World population prospects 2019. Highlights N. Y. U. Nations Dep. Econ. Soc. Aff.11, 125 (2019). [Google Scholar]
- 2.McClements, D. J. et al. Building a resilient, sustainable, and healthier food supply through innovation and technology. Annu. Rev. Food Sci. Technol.12, 1–28 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Godfray, H. C. J. et al. Food security: the challenge of feeding 9 billion people. Sci.327, 812–818 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Dickie, F., Miyamoto, M. & Collins, C. M. T. The potential of insect farming to increase food security. Edible Insects 1–10 (2019).
- 5.Ishara, J., Ogunyiola, A., Matendo, R., Kiyala, J. C. K. & Karume, K. Climate Change and Its Implications on Food Security in the Great Lakes Region. In Climate Change and Socio-political Violence in Sub-Saharan Africa in the Anthropocene: Perspectives from Peace Ecology and Sustainable Development 113–140 (Springer, 2024).
- 6.Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep.10, 13768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange, K. W. & Nakamura, Y. Edible insects as future food: chances and challenges. J. Futur. foods1, 38–46 (2021). [Google Scholar]
- 8.Ishara, J. et al. The contribution of commonly consumed edible insects to nutrition security in the Eastern DR Congo. Sci. Rep.14, 16186 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Huis, A., Rumpold, B., Maya, C. & Roos, N. Nutritional qualities and enhancement of edible insects. Annu. Rev. Nutr.41, 551–576 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Mishyna, M., Chen, J. & Benjamin, O. Sensory attributes of edible insects and insect-based foods–Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol.95, 141–148 (2020). [Google Scholar]
- 11.Dobermann, D., Swift, J. A. & Field, L. M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 293–308. 10.1111/nbu.12291. (2017)
- 12.Tanga, C. M. et al. Edible insect farming as an emerging and profitable enterprise in East Africa. Curr. Opin. insect Sci.48, 64–71 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Panassiti, B. et al. Insects benefit from agri-environmental schemes aiming at grassland extensification. Agric. Ecosyst. Environ.356, 108613 (2023). [Google Scholar]
- 14.Nowakowski, A. C., Miller, A. C., Miller, M. E., Xiao, H. & Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr.62, 3499–3508 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Koutsos, E., Modica, B. & Freel, T. Immunomodulatory potential of black soldier fly larvae: applications beyond nutrition in animal feeding programs. Transl. Anim. Sci.6, txac084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingqian, N. et al. Insect bioactive compounds and their potential use in animal diets and medicine. Entomol. Res. (2023).
- 17.Seabrooks, L. & Hu, L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B7, 409–426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbarian, M., Khani, A., Eghbalpour, S. & Uversky, V. N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci.23, 1445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megido, C. R. et al. Edible insects acceptance by B elgian consumers: promising attitude for entomophagy development. J. Sens. Stud.29, 14–20 (2014). [Google Scholar]
- 20.Payne, C. L. R., Scarborough, P., Rayner, M. & Nonaka, K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci. Technol.47, 69–77 (2016). [Google Scholar]
- 21.Wendin, K. M. E. & Nyberg, M. E. Factors influencing consumer perception and acceptability of insect-based foods. Curr. Opin. food Sci.40, 67–71 (2021). [Google Scholar]
- 22.Benndorf, R. et al. Natural products from Actinobacteria associated with fungus-growing termites. Antibiotics7, 83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo, S.-L. et al. Isocoumarins from American cockroach (Periplaneta americana) and their cytotoxic activities. Fitoterapia95, 115–120 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Mebs, D., Pogoda, W., Schneider, M. & Kauert, G. Cantharidin and demethylcantharidin (palasonin) content of blister beetles (Coleoptera: Meloidae) from southern Africa. Toxicon53, 466–468 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Wang, J. et al. Functional study on the mutations in the silkworm (Bombyx mori) acetylcholinesterase type 1 gene (ace 1) and its recombinant proteins. Mol. Biol. Rep.41, 429–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellner, R. L. L. & Dettner, K. Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): evidence for polymorphism of hemolymph toxin. J. Chem. Ecol.21, 1719–1733 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Dai, C., Ma, H., Luo, L. & Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol.236, 681–689 (2013). [Google Scholar]
- 28.Cito, A., Botta, M., Francardi, V. & Dreassi, E. Insects as source of angiotensin converting enzyme inhibitory peptides. J. Insects Food Feed3, 231–240 (2017). [Google Scholar]
- 29.Hadi, J. & Brightwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods10, 1226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessina, F. et al. Antihypertensive, cardio-and neuro-protective effects of Tenebrio molitor (Coleoptera: Tenebrionidae) defatted larvae in spontaneously hypertensive rats. PLoS One.15, e0233788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beemelmanns, C. et al. Macrotermycins A–D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org. Lett.19, 1000–1003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, S. R. et al. Natalenamides A–C, cyclic tripeptides from the termite-associated Actinomadura sp. RB99. Molecules23, 3003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan, Y.-M. et al. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg. Med. Chem. Lett.25, 2469–2472 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Richter, H., Gover, O. & Schwartz, B. Anti-inflammatory activity of black soldier fly oil associated with modulation of tlr signaling: A metabolomic approach. Int. J. Mol. Sci.24, 10634 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang, J.-J. et al. Constituents from the edible Chinese black ants (Polyrhachis dives) showing protective effect on rat mesangial cells and anti-inflammatory activity. Food Res. Int.67, 163–168 (2015). [Google Scholar]
- 36.Wijesekara, T. & Xu, B. New insights into sources, bioavailability, health-promoting effects, and applications of chitin and chitosan. J. Agric. Food Chem.72, 17138–17152 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Eunice Boko, A. C., Blei, S. H., Koko, A. C. & Angaman, D. M. Evaluating Nudaurelia dione (Saturniidae), an Edible Insect, for Sustainable Nutrition: Composition, Benefits, and Antinutritional Insights. J. Food Biochem.2024, 5559567 (2024). [Google Scholar]
- 38.Mu, L., Wu, L., Wu, S., Ye, Q. & Zhong, Z. Progress in chitin/chitosan and their derivatives for biomedical applications: Where we stand. Carbohydr. Polym. 122233 (2024). [DOI] [PubMed]
- 39.Ahn, M. Y., Han, J. W., Hwang, J. S., Yun, E. Y. & Lee, B. M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Heal. Part A.77, 1332–1345 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Ahn, M. Y., Hwang, J. S., Kim, M.-J. & Park, K.-K. Antilipidemic effects and gene expression profiling of the glycosaminoglycans from cricket in rats on a high fat diet. Arch. Pharm. Res.39, 926–936 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Ahn, M. Y. et al. Anti-diabetic activity of field cricket glycosaminoglycan by ameliorating oxidative stress. BMC Complement. Med. Ther.20, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan, Y.-M., Luo, Q., Li, J.-J., Tu, Z.-C. & Cheng, Y.-X. Novel spirooxindole alkaloid derivatives from the medicinal insect Blaps japanensis and their biological evaluation. Bioorg. Chem.141, 106845 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Lu, J. et al. Probing the antioxidant activity of functional proteins and bioactive peptides in Hermetia illucens larvae fed with food wastes. Sci. Rep.12, 2799 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dontsov, A. E. et al. Ommochromes from the compound eyes of insects: physicochemical properties and antioxidant activity. Biochem. 85, 668–678 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Zielińska, E., Baraniak, B. & Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients9, 970 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Mattia, C., Battista, N., Sacchetti, G. & Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr.6, 106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, Z. et al. Antibacterial and immunomodulatory activities of insect defensins-DLP2 and DLP4 against multidrug-resistant Staphylococcus aureus. Sci. Rep.7, 12124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ai, H., Wang, F., Zhang, N., Zhang, L. & Lei, C. Antiviral, immunomodulatory, and free radical scavenging activities of a protein-enriched fraction from the larvae of the housefly, Musca domestica. J. Insect Sci.13, 112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, Z. et al. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia. Pac. Entomol.22, 633–637 (2019). [Google Scholar]
- 50.Chen, Y. et al. Purification of novel polypeptides from bee pupae and their immunomodulatory activity in vivo and in vitro. J. Insects Food Feed8, 1117–1132 (2022). [Google Scholar]
- 51.Saidemberg, D. M., da Silva-Filho, L. C., Tognoli, L. M. M. C., Tormena, C. F. & Palma, M. S. Polybioside, a neuroactive compound from the venom of the social wasp Polybia paulista. J. Nat. Prod.73, 527–531 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Bosque, I., Gonzalez-Gomez, J. C., Loza, M. I. & Brea, J. Natural tetraponerines: A general synthesis and antiproliferative activity. J. Org. Chem.79, 3982–3991 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Lu, J. et al. Sesquiterpene acids from Shellac and their bioactivities evaluation. Fitoterapia97, 64–70 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Vihakas, M. A., Kapari, L. & Salminen, J.-P. New types of flavonol oligoglycosides accumulate in the hemolymph of birch-feeding sawfly larvae. J. Chem. Ecol.36, 864–872 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Cheseto, X. et al. Potential of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) as an unconventional source of dietary and therapeutic sterols. PLoS One10, e0127171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Islam, M. M. & Yang, C.-J. Efficacy of mealworm and super mealworm larvae probiotics as an alternative to antibiotics challenged orally with Salmonella and E. coli infection in broiler chicks. Poult. Sci.96, 27–34 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Stull, V. J. et al. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep.8, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borrelli, L. et al. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep.7, 16269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liaqat, F. & Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym.184, 243–259 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Nino, M. C., Reddivari, L., Osorio, C., Kaplan, I. & Liceaga, A. M. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed7, 1077–1088 (2021). [Google Scholar]
- 61.Torres-Castillo, J. A. & Olazarán-Santibáñez, F. E. Insects as source of phenolic and antioxidant entomochemicals in the food industry. Front. Nutr.10, 1133342 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim, J.-K. et al. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem. Biophys. Res. Commun.345, 1215–1223 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Kreuzenbeck, N. B. et al. Isolation, (bio)synthetic studies and evaluation of antimicrobial properties of drimenol-type sesquiterpenes of Termitomyces fungi. Commun. Chem.6, 79 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, X. et al. Diversity, bacterial symbionts, and antimicrobial potential of termite-associated fungi. Front. Microbiol.11, 300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou, Y. et al. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods11, 3961 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang, C. K. et al. Antibacterial cotton fibers treated with silver nanoparticles and quaternary ammonium salts. Carbohydr. Polym.151, 1012–1018 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Lu, J. et al. Identification of N-acetyldopamine dimers from the dung beetle Catharsius molossus and their COX-1 and COX-2 inhibitory activities. Molecules20, 15589–15596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An, J. S. et al. Lenzimycins A and B, metabolites with antibacterial properties from Brevibacillus sp. associated with the dung beetle Onthophagus lenzii. Front. Microbiol.11, 599911 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Correa, Y. et al. Identification and characterization of compounds from Chrysosporium multifidum, a fungus with moderate antimicrobial activity isolated from Hermetia illucens gut microbiota. PLoS One14, e0218837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mudalungu, C. M., Tanga, C. M., Kelemu, S. & Torto, B. An overview of antimicrobial compounds from African edible insects and their associated microbiota. Antibiotics10, 621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettit, G. R., Ye, Q., Herald, D. L., Hogan, F. & Pettit, R. K. Antineoplastic agents. 573. isolation and structure of papilistatin from the papilionid butterfly Byasa polyeuctes termessa. J. Nat. Prod.73, 164–166 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klassen, J. L., Lee, S. R., Poulsen, M., Beemelmanns, C. & Kim, K. H. Efomycins K and L from a termite-associated Streptomyces sp. M56 and their putative biosynthetic origin. Front. Microbiol.10, 1739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong, Q.-F. et al. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresour. Technol.99, 8521–8523 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Harikrishnan, R., Kim, J.-S., Balasundaram, C. & Heo, M.-S. Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture326, 46–52 (2012). [Google Scholar]
- 75.Marono, S. et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci.96, 1783–1790 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Liu, Y. et al. Hormonal and nutritional regulation of insect fat body development and function. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am.71, 16–30 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Zhao, P. et al. Genome-wide identification and immune response analysis of serine protease inhibitor genes in the silkworm, Bombyx mori. PLoS One7, e31168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hahn, T. et al. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol.95, 2775–2795 (2020). [Google Scholar]
- 79.Finke, M. D. Complete nutrient content of four species of feeder insects. Zoo. Biol.32, 27–36 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Belluco, S. et al. Edible insects in a food safety and nutritional perspective: a critical review. Compr. Rev. food Sci. food Saf.12, 296–313 (2013). [Google Scholar]
- 81.Kulma, M., Kou, L., Homolková, D. & Plachý, V. Effect of developmental stage on the nutritional value of edible insects. A case study with Blaberus craniifer and Zophobas morio. J. Food Compos. Anal. 92, (2020).
- 82.Kong, B. & Xiong, Y. L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem.54, 6059–6068 (2006). [DOI] [PubMed] [Google Scholar]
- 83.da Silva Lucas, A. J., de Oliveira, L. M., Da Rocha, M. & Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem.311, 126022 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Teixeira, C. S. S., Villa, C., Costa, J., Ferreira, I. M. & Mafra, I. Edible insects as a novel source of bioactive peptides: A systematic review. Foods12, 2026 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Psarianos, M., Aghababaei, F. & Schlüter, O. K. Bioactive compounds in edible insects: Aspects of cultivation, processing and nutrition. Food Res. Int. 115802 (2025). [DOI] [PubMed]
- 86.Gullón, P., Gullón, B., Romaní, A., Rocchetti, G. & Lorenzo, J. M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol.101, 182–197 (2020). [Google Scholar]
- 87.Karrar, E. et al. Lipid-soluble vitamins from dairy products: extraction, purification, and analytical techniques. Food Chem.373, 131436 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Farooq, S., Abdullah, Zhang, H. & Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf.20, 4250–4277 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Rose, A., Jaczynski, J. & Matak, K. Extraction of lipids from insect powders using a one-step organic solvent extraction process. Futur. Foods4, 100073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.An, R. et al. Isolation, purification and identification of biological compounds from Beauveria sp. and their evaluation as insecticidal effectiveness against Bemisia tabaci. Sci. Rep.11, 12020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giovannoni, S. et al. Determination of variability of terpenes and terpenoids in Cannabis sativa by gas chromatography-flame ionization detection and gas chromatography-mass spectrometry. J. Chromatogr. A1687, 463669 (2023). [DOI] [PubMed] [Google Scholar]
- 92.da Silva Lucas, A. J. et al. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem.343, 128550 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Yea, C. S., Ahmadi, R., Zarei, M. & Muhialdin, B. J. Fractionation and Purification of Bioactive Peptides. in Bioactive Peptides from Food 267–298 (CRC Press, 2022).
- 94.Gupta, P. et al. Mass spectrometry-based technology and workflows for studying the chemistry of fungal endophyte derived bioactive compounds. ACS Chem. Biol.16, 2068–2086 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Shakir, N. et al. Impact of NaCl stress on phytoconstituents and bioactivity of Matricaria chamomilla: a multi-analytical approach. Sci. Rep.14, 19717 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rivas-Navia, D. M. et al. Bioactive compounds of insects for food use: Potentialities and risks. J. Agric. Food Res.14, 100807 (2023). [Google Scholar]
- 97.Zhang, J., Li, M., Han, Z. & Shao, J.-H. A review of insect peptides: From extraction and preparation to bioactivities. Curr. Opin. Food Sci. 101301 (2025).
- 98.Stull, V. J. & Weir, T. L. Chitin and omega-3 fatty acids in edible insects have underexplored benefits for the gut microbiome and human health. Nat. Food4, 283–287 (2023). [DOI] [PubMed] [Google Scholar]
- 99.Mudalungu, C. M., Mokaya, H. O. & Tanga, C. M. Beneficial sterols in selected edible insects and their associated antibacterial activities. Sci. Rep.13, 10786 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siddiqui, S. A. et al. Legal situation and consumer acceptance of insects being eaten as human food in different nations across the world–A comprehensive review. Compr. Rev. Food Sci. Food Saf. (2023). [DOI] [PubMed]
- 101.Sidali, K. L., Pizzo, S., Garrido-Pérez, E. I. & Schamel, G. Between food delicacies and food taboos: A structural equation model to assess Western students’ acceptance of Amazonian insect food. Food Res. Int.115, 83–89 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Kelemu, S. et al. African edible insects for food and feed: inventory, diversity, commonalities and contribution to food security. J. Insects Food Feed1, 103–119 (2015). [Google Scholar]
- 103.Ishara, J. et al. Inventory reveals wide biodiversity of edible insects in the Eastern Democratic Republic of Congo. Sci. Rep.12, 1576 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J.18, 1–11 (2020). [Google Scholar]
- 105.Ng’ang’a, J. et al. Microbial quality of edible grasshoppers Ruspolia differens (Orthoptera: Tettigoniidae): From wild harvesting to fork in the Kagera Region, Tanzania. J. Food Saf.39, e12549 (2019). [Google Scholar]
- 106.Borges, M. M., da Costa, D. V., Trombete, F. M. & Câmara, A. K. F. I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci.46, 100864 (2022). [Google Scholar]
- 107.Van Huis, A. and Oonincx D. G. A. B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 10.1007/s13593-017-0452-8 (2017)
- 108.Ishara, J. et al. Insights into the effects of geographical sourcing area on nutrient composition and sensory attributes of nine edible insects. Sci. Rep.15, 11610 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hopkins, I., Farahnaky, A., Gill, H., Newman, L. P. & Danaher, J. Australians’ experience, barriers and willingness towards consuming edible insects as an emerging protein source. Appetite169, 105832 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Mishyna, M., Chen, J. & Benjamin, O. Sensory attributes of edible insects and insect-based foods – Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. 10.1016/j.tifs.2019.11.016 (2019)
- 111.Melgar-lalanne, G., Hernandez-Alvarez, A.-J. & Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 18, (2019). [DOI] [PubMed]
- 112.Jiang, N. et al. Design, synthesis and antiproliferative activity of novel 2-substituted-4-amino-6-halogenquinolines. Molecules17, 5870–5881 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu, H.-J., Xu, T., Yan, Y.-M., Tu, Z.-C. & Cheng, Y.-X. Neolignans and norlignans from insect medicine Polyphaga plancyi and their biological activities. Nat. Products Bioprospect.11, 51–62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim, K. H. et al. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci.5, 4333–4338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang, H. R. et al. Termisoflavones A–C, isoflavonoid glycosides from termite-associated Streptomyces sp. RB1. J. Nat. Prod.79, 3072–3078 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Wyche, T. P. et al. Linear peptides are the major products of a biosynthetic pathway that encodes for cyclic depsipeptides. Org. Lett.19, 1772–1775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carr, G. et al. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett.14, 2822–2825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.von Bonin, A. et al. Efomycine M: an inhibitor of selectins?. Nat. Med.12, 873 (2006). [DOI] [PubMed] [Google Scholar]
- 119.Twu, N.-F., Srinivasan, R., Chou, C.-H., Wu, L.-S. & Chiu, C.-H. Cantharidin and norcantharidin inhibit caprine luteal cell steroidogenesis in vitro. Exp. Toxicol. Pathol.64, 37–44 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Kim, S.-H. et al. Tripartin, a histone demethylase inhibitor from a bacterium associated with a dung beetle larva. Org. Lett.15, 1834–1837 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Kim, M. J. et al. Population genetic characterization of the endangered dung beetle Copris tripartitus (Coleoptera: Scarabaeidae) using novel microsatellite markers. J. Asia. Pac. Entomol.25, 101899 (2022). [Google Scholar]
- 122.Um, S. et al. Naphthoquinone–oxindole alkaloids, coprisidins A and B, from a gut-associated bacterium in the dung beetle, Copris tripartitus. Org. Lett.18, 5792–5795 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Park, S.-H. et al. Tripartilactam, a cyclobutane-bearing tricyclic lactam from a Streptomyces sp. in a dung beetle’s brood ball. Org. Lett.14, 1258–1261 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Blodgett, J. A. V. et al. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl Acad. Sci.107, 11692–11697 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prasad, S. B. & Verma, A. K. Cantharidin-mediated ultrastructural and biochemical changes in mitochondria lead to apoptosis and necrosis in murine Dalton’s lymphoma. Microsc. Microanal.19, 1377–1394 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Zhao, Y. et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab.21, 777–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dettner, K. et al. Occurrence of terpene anhydride Palasonin and Palasoninimide in blister beetle Hycleus lunata (Coleoptera: Meloidae). Biochem. Syst. Ecol.31, 203–205 (2003). [Google Scholar]
- 128.Lang, E. & Lang, F. Triggers, inhibitors, mechanisms, and significance of eryptosis: the suicidal erythrocyte death. Biomed Res. Int. 2015, (2015). [DOI] [PMC free article] [PubMed]
- 129.Rivero-Pino, F., Guadix, A. & Guadix, E. M. Identification of novel dipeptidyl peptidase IV and α-glucosidase inhibitory peptides from Tenebrio molitor. Food Funct.12, 873–880 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Zielińska, E., Baraniak, B. & Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol.53, 2542–2551 (2018). [Google Scholar]
- 131.Zielińska, E., Karaś, M., Baraniak, B. & Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol.246, 1361–1369 (2020). [Google Scholar]
- 132.Tang, J.-J. et al. Dopamine derivatives from the insect Polyrhachis dives as inhibitors of ROCK1/2 and stimulators of neural stem cell proliferation. Tetrahedron70, 8852–8857 (2014). [Google Scholar]
- 133.Shi, Y.-N. et al. Bioactive compounds from the insect Aspongopus chinensis. Bioorg. Med. Chem. Lett.24, 5164–5169 (2014). [DOI] [PubMed] [Google Scholar]
- 134.de Carvalho, F. M. et al. Brazilian red propolis: Extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomolecules10, 726 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arbiser, J. L. et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood109, 560–565 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang, Y. et al. Molecular mechanisms of novel peptides from silkworm pupae that inhibit α-glucosidase. Peptides76, 45–50 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Kong, W.-H. et al. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem.56, 2613–2619 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Liu, S. et al. Antioxidant activity and phenolic compounds of Holotrichia parallela Motschulsky extracts. Food Chem.134, 1885–1891 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Pettit, G. R., Meng, Y., Herald, D. L., Knight, J. C. & Day, J. F. Antineoplastic Agents. 553. The Texas Grasshopper Brachystola m agna. J. Nat. Prod.68, 1256–1258 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hall, F., Reddivari, L. & Liceaga, A. M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients12, 3588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed in the current study are available from the corresponding author upon reasonable request.