Abstract
Myocardial fibrosis can occur in patients who have hypertrophic cardiomyopathy in the absence of epicardial coronary disease. In such patients, myocardial fibrosis has been linked to a poorer prognosis than in those without fibrosis. Gadolinium-DTPA delayed-enhancement magnetic resonance imaging (de-MRI) accurately identifies regions of myocardial fibrosis. We used de-MRI to screen for myocardial fibrosis in 8 patients with nonobstructive hypertrophic cardiomyopathy that had been diagnosed by 2-dimensional echocardiography.
After localization of the heart and acquisition of electrocardiographically gated cine images, gadolinium-DTPA (0.2 mmol/kg) was administered to the patient. Fifteen minutes later, de-MRI images were obtained using a T1-weighted, inversion-recovery, fast, low-angle shot sequence. Images were gated to end-diastole and obtained during a single breath-hold. The inversion time was modified iteratively to obtain maximal nulling of the signal from the ventricular myocardium. Regions of myocardium with abnormally high signals (>300% of remote normal myocardium) were designated as fibrotic.
Eight patients with hypertrophic cardiomyopathy underwent de-MRI. The mean age was 52 years, the mean left ventricular mass was 201 grams, and the mean ejection fraction was 0.68. In the 6 patients with recent clinical deterioration, de-MRI showed clearly delineated areas of myocardial fibrosis; no such areas were seen in the 2 asymptomatic patients.
We conclude that patients with symptomatic hypertrophic cardiomyopathy display regions of abnormal signal intensity on de-MRI that likely represent fibrosis. This technique may provide useful information in the evaluation of such patients and warrants further study. (Tex Heart Inst J 2002;29:176–80)
Key words: Cardiomyopathy, hypertrophic; endomyocardial fibrosis; gadolinium DTPA/diagnostic use; magnetic resonance imaging, delayed-enhancement
Hypertrophic cardiomyopathy (HCM) is a disease of the myocardium characterized by inappropriate hypertrophy; intramyocardial, small-vessel coronary artery disease (CAD); and myocardial fibrosis. Evidence of myocardial ischemia and fibrosis are seen with increased frequency and severity in patients who have suffered the most severe sequelae of HCM. 1–5 These sequelae include systolic and diastolic left ventricular (LV) dysfunction, angina pectoris, syncope, and sudden cardiac death. The diastolic dysfunction has been ascribed to the mechanical effects of extensive ventricular hypertrophy and interstitial collagen deposition. Angina pectoris suggestive of myocardial ischemia frequently occurs in the absence of epicardial CAD. Indeed, myocardial ischemia secondary to intramyocardial small-vessel CAD and to the increased oxygen requirements of the hypertrophied myocardium may contribute to the development of myocardial fibrosis, LV dysfunction, and ventricular arrhythmias. Therefore, the detection of ischemia, or its sequela fibrosis, in patients with HCM might provide useful diagnostic and prognostic information. Gadolinium-DTPA (Gd-DTPA) delayed-enhancement magnetic resonance imaging (de-MRI) is a promising technique for imaging regions of both irreversible and reversible myocardial injury and fibrosis. 6,7 We report the use of de-MRI to identify myocardial fibrosis in 8 patients with echocardiographically diagnosed nonobstructive HCM, and we describe its value for the study of patients who have this disease.
Methods
Eight patients with nonobstructive HCM diagnosed by 2-dimensional echocardiography were referred to our institution for evaluation of LV mass, function, and physical characteristics. After localization of the heart, gated cine images were obtained using a cine-TFE pulse sequence with the following acquisition parameters: TR/TE/α = 4.8 msec/1.8 msec/25°, voxel size: 1.2 × 1.2 × 10 mm, temporal resolution of 40–60 msec depending on the patient's heart rate. Cine images were obtained in the following orientations: 2-chamber long-axis, 4-chamber long-axis, LV outflow tract, and short-axis stack covering the entire LV. Gadolinium-DTPA (0.2 mmol/kg) was administered to the patient. After a 15-minute delay, images were obtained in the same orientations using an inversion-recovery T1-weighted sequence (de-MRI) with the following acquisition parameters: TR/TE/α = 7 msec/3 msec/15°, voxel size: 1.2 × 1.2 × 10 mm, 32 lines of k-space collected for each R-R interval in end-diastole, acquisition time 16 heartbeats (single breath-hold). Images obtained with de-MRI after a 15-minute delay from the time of Gd-DTPA administration show increased signal intensity of acutely necrotic or chronically scarred myocardial tissue compared with normal myocardium. The inversion time was modified iteratively to obtain maximal nulling of the LV myocardium, with an average value of 225 msec. Left ventricular mass and end-diastolic and end-systolic volumes were derived, with the use of Simpson's rule, from epicardial and endocardial tracings, which were obtained from a complete set of short-axis cine images.
Results
The results are summarized in Table I. There were 8 patients (6 men and 2 women), most of whom had presented with recent clinical deterioration (within the past 6 months before the study). The mean age was 52 ± 12 (SD) years. All patients had normal coronary angiograms except for patient 1, who had nonocclusive disease. Although patient 6 reported no symptoms, antero-apical hypokinesis was detected. The mean LV mass for the 8 patients was 201 ± 41 grams, and the mean LV ejection fraction was 0.68 ± 0.054. With de-MRI, areas of hyperenhancement that likely represented fibrosis were detected in the 6 patients who had evidence of recent clinical deterioration (Figs. 1 and 2). No such areas were detected in patients 7 and 8 (asymptomatic).
TABLE I. Summary of Delayed-Enhancement MRI Results in 8 Patients with Hypertrophic Cardiomyopathy
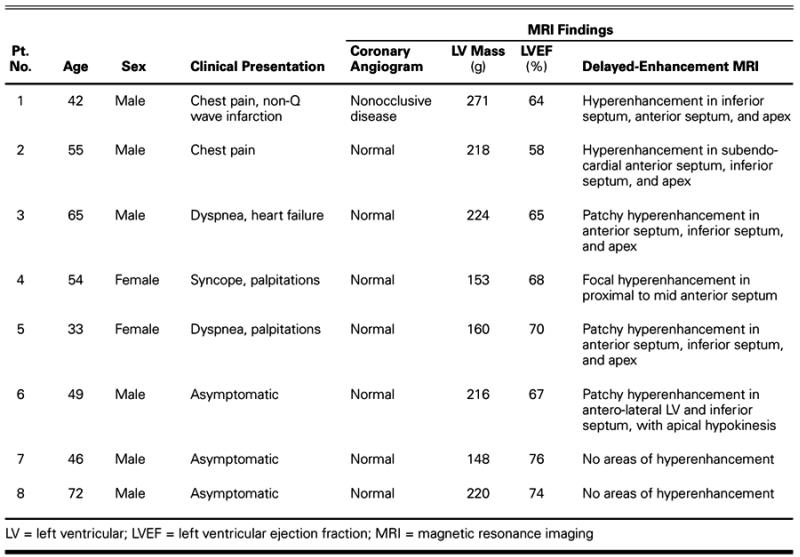
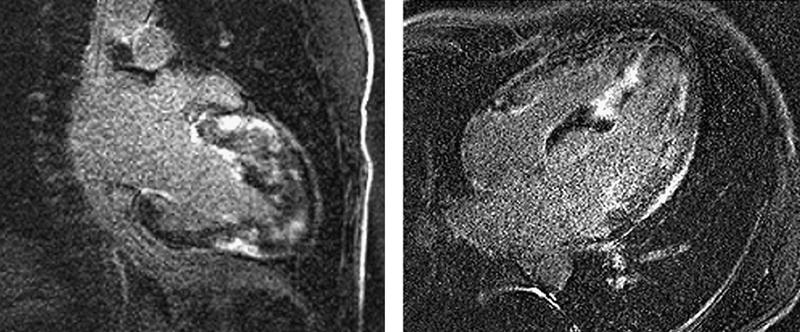
Fig. 1 De-MRI from patient 1, acquired in the 2-chamber long-axis (left) and 4-chamber projections, shows patchy areas of fibrosis throughout the anterior, lateral, and inferior walls, and in the distal half of the interventricular septum.
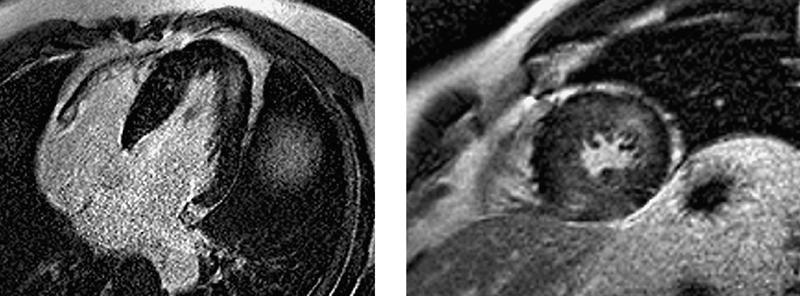
Fig. 2 De-MRI from patient 6, acquired in the 4-chamber (left) and short-axis projections, shows fine sand-like intramyocardial fibrosis in the distal anterolateral wall and inferior septum.
Discussion
The clinical manifestations of HCM vary widely within the population of affected patients. Beyond a personal or family history of syncope or sudden death, there is no reliable predictive instrument that can identify individuals with a high risk of life-threatening complications. Measures of disease severity, such as LV mass, septal thickness, and outflow tract obstruction, although clearly related to the severity of functional limitation, are of limited use in predicting subsequent deterioration of contractile function or sudden cardiac death. Genetic analysis of the mutations responsible for disease in an individual or family members offers hope but has not yet reached clinical application. The importance of identifying patients likely to suffer life-threatening ventricular arrhythmias has increased with the development of successful therapeutic options such as implantable cardioverter-defibrillators.
Autopsy studies have introduced other variables that might be used to measure disease severity and predict mortality risk. The histomorphologic characteristics of HCM are hypertrophy, myofibrillar disarray, interstitial fibrosis, and intramyocardial arteriosclerosis. Hypertrophy, usually measured by echocardiography, is a weak correlate of disease severity and outcome, and myofibrillar disarray is not currently measurable without tissue. 8 Intramyocardial CAD is a common observation in autopsy reports from patients of almost every age. Moreover, myocardial fibrosis, which tends to appear along with intramyocardial CAD, has been associated with more severe manifestations of the disease, such as end-stage heart failure and sudden cardiac death. 9
Myocardial fibrosis is more extensive in patients with HCM than in those with other forms of LV hypertrophy. 10 Both matrix collagen and replacement fibrosis are also found in increased quantities in patients with HCM. 11,12 In addition, a greater extent of myocardial fibrosis is found in the hearts of patients with severe heart failure or sudden cardiac death compared with those who died of noncardiac events. 13 The cause of the increased connective tissue volume is unknown. However, intramyocardial medial and intimal hyperplasia, mural fibrosis, wall thickening, and reduction in arteriolar density correlate with the degree and location of myocardial fibrosis, which suggests a relationship of myocardial fibrosis to ischemia. 9,14–16
Physiologic evidence of the impact of intramyocardial CAD has been established using direct measurements of the coronary physiology and by the use of Tl-201 scintigraphy. Coronary flow reserve, velocity, and resistance are all diminished or impaired in response to pharmacologic and nonpharmacologic forms of stress. 5,17,18 Correlations between perfusion abnormalities and clinical presentations of sudden cardiac death, syncope, arrhythmias, and contractile failure have also been described. 2–4,19 Chronic or recurrent ischemic injury may result in the development of myocardial fibrosis. A similar pathogenesis has been proposed for patients with atherosclerosis in whom repeated ischemic injury may result in fibrosis, eventually leading to contractile failure.
Delayed-hyperenhancement, inversion-recovery, T1-weighted imaging sequences modified for gated-imaging of the myocardium—or de-MRI—may improve detection of myocardial fibrosis. After the administration of Gd-DTPA, increased signal intensity is detected from infarcted or fibrotic myocardium. Kim and colleagues 6 used de-MRI to gather images of experimental myocardial infarction in dogs at 1 day, 3 days, and 8 weeks. They found a strong correlation between regions of delayed enhancement and histologic evidence of myocardial necrosis at 1 and 3 days and of collagenous fibrosis at 8 weeks. 6 More recently, the same investigators used de-MRI in 50 patients who had CAD and regional wall motion abnormalities; de-MRI accurately identified regions of reversible myocardial dysfunction before revascularization (that is, the regions that were dysfunctional and did not hyperenhance). 7 Therefore, differential contrast imaging after Gd-DTPA administration may be of value for the identification of infarcted, viable, or fibrotic myocardium.
In each of the 6 patients with HCM and evidence of recent clinical deterioration, de-MRI clearly delineated regions of increased signal intensity similar in appearance to signals seen with experimental myocardial infarction. 6 In only one of the patients (patient 1) was biochemical evidence consistent with recent myocardial infarction. Therefore, the abnormal appearance of LV myocardium on de-MRI in the remaining 5 patients strongly suggests distinct areas of fibrosis. Considering the importance of fibrosis as suggested by autopsy studies, a noninvasive technique such as de-MRI may be of use in the clinical evaluation of patients with HCM.
The sensitivity of de-MRI for the detection of fibrosis, particularly the degree of interstitial fibrosis routinely observed in patients with HCM, has not been ascertained. Moreover, although the observations from Tl-201 scintigraphy suggest that myocardial infarction and fibrosis are associated with the more severe manifestations of the disease, there is as yet no evidence that the identification of myocardial fibrosis is of prognostic importance. Nonetheless, the capability of de-MRI to noninvasively determine the extent of intramyocardial fibrosis with higher resolution than has previously been available suggests that this method is a potentially valuable tool in the prognostic evaluation of patients with HCM.
In summary, we have described 6 patients with nonobstructive HCM in whom gadolinium-DTPA delayed-enhancement magnetic resonance imaging detected evidence of myocardial fibrosis or necrosis after clinical deterioration. The use of this imaging method for the prognostic evaluation of HCM warrants further study.
Footnotes
Address for reprints: James M. Wilson, MD, 6624 Fannin, Suite 2480, Houston, TX 77030
References
- 1.Nagata S, Park Y, Minamikawa T, Yutani C, Kamiya T, Nishimura T, et al. Thallium perfusion and cardiac enzyme abnormalities in patients with familial hypertrophic cardiomyopathy. Am Heart J 1985;109:1317–22. [DOI] [PubMed]
- 2.von Dohlen TW, Prisant LM, Frank MJ. Significance of positive or negative thallium-201 scintigraphy in hypertrophic cardiomyopathy. Am J Cardiol 1989;64:498–503. [DOI] [PubMed]
- 3.Mori T, Yamabe H, Yokota Y, Fukuzaki H. Clinical significance of dipyridamole Tl-201 emission computed tomography perfusion abnormality for evaluating pathophysiological and pathological aspects in hypertrophic cardiomyopathy. Jpn Circ J 1988;52(2):111–8. [DOI] [PubMed]
- 4.Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1993;22:796–804. [DOI] [PubMed]
- 5.Cannon RO 3rd, Rosing DR, Maron BJ, Leon MB, Bonow RO, Watson RM, Epstein SE. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation 1985;71:234–43. [DOI] [PubMed]
- 6.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. [DOI] [PubMed]
- 7.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53. [DOI] [PubMed]
- 8.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation 1982;65:1388–94. [DOI] [PubMed]
- 9.Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 1986;8:545–57. [DOI] [PubMed]
- 10.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J 1986;55:575–81. [DOI] [PMC free article] [PubMed]
- 11.Factor SM, Butany J, Sole MJ, Wigle ED, Williams WC, Rojkind M. Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1991;17:1343–51. [DOI] [PubMed]
- 12.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000;35:36–44. [DOI] [PubMed]
- 13.Emoto R, Yokota Y, Fukuzaki H. Prognosis in hypertrophic cardiomyopathy—echocardiographic follow-up and histopathological study. Jpn Circ J 1989;53(9):1031–44. [DOI] [PubMed]
- 14.Schwartzkopff B, Mundhenke M, Strauer BE. Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol 1998;31:1089–96. [DOI] [PubMed]
- 15.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Matsuda M, Hamashima Y, Kawai C. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardio-myopathy. Circulation 1987;75:1130–9. [DOI] [PubMed]
- 16.Liu SK, Roberts WC, Maron BJ. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiol 1993;72:944–51. [DOI] [PubMed]
- 17.Kyriakidis MK, Dernellis JM, Androulakis AE, Kelepeshis GA, Barbetseas J, Anastakasis AN, et al. Changes in phasic coronary blood flow velocity profile and relative coronary flow reserve in patients with hypertrophic obstructive cardiomyopathy. Circulation 1997;96:834–41. [DOI] [PubMed]
- 18.Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998;97:230–3. [DOI] [PubMed]
- 19.Cannon RO 3rd, Dilsizian V, O'Gara PT, Udelson JE, Schenke WH, Quyyumi A, et al. Myocardial metabolic, hemodynamic, and electrocardiographic significance of reversible thallium-201 abnormalities in hypertrophic cardiomyopathy. Circulation 1991;83:1660–7. [DOI] [PubMed]


