Abstract
Background
Aboriginal populations in North America are exhibiting an increased prevalence of cardiovascular disease and associated traditional and nontraditional cardiovascular risk factors, trends believed to be due to the influence of Western lifestyle habits. Because these influences are present at an early age, we sought to study the patterns of one such habit, cigarette smoking, among Aboriginal Canadian youths and to assess the associated accrual of cardiovascular risk factors at an early age.
Methods
Patterns of cigarette smoking were assessed in a population-based, cross-sectional study involving 236 youths aged 10–19 (mean 14.9) years in the Oji-Cree community of Sandy lake, in northwestern Ontario. Participants underwent clinical and metabolic evaluation with assessment of cardiovascular risk factors.
Results
The prevalence of cigarette smoking among the study participants was considerably higher than age-specific national averages, with fully 50% of the participants overall and 82% of the adolescent participants (aged 15–19) being current smokers. Compared with their peers, children smoking 6 or more cigarettes per day had an enhanced cardiovascular risk profile consisting of a higher mean systolic blood pressure (111 v. 107.5 mm Hg, p = 0.036), a higher mean plasma homocysteine level (8.7 v. 7.6 μmol/L, p = 0.008) and a lower mean serum folate level (4.5 v. 5.4 mmol/L, p = 0.007), after adjustment for age, sex and body mass index. In separate multiple linear regression analyses, current cigarette exposure (number of cigarettes smoked per day) emerged as an independent determinant of both systolic blood pressure and plasma homocysteine level.
Interpretation
In this Aboriginal community with remarkably high rates of cigarette smoking among its youth, an independent dose–response relation was found between current smoking exposure and both traditional (systolic blood pressure) and nontraditional (homocysteine level) cardiovascular risk factors. The association of cigarette smoking with an enhanced cardiovascular risk profile at an early age may be a factor contributing to the high prevalence of cardiovascular disease in this Aboriginal population.
Although the incidence of cardiovascular disease and associated death rates have been steadily declining in North America in recent decades, the opposite trend has been occurring in Aboriginal populations in Canada and the United States.1,2,3 For example, in Ontario, hospital admissions because of ischemic heart disease doubled in Aboriginal communities between 1981 and 1997, whereas over the same period the rates in the general population declined.1 A major contributing factor appears to be the development of an excessive burden of cardiovascular risk factors in North American Aboriginal populations, most notably dramatic increases in the prevalence of diabetes mellitus.4,5,6,7 Increased rates of other traditional cardiovascular risk factors (e.g., hypertension, obesity and albuminuria) have also been reported.5,6,7 In addition, novel nontraditional cardiovascular risk factors related to chronic subclinical inflammation (C-reactive protein), adipocyte dysfunction (adiponectin and leptin) and prothrombotic activity (homocysteine) may be further contributing to the burden of vascular disease.8
Indeed, increased serum concentrations of C-reactive protein, an independent predictor of ischemic heart disease, have been documented in adult Oji-Cree of the Sandy Lake First Nation community in northwestern Ontario.9 In the same population, hypoadiponectinemia, an emerging predictor of vascular events, has been associated with an enhanced cardiovascular risk profile, including hyperglycemia, insulin resistance, obesity, hypertriglyceridemia and decreased levels of high-density lipoprotein (HDL) cholesterol.10 Overall, both traditional and nontraditional cardiovascular risk factors appear to be contributing to the increased prevalence of cardiovascular disease in Aboriginal populations.
The rapid accumulation of vascular risk factors in North American indigenous populations has been linked to dramatic changes in culture and lifestyle experienced by Aboriginal people in the past half century. It has been suggested that, in concert with underlying genetic susceptibility, the adoption of dietary habits and sedentary lifestyles typical of Western society has led to the proliferation of vascular and metabolic risk factors and disease in Aboriginal populations. Furthermore, this pathologic gene–environment interaction is occurring at an early age.11 Thus, modifiable risk factors associated with lifestyle among Aboriginal children warrant study and may identify important targets for interventions aimed at preventing future disease.
One such lifestyle factor is cigarette smoking. Cigarette smoking is a primary cause of preventable death in Western society and is associated with both cardiovascular disease and cancer, the 2 leading causes of death in North American Aboriginal populations.12 In the Strong Heart Study, high rates of smoking were reported in American Aboriginal communities, although the numbers of cigarettes smoked per day were below the US average.6 In the Oji-Cree community of Sandy Lake, the prevalence of smoking has been reported to exceed 80% and 70% among men and women, respectively.13 Given such widespread cigarette smoking among Aboriginal adults, it would be of interest to know the smoking patterns of Aboriginal children, particularly since habitual smoking is rarely initiated after the age of 25.14 In this report, we investigate the pattern of cigarette smoking among Aboriginal children and adolescents participating in a population-based study in the Oji-Cree Sandy Lake community and assess the associated accrual of traditional and nontraditional cardiovascular risk factors at an early age.
Methods
The methods of the Sandy Lake Health and Diabetes Project have previously been described in detail.5,8,13 In brief, between July 1993 and December 1995, 728 (71.5%) of 1018 eligible residents (aged 10–79 years) of Sandy Lake, an Aboriginal community in northwestern Ontario, participated in a population-based cross-sectional survey to determine the prevalence of type 2 diabetes and its associated risk factors. The analyses in this report are based on the subgroup of 236 youths aged 10–19 years at the time of the survey, who represented 72.6% of the eligible population in this age range. Signed, informed consent was obtained from all participants or their parents or guardians, and the study was approved by the Sandy Lake First Nation Band Council and the University of Toronto Ethics Review Committee. All interviews and examinations were conducted by trained community members working with the Sandy Lake Health and Diabetes Project.
Study participants underwent oral glucose tolerance testing, with measurement of blood pressure (using an appropriately sized pediatric cuff) and body anthropometry (height, weight and waist-circumference measurement), as previously described.8,13 Diabetes and impaired glucose tolerance were diagnosed according to 1985 World Health Organization criteria.15 Measurements of fasting glucose, insulin, total cholesterol, triglycerides, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, C-reactive protein, adiponectin and vitamin B6 were obtained, as previously described.9,10 Serum levels of vitamin B12 and folate were measured using the Quantaphase II radioassay (Bio-Rad Laboratories, Mississauga, Ont.). Plasma homocysteine levels were measured using the fluorescence polarization immunoassay (Abbott IMx Homocysteine, Abbott Laboratories, Abbott Park, Ill.; reagent no. 69-4242/R4, Axis-Shield ASA, Oslo). Apolipoprotein B levels were determined using nephelometry.16 Insulin resistance was estimated using the homeostasis model assessment index (HOMA-IR).17
“Current smoking” was defined in the interviewer-administered questionnaire as current use, at the time of the survey, of cigarettes, cigars, pipes or chewing tobacco. Because the use of cigars, pipes and chewing tobacco was rare in this pediatric population, the current analysis was restricted to cigarette smoking. “Ever smoking” was defined by any history of ever using cigarettes, pipes, cigars or chewing tobacco on a daily basis. “Cigarettes per day” was defined as the number of cigarettes currently smoked per day (current exposure). “Pack-years,” a measure of cumulative smoking exposure, was defined as the product of the number of years of smoking and the number of packs of cigarettes smoked per day.
The distributions of continuous variables were assessed for normality, and the natural log-transformations of skewed variables (C-reactive protein level, homocysteine level, vitamin B12 level, number of cigarettes per day and HOMA-IR) were used in subsequent analyses, with back-transformed results from multivariate analyses presented in tables. In analyses restricted to normoglycemic participants (because of the potential confounding effects of glucose intolerance), univariate associations of smoking exposure with traditional and nontraditional cardiovascular risk factors were assessed using Spearman correlation analysis. Differences in cardiovascular risk factors between participants with low (≤ 5 cigarettes per day) and high (≥ 6 cigarettes per day) current smoking exposure were assessed by analysis of variance, with adjustment for age, sex and body mass index (BMI). We chose this smoking exposure threshold of 5 cigarettes per day by rounding up from the mean exposure of 4.2 cigarettes per day in this population. Multiple linear regression analysis was used to determine the factors that were significantly and independently associated with variation in systolic blood pressure and log-transformed homocysteine level. A forward selection approach was used with a p value threshold of 0.1 for entry into the model. For the systolic blood pressure model, the following independent variables were considered: age, sex, BMI, total cholesterol:HDL ratio, LDL cholesterol level, HOMA-IR, C-reactive protein, adiponectin, folate, vitamin B12 and homocysteine levels, and number of cigarettes per day. For the homocysteine model, the following independent variables were considered: age, sex, BMI, systolic blood pressure, total cholesterol:HDL ratio, LDL cholesterol level, HOMA-IR, C-reactive protein, adiponectin, folate, vitamin B12 and vitamin B6 levels, and number of cigarettes per day.
Results
Table 1shows the demographic, clinical and metabolic characteristics of the 236 study participants aged 10–19 years. The mean age was 14.9 years, and 61% of the participants were female. Overall, 56% of the participants reported a history of cigarette use at some point in their lives, and 50% described themselves as current smokers. The mean current usage was 4.2 cigarettes per day, with a mean cumulative exposure of 0.8 pack-years.
Table 1
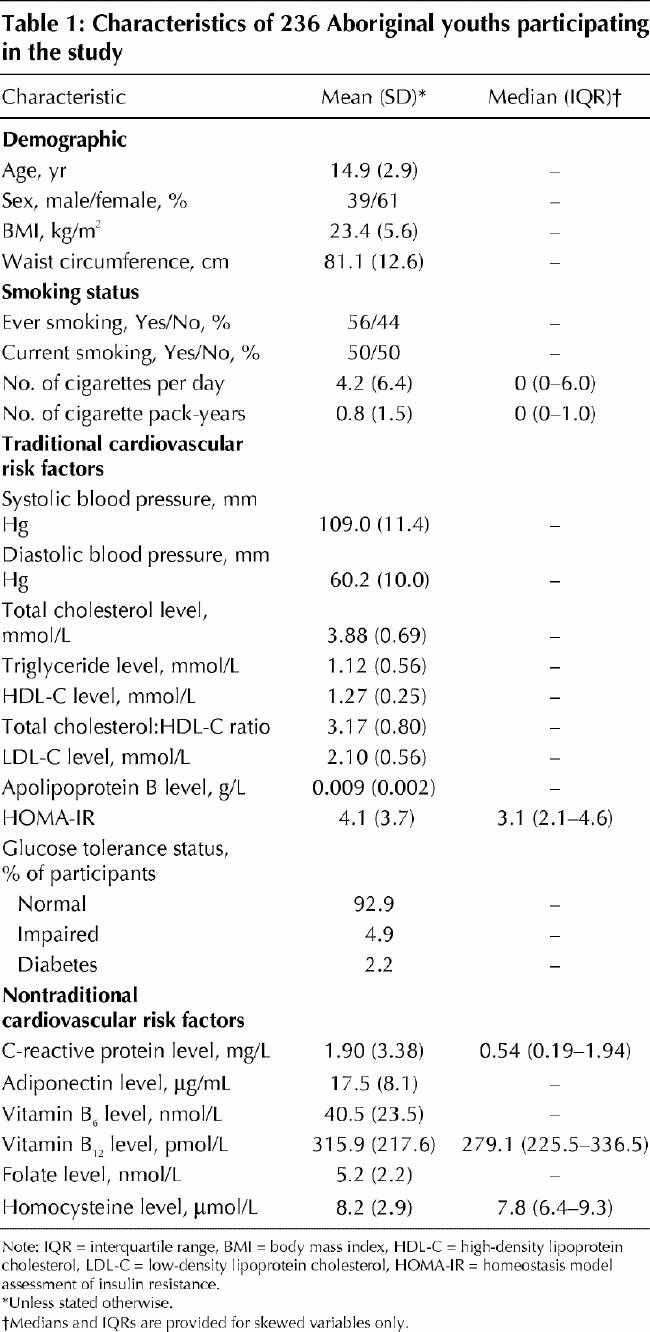
As shown in Fig. 1, the prevalence rates of current smoking among the study participants of this Aboriginal community were considerably higher than age-specific national averages from Health Canada's 1994 Youth Smoking Survey.18 Overall, 82% of the adolescent participants (aged 15–19 years) reported current smoking. The age-specific prevalence of smoking increased dramatically between the ages of 12 and 15 years.
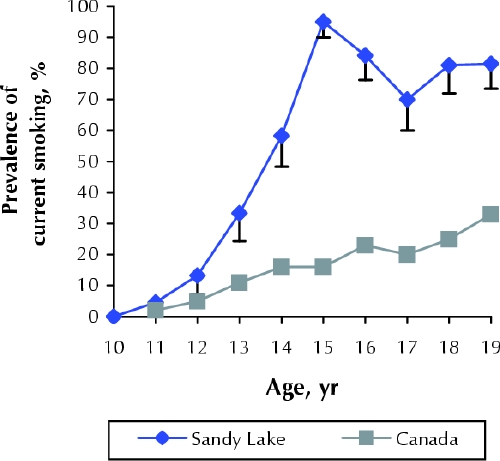
Fig. 1: Prevalence of current smoking among 236 youths aged 10–19 years in the Oji-Cree community of Sandy Lake, in northwestern Ontario, and age-specific national averages from Health Canada's 1994 Youth Smoking Survey.18
To study the potential pathophysiologic correlates of smoking exposure in this pediatric population, Spearman univariate correlations were derived between the numbers of cigarettes currently smoked per day and a series of traditional and nontraditional cardiovascular risk factors in normoglycemic children (n = 220). Current smoking exposure was significantly associated with age (r = 0.62, p < 0.001), increased adiposity (BMI and waist circumference) (both r values ≥ 0.18, p < 0.01) and systolic blood pressure (r = 0.15, p < 0.002). Among nontraditional cardiovascular risk factors, current smoking exposure was positively correlated with total homocysteine concentration (r = 0.31, p < 0.001) and inversely associated with serum levels of folate, vitamin B12, vitamin B6 and adiponectin (all |r| values ≥ 0.18, p < 0.01).
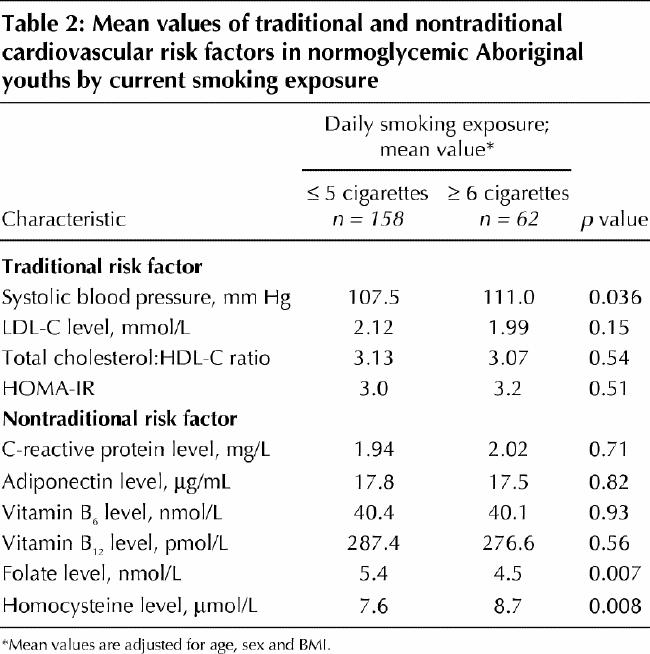
To study the implications of smoking in this pediatric population further, cardiovascular risk profiles were compared between normoglycemic children with high levels of smoking exposure (≥ 6 or more cigarettes per day) and those with lower levels of exposure (≤ 5 cigarettes per day) (Table 2). After adjustment for age, sex and BMI, children smoking 6 or more cigarettes per day were found to have a significantly higher mean systolic blood pressure and plasma homocysteine level and a significantly lower mean serum folate level than children with low levels of smoking exposure. Other cardiovascular risk factors (e.g., lipid levels, insulin resistance, and serum levels of C-reactive protein, adiponectin, and vitamins B6 and B12) did not differ significantly between the 2 groups.
Table 2
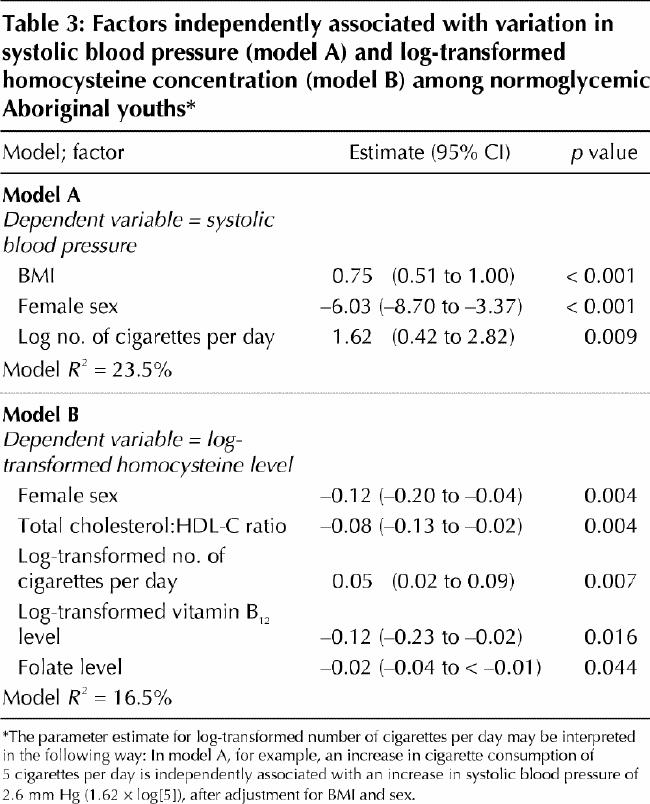
To assess the independent contributions of smoking exposure to cardiovascular risk profile, multiple linear regression models were constructed with the use of demographic variables (age and sex), traditional cardiovascular risk factors (systolic blood pressure, total cholesterol:HDL ratio, LDL cholesterol level, HOMA-IR and BMI) and nontraditional cardiovascular risk factors (serum levels of C-reactive protein, adiponectin, folate, vitamin B12 and homocysteine). On multiple linear regression analysis with systolic blood pressure as the dependent variable (Table 3, model A), current smoking exposure (cigarettes per day) emerged as an independent positive correlate of systolic blood pressure, as did BMI and male sex. Similarly, with log-transformed homocysteine concentration as the dependent variable (Table 3, model B), current smoking exposure emerged as a positive independent correlate of homocysteine concentration; female sex, total cholesterol:HDL ratio, and log-transformed vitamin B12 and folate levels were identified as negative independent covariates.
Table 3
Interpretation
In this study, we documented remarkably high rates of cigarette smoking among youths aged 10–19 years in an Aboriginal Canadian community facing a growing prevalence of cardiovascular disease. We also found an independent dose–response relation between smoking exposure (number of cigarettes per day) and both traditional (systolic blood pressure) and nontraditional (homocysteine concentration) cardiovascular risk factors in this pediatric population. These findings suggest that widespread cigarette smoking among Aboriginal youths is associated with an enhanced cardiovascular risk profile at an early age and may be a factor contributing to the considerable burden of cardiovascular disease in Aboriginal populations.
The rates of current smoking in the Sandy Lake community (50% among youths aged 10–19 and 82% among adolescents aged 15–19) were markedly higher than rates obtained during a similar period as part of the 1994 Youth Smoking Survey18 (15% and 24%, respectively). Indeed, the rates in Sandy Lake exceeded those of all 75 sites from 43 countries participating in the Global Youth Tobacco Survey.19 Our findings are consistent with those from previous studies demonstrating high prevalence rates of smoking among North American Aboriginal youths.18,20,21 However, our study went further by demonstrating a relation between daily smoking exposure and enhanced cardiovascular risk profile among our study participants, a particularly salient concern given the growing prevalence of diabetes and cardiovascular disease in North American indigenous populations. The importance of this issue is further underscored by longitudinal evidence linking childhood cardiovascular risk factors with future atherosclerotic disease in adults.22,23,24,25
We found that the enhanced cardiovascular risk profile associated with smoking exposure among the youths in our study consisted of increased systolic blood pressure and elevated plasma homocysteine concentration. Although previous studies have shown variable associations between smoking and systolic blood pressure in other populations,26,27 our findings suggest that a positive independent, dose-dependent relation exists between the number of cigarettes smoked per day and systolic blood pressure in this Aboriginal pediatric population. It is unclear whether this relation is unique to this population. Although the cross-sectional nature of the study precludes comment on causality, the high prevalence of cigarette smoking reported herein raises the possibility that the prevalence of hypertension in this community may increase as the study population ages.
Smoking has previously been associated with increased homocysteine concentrations in both adults and children.28,29,30,31 The mechanisms underlying this association are unclear, although reduced dietary intake of fruits, vegetables and vitamins and decreased serum levels of vitamin B12 and folate in smokers have been suggested as possible contributory factors.28,29 Interestingly, a dose-dependent relation between the number of cigarettes smoked per day and plasma homocysteine concentrations has been reported in adults.28 Our study extends this dose-dependent relation to a pediatric population in a community facing a growing prevalence of cardiovascular disease.
We recognize that the cross-sectional nature of our study limits our ability to address causality between factors reported herein. Nevertheless, our findings identify important issues for further study and highlight potential public health interventions to consider for this population, including smoking cessation programs, blood pressure surveillance in young adults and possibly homocysteine-lowering therapy. Another limitation of our study is that the data are from 1994 and may not reflect current smoking patterns among Aboriginal youths. On the other hand, the age of the data should not affect the key biologic finding of independent relations between cigarette smoking and specific cardiovascular risk factors in Aboriginal youths.
In summary, we report high rates of cigarette smoking among youths aged 10–19 years in an Aboriginal Canadian community facing a growing prevalence of cardiovascular disease. We found a dose-dependent relation between daily smoking exposure and both traditional (systolic blood pressure) and nontraditional (homocysteine) cardiovascular risk factors in this pediatric population. Prevention and cessation of smoking thus emerge as important public health priorities to consider in this population. Accordingly, plans are underway to target youth smoking as part of an ongoing, community-based intervention program aimed at addressing lifestyle factors associated with type 2 diabetes and other chronic diseases in the Sandy Lake community.32,33
Acknowledgments
We gratefully acknowledge the following groups and individuals: the chief, council and community members of Sandy Lake First Nation, the Sandy Lake community surveyors (Mary Mamakeesic, Tina Noon, Ken Goodwin, Edith Fiddler) and Annette Barnie.
Footnotes
This article has been peer reviewed.
Contributors: Ravi Retnakaran contributed to the data analysis and drafted the article. Anthony Hanley, Philip Connelly, Stewart Harris and Bernard Zinman were involved in the conception and design of the study and the data analysis. All of the authors contributed to the critical revision of the manuscript for intellectual content and approved the final version.
This work was supported by the National Institutes of Health (grants 91-DK-01 and 1-R21-DK-44597-01), the Ontario Ministry of Health and Long-Term Care (grant 04307) and the Canadian Institutes of Health Research (CIHR). Ravi Retnakaran is supported by a CIHR Fellowship. Anthony Hanley is a Scholar of the Canadian Diabetes Association and a Banting and Best Diabetes Centre New Investigator. Stewart Harris is a Career Scientist with the Ontario Ministry of Health and Long-Term Care. Bernard Zinman holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto.
Competing interests: None declared.
Correspondence to: Dr. Bernard Zinman, Mount Sinai Hospital, Lebovic Building, 5th floor, Rm. L5-024, 600 University Ave., Toronto ON M5G 1X5; fax 416 586-4740; zinman@mshri.on.ca
References
- 1.Shah B, Hux J, Zinman B. Increasing rates of ischemic heart disease in the Native population of Ontario, Canada. Arch Intern Med 2000;160:1862-6. [DOI] [PubMed]
- 2.Howard BV, Lee E, Cowan L, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians: the Strong Heart Study. Circulation 1999;99:2389-95. [DOI] [PubMed]
- 3.Sewell J, Malasky B, Gedney C, Gerber TM, Brody EA, Pacheco EA, et al. The increasing incidence of coronary artery disease and cardiovascular risk factors among a southwest Native American Tribe: the White Mountain Apache Heart Study. Arch Intern Med 2002;162:1368-72. [DOI] [PubMed]
- 4.Young TK, Reading J, Elias B, O'Neil JD. Type 2 diabetes in Canada's First Nations: status of an epidemic in progress. CMAJ 2000;163(5):561-6. [PMC free article] [PubMed]
- 5.Harris SB, Gittelsohn J, Hanley AJG, Barnie A, Wolever TMS, Gao XJ, et al. The prevalence of NIDDM and associated risk factors in Native Canadians. Diabetes Care 1997;20:185-7. [DOI] [PubMed]
- 6.Welty T, Lee E, Yeh J, Cowan LD, Go O, Fabsitz RR, et al. Cardiovascular disease risk factors among American Indians: the Strong Heart Study. Am J Epidemiol 1995;142:269-87. [DOI] [PubMed]
- 7.Welty T, Rhoades D, Yeh F, Lee ET, Cowan LD, Fabsitz RR, et al. Changes in cardiovascular disease risk factors among American Indians: the Strong Heart Study. Ann Epidemiol 2002;12:97-106. [DOI] [PubMed]
- 8.Hanley AJ, Harris SB, Gao XJ, Kwan J, Zinman B. Serum immunoreactive leptin concentrations in a Canadian Aboriginal population with high rates of NIDDM. Diabetes Care 1997;20:1408-15. [DOI] [PubMed]
- 9.Connelly PW, Hanley AJ, Harris SB, Hegele RA, Zinman B. Relation of waist circumference and glycemic status to C-reactive protein in Sandy Lake Oji-Cree. Int J Obes Relat Metab Disord 2003;27:347-54. [DOI] [PubMed]
- 10.Hanley AJ, Connelly PW, Harris SB, Zinman B. Adiponectin in a Native Canadian population experiencing rapid epidemiological transition. Diabetes Care 2003;26:3219-25. [DOI] [PubMed]
- 11.Hanley AJ, Harris SB, Gittelsohn J, Wolever T, Saksvig R, Zinman B. Overweight among children and adolescents in a Native Canadian community: prevalence and associated factors. Am J Clin Nutr 2000;71:693-700. [DOI] [PubMed]
- 12.Young TK. The health of Native Americans: towards a biocultural epidemiology. New York: Oxford University Press; 1994.
- 13.Harris SB, Zinman B, Hanley AJ, Gittelsohn J, Hegele R, Connelly PW, et al. The impact of diabetes on cardiovascular risk factors and outcomes in a Native Canadian population. Diabetes Res Clin Pract 2002;55:165-73. [DOI] [PubMed]
- 14.US Department of Health and Human Services. Preventing tobacco use among young people: a report of the Surgeon General. Washington: US Department of Health and Human Services; 1994.
- 15.World Health Organization. Diabetes mellitus: report of a WHO study group [Technical Report series no 727]. Geneva: WHO; 1985. [PubMed]
- 16.Lipid Research Clinics Program. Manual of laboratory operations: lipid and lipoprotein analysis. 2nd ed. Bethesda (MD): Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services; 1982.
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9. [DOI] [PubMed]
- 18.Stephens T, Morin M, editors. Youth Smoking Survey, 1994: technical report. Ottawa: Health Canada; 1996. Cat no H49-98/1-1994E.
- 19.Global Youth Tobacco Survey Collaborative Group. Tobacco use among youth: a cross country comparison. Tob Control 2002;11:252-70. [DOI] [PMC free article] [PubMed]
- 20.LeMaster P, Connell C, Mitchell C, Manson S. Tobacco use among American Indian adolescents: protective and risk factors. J Adolesc Health 2002; 30: 426-32. [DOI] [PubMed]
- 21.US Centers for Disease Control. Tobacco, alcohol, and other drug use among high school students in Bureau of Indian Affair-funded schools — United States 2001. MMWR Morb Mortal Wkly Rep 2003;52:1070-2. [PubMed]
- 22.Raitakari O, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 2003;290:2277-83. [DOI] [PubMed]
- 23.Li S, Chen W, Srinivasan S, Bond MG, Tang R, Urbina EM, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 2003;290:2271-6. [DOI] [PubMed]
- 24.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors from childhood through middle age: the Muscatine Study. Circulation 2001;104:2815-9. [DOI] [PubMed]
- 25.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol 1996;27:277-84. [DOI] [PubMed]
- 26.Green MS, Jucha E, Luz Y. Blood pressure in smokers and non-smokers: epidemiologic findings. Am Heart J 1986;111:932-40. [DOI] [PubMed]
- 27.Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension 2001;37:187-93. [DOI] [PubMed]
- 28.Nygard O, Vollset S, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profile: the Hordaland Homocysteine Study. JAMA 1995;274:1526-33. [DOI] [PubMed]
- 29.Osganian S, Stampfer M, Spiegelman D, Rimm E, Cutler JA, Feldman HA, et al. Distribution of and factors associated with serum homocysteine levels in children: Child and Adolescent Trial for Cardiovascular Health. JAMA 1999; 281: 1189-96. [DOI] [PubMed]
- 30.Ganji V, Kafai M. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2003;77:826-33. [DOI] [PubMed]
- 31.Bates C, Mansoor M, Gregory J, Pentieva K, Prentice A. Correlates of plasma homocysteine, cysteine, and cysteinyl-glycine in respondents in the British National Diet and Nutrition Survey of young people aged 4-18 years and a comparison with the survey of people aged 65 years and over. Br J Nutr 2002; 87:71-9. [DOI] [PubMed]
- 32.Harris SB, Zinman B. Primary prevention of type 2 diabetes in high-risk populations. Diabetes Care 2000;23:879-81. [DOI] [PubMed]
- 33.Gittelsohn J, Harris SB, Whitehead S, Wolever T, Hanley AJ, Barnie A, et al. Developing diabetes interventions in an Ojibwa-Cree community in northern Ontario: linking qualitative and quantitative data. Chronic Dis Can 1995; 16: 157-64.


