Abstract
ECTOPIC PREGNANCY IS A LIFE- AND FERTILITY-threatening condition that is commonly seen in Canadian emergency departments. Increases in the availability and use of hormonal markers, coupled with advances in formal and emergency ultrasonography have changed the diagnostic approach to the patient in the emergency department with first-trimester bleeding or pain. Ultrasonography should be the initial investigation for symptomatic women in their first trimester; when the results are indeterminate, the serum β human chorionic gonadotropin (β-hCG) concentration should be measured. Serial measurement of β-hCG and progesterone concentrations may be useful when the diagnosis remains unclear. Advances in surgical and medical therapy for ectopic pregnancy have allowed the proliferation of minimally invasive or noninvasive treatment. Guidelines for laparoscopy and for methotrexate therapy are provided.
Ectopic pregnancy, in which the gestational sac is outside the uterus, is the most common life-threatening emergency in early pregnancy. The incidence in the United States has increased greatly in the last few decades, from 4.5 per 1000 pregnancies in 1970 to an estimated 19.7 per 1000 pregnancies in 1992.1,2 Although spontaneous resolution of ectopic pregnancy can occur, patients are at risk of tubal rupture and catastrophic hemorrhage.3,4 Ectopic pregnancy remains an important cause of maternal death, accounting for about 4% of the approximately 20 annual pregnancy-related deaths in Canada.5 Despite the relatively high frequency of this serious condition, early detection can be challenging. In up to half of all women with ectopic pregnancy presenting to an emergency department, the condition is not identified at the initial medical assessment.6 Although the incidence of ectopic pregnancy in the general population is about 2%, the prevalence among pregnant patients presenting to an emergency department with first-trimester bleeding or pain, or both, is 6% to 16%.7,8,9,10,11,12,13,14 Thus, greater suspicion and a lower threshold for investigation are justified.
The availability of newer hormonal markers and ultrasound imaging has increased the complexity of the diagnostic workup in patients suspected of having an ectopic pregnancy, and the evolution of less invasive surgical techniques and noninvasive medical management has altered the treatment landscape. In this review we summarize the current literature examining the impact of recent advances in the diagnosis and treatment of ectopic pregnancy. Articles cited were identified with use of the keywords “ectopic pregnancy,” “epidemiology,” “diagnosis,” “radiography,” “ultrasonography,” “therapy,” “surgery,” “methotrexate,” “emergency department” and “emergency” in searches of MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews.
Diagnosis
Historical features and physical findings
Ectopic pregnancy is usually diagnosed in the first trimester of pregnancy. The most common gestational age at diagnosis is 6 to 10 weeks, but fetal viability can be discovered until the time of delivery.15,16 Ectopic pregnancy has about the same frequency across a wide range of maternal ages and ethnic origins. Documentation of risk factors (Table 19,17,18) is an essential part of history-taking, and asymptomatic clinic patients with risk factors may benefit from routine early imaging.19 However, more than half of identified ectopic pregnancies are in women without known risk factors.8,9
Table 1
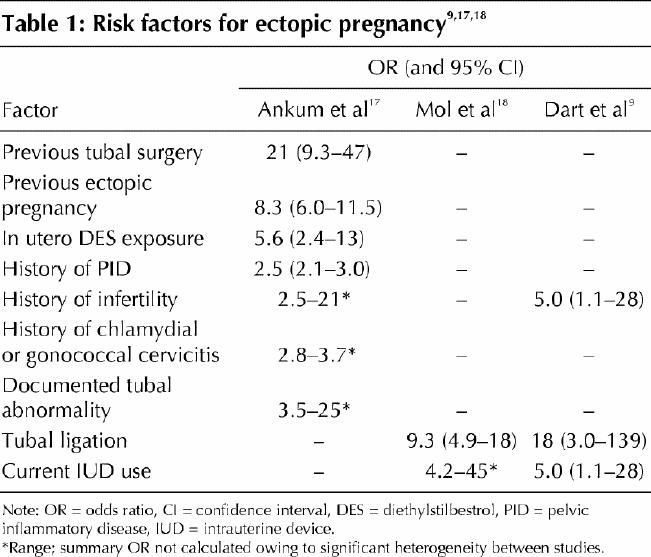
The physical findings depend on whether tubal rupture has occurred. Women with intraperitoneal hemorrhage present with significant abdominal pain and tenderness, along with various degrees of hemodynamic instability. However, women without rupture may also present with pelvic pain or vaginal bleeding, or both.8,9,20,21 Several investigators have measured the predictive value of specific risk factors and physical findings alone or in combination: no combination correctly and consistently ruled out ectopic pregnancy.8,9,21 Given the high prevalence of ectopic pregnancy among pregnant women presenting to emergency departments, further investigation is prudent for all patients presenting with first-trimester bleeding or pain.
Use of β human chorionic gonadotropin measurement
It is important to confirm pregnancy. In the emergency department, pregnancy is diagnosed by determining the urine or serum concentration of β human chorionic gonadotropin (β-hCG). This hormone is detectable in urine and blood as early as 1 week before an expected menstrual period. Serum testing detects levels as low as 5 IU/L, whereas urine testing detects levels as low as 20–50 IU/L.22 In most cases, screening is done with a urine test, since obtaining the results of a serum test is time-consuming and is not always possible in the evening and at night. However, if pregnancy is strongly suspected, even when the urine test has a negative result, serum testing will be definitive.
A single serum measurement of the β-hCG concentration, however, cannot identify the location of the gestational sac. Although women with an ectopic pregnancy tend to have lower β-hCG levels than those with an intrauterine pregnancy, there is considerable overlap (Table 2).23,24
Table 2
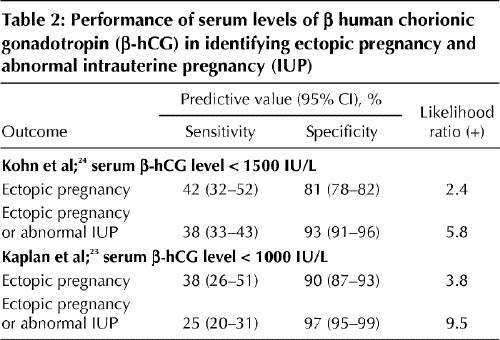
If a low serum β-hCG level (< 1000 IU/L) is associated with a higher relative risk of ectopic pregnancy, then can very low levels predict a benign clinical course? In general, no. Although a single very low serum level (< 100 IU/L) has been felt to be reassuring, in a review of 716 admitted patients with ectopic pregnancy, 29% of those with such a level were found to have tubal rupture at laparoscopy.25 The risk of tubal rupture was similar across a wide range of β-hCG values. Another study identified 38 instances of rupture among women with serum levels ranging from 10 to 189 720 IU/L.7 Thus, a single serum β-hCG measurement cannot exclude ectopic pregnancy or predict the risk of rupture unless it is less than 5 IU/L.
Serial β-hCG measurement is often used for women with first-trimester bleeding or pain, or both, but, as with a single measurement, serial measurement cannot confirm the location of the gestational sac. In a normal pregnancy, the first-trimester β-hCG concentration rapidly increases, doubling about every 2 days. An increase over 48 hours of at least 66% has been used as a cutoff point for viability.20,26,27 Ectopic pregnancy may present with rising, falling or plateau β-hCG levels; thus, serial measurement is most useful to confirm fetal viability rather than to identify ectopic pregnancy. In a patient with a subnormal increase in β-hCG concentration, nonviability is assumed, and more invasive investigations can be used to clarify the nature of the abnormality (i.e., miscarriage v. ectopic pregnancy). However, over-reliance on the doubling time may result in the interruption of a normal pregnancy through diagnostic dilatation and curettage (D&C) or administration of methotrexate. A recent study identified patients with only a 53% increase in serum β-hCG levels over 2 days who had a viable intrauterine pregnancy.28 Thus, demonstration of normal doubling of serum levels over 48 hours supports a diagnosis of fetal viability but does not rule out ectopic pregnancy, and a rising β-hCG concentration that fails to reach 50% suggests a failing or ectopic pregnancy, as does a plateau. Falling levels confirm nonviability but do not rule out ectopic pregnancy.
Use of progesterone measurement
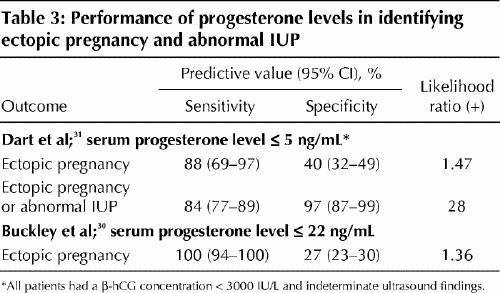
Measurement of the serum concentration of progesterone has been investigated as a potentially useful adjunct to serum β-hCG measurement, since progesterone levels are stable and independent of gestational age in the first trimester.14 A meta-analysis, published in 1998, of studies assessing a single progesterone level demonstrated good capacity of low levels (≤ 5 ng/mL) to correctly diagnose pregnancy failure, but this cutoff was unable to discriminate between ectopic pregnancy and intrauterine pregnancy.29 Both high (> 22 ng/mL) and low (≤ 5 ng/mL) cutoff points have since been studied for their ability to correctly identify nonviable pregnancy and ectopic pregnancy (Table 3).30,31 Rapid progesterone analysis can identify 2 important subgroups of patients in the emergency department with symptomatic first-trimester bleeding or pain, or both: stable patients with progesterone levels above 22 ng/mL, who have a high (but not certain) likelihood of viable intrauterine pregnancy; and patients with levels of 5 ng/mL or less, who almost certainly have a nonviable pregnancy. Invasive diagnostic testing (e.g., D&C) could be postponed in the former patients but offered to the latter, as could treatment with methotrexate, without fear of interrupting a potentially viable intrauterine pregnancy.
Table 3
Ultrasound imaging
Transvaginal ultrasonography has transformed the assessment of women with problematic early pregnancy, allowing earlier, clearer visualization of both normally developing embryos and abnormalities. A normal gestational sac, an ovoid collection of fluid adjacent to the endometrial stripe, can be visualized by means of the transvaginal probe at a gestational age of about 5 weeks. It can often be seen when 2 or 3 mm in diameter and should be consistently seen at 5 mm. Since the hormonal environment in ectopic pregnancy can produce an intrauterine fluid collection that mimics a gestational sac (the “pseudogestational sac” shown in Fig. 1, arrow), a sac alone cannot confirm intrauterine pregnancy.32
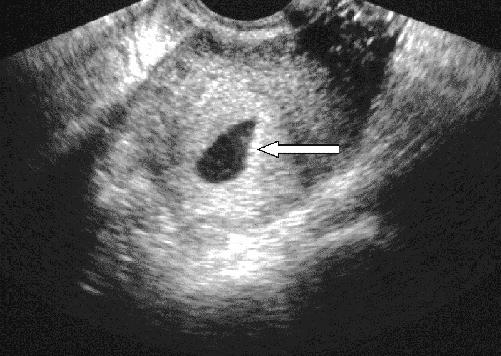
Fig. 1: Transvaginal ultrasound image, showing intrauterine fluid collection without yolk sac or fetal pole: “pseudogestational sac” (arrow).
As the embryo matures, more sonographic signs become visible. Once the sac is implanted within the endometrium, its position relative to the endometrial wall changes, producing the intradecidual-sac sign and then the double decidual-sac sign. The earliest embryonic landmark, the yolk sac, appears when the sac is 8 mm or more in diameter, usually during the fifth week of gestation (Fig. 2). Cardiac activity can be seen with endovaginal scanning when the embryo reaches 4 to 5 mm in diameter, at a gestational age of 6–6.5 weeks.32
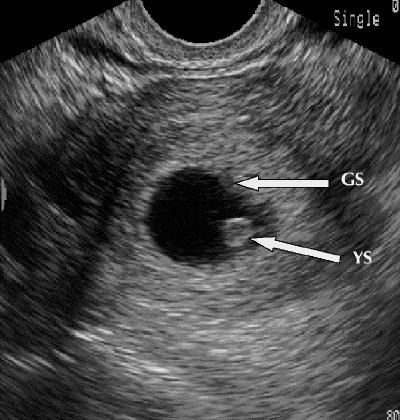
Fig. 2: Transvaginal ultrasound image, showing early intrauterine gestational sac (GS) with yolk sac (YS).
The concept of the discriminatory threshold (the β-hCG level at which an intrauterine gestational sac can be reliably seen in a normal pregnancy) has existed since the early 1980s.33 A β-hCG level that has risen above the discriminatory threshold in the absence of sonographic signs of early pregnancy is considered presumptive evidence of an ectopic pregnancy. With the evolution in ultrasound technology, the discriminatory threshold has dropped from 6500 IU/L with a transabdominal approach to between 1000 and 2000 IU/L with transvaginal imaging.34 This threshold is user- and machine-dependent and thus will vary slightly from institution to institution. Caution should be used in assuming an ectopic pregnancy when a nondiagnostic ultrasound image accompanies a single β-hCG level above the discriminatory threshold: several articles have reported a small number of patients with indeterminate ultrasound images and β-hCG levels above the threshold who have been eventually found to have a viable intrauterine pregnancy.34,35,36 In addition, there could be unseen multiple intrauterine gestational sacs, since the β-hCG values relative to gestational age are higher in patients with multiple embryos.37
Ultrasonographic identification of an intrauterine pregnancy (gestational sac plus yolk sac or other embryonic sign) rules out ectopic pregnancy in most patients.32 The exception is in patients with ovulation induction and assisted conception, who are at risk of heterotopic pregnancy (dizygotic twins, 1 intrauterine and 1 extrauterine). Although this phenomenon is exceedingly rare in the general population (estimated frequency 1 per 3889 to 30 000 pregnancies),38 in the setting of assisted reproduction it may occur in 1 in 100 pregnancies.39
The spectrum of sonographic findings in ectopic pregnancy is broad. Identification of an extrauterine gestational sac containing a yolk sac (with or without an embryo) confirms the diagnosis. Suggestive findings include an empty uterus, cystic or solid adnexal or tubal masses (including the tubal-ring sign, representing a tubal gestational sac), hematosalpinx and echogenic or sonolucent cul-de-sac fluid (Fig. 3).
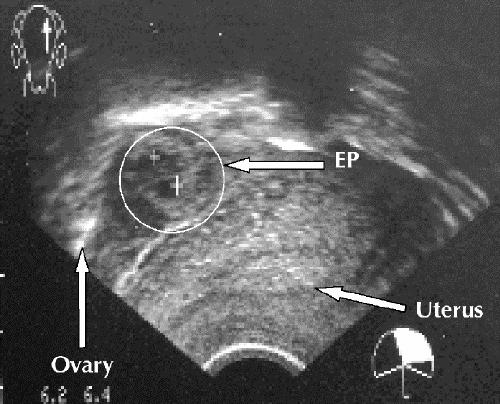
Fig. 3: Transvaginal ultrasound image, showing empty uterus and complex adnexal mass (ectopic pregnancy [EP]) separate from ovary.
Many prospective studies have shown that “formal” transvaginal ultrasound imaging (i.e., that performed by ultrasound technicians and interpreted by radiologists) in the emergency department has high accuracy in confirming intrauterine and ectopic pregnancy. Most protocols can establish a diagnosis with the initial scan in more than 75% of emergency department patients.23,35,40 A diagnosis can often be established even in the subgroup of patients with β-hCG levels below the discriminatory threshold. In some studies, transvaginal scanning has identified up to one-third of the patients with below-threshold β-hCG levels who had ectopic pregnancy.35,41 Given the likelihood of a definitive diagnosis, even with below-threshold β-hCG levels, ultrasonography is the best initial investigation in problematic early pregnancy.
Because expertise in transvaginal ultrasonography is not available in all hospitals and may not be quickly available in some larger centres, there have been several studies of ultrasonography performed by emergency physicians in the assessment of patients with first-trimester bleeding or pain. Ultrasonography in the emergency department (ED-based ultrasonography) has evolved over the last decade and is now part of the diagnostic work-up for many clinical problems in major Canadian centres, as well as in a large number of smaller community emergency departments (Dr. Ray Wiss, Emergency Department Echo course director: personal communication, 2005). The evaluation involves 2 “Yes/No” questions: Can an intrauterine pregnancy be identified? Is there free pelvic or intra-abdominal fluid? This approach is in contrast to the goal of a “formal” pelvic ultrasound study, which is to describe the anatomic appearance and visible abnormalities of the uterus, adnexa and cul-de-sac. Absence of an intrauterine pregnancy translates to a risk of ectopic pregnancy of about 36%,42 and free fluid in the cul-de-sac represents an even higher risk. Several studies have documented the ability of emergency physicians to quickly and accurately identify both intrauterine pregnancy and intra-abdominal free fluid by means of ED-based ultrasonography after brief standardized training.12,42,43 The addition of ED-based ultrasonography to structured protocols for assessing symptomatic patients in the 1st trimester of pregnancy has led to a dramatically decreased stay in the emergency department44 as well as a decrease in the incidence of complications associated with missed ectopic pregnancy and tubal rupture.12
Transvaginal ultrasonography should therefore be the initial investigation for pregnant patients presenting to the emergency department with first-trimester bleeding or pain. Not only is it highly accurate in identifying ectopic pregnancy, but also it offers patients what they are most expecting from their visit: information about the health and viability of their pregnancy. No combination of history-taking, physical examination and laboratory tests can make the same claim. The use of ED-based ultrasonography offers rapid bedside detection of a viable intrauterine pregnancy or a high risk of ectopic pregnancy. Emergency physicians without access to bedside ED-based ultrasonography should arrange formal ultrasonography for all patients with early-pregnancy complaints. This investigation can be performed during the initial visit or, if the patient is stable and has minimal symptoms, the next day in an outpatient visit. However, in the case of outpatient investigation, mechanisms for timely follow-up, re-examination and further investigation must be in place.
Fig. 4 outlines the recommended approach to imaging and follow-up.
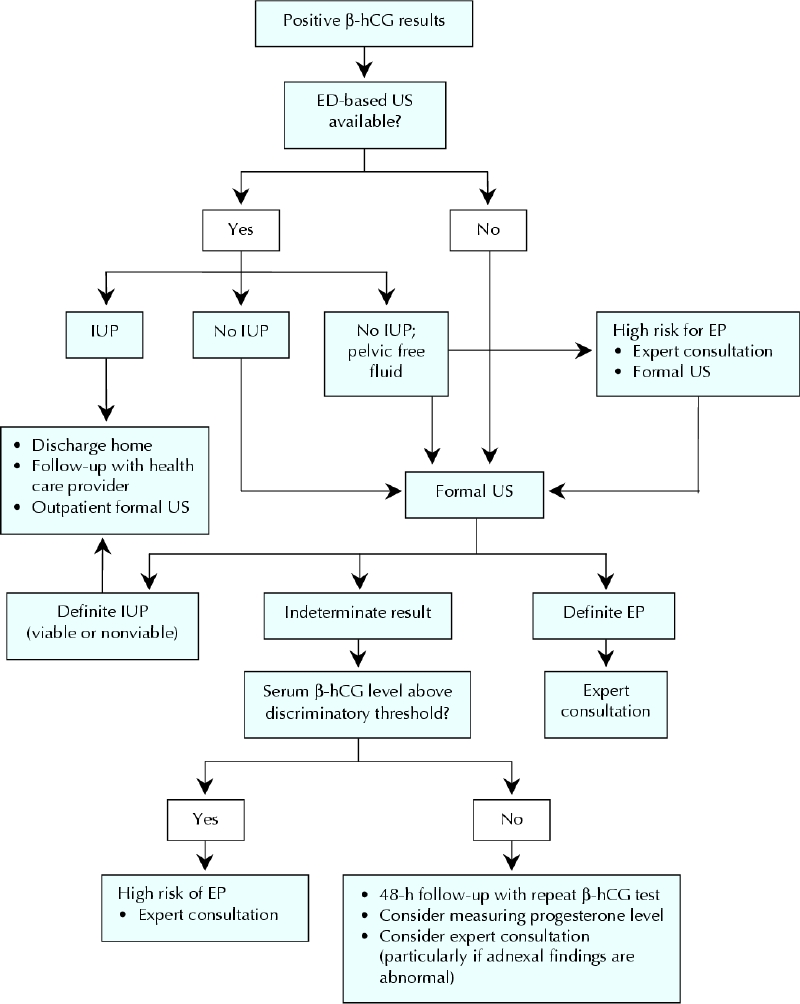
Fig. 4: Recommended approach to investigating first-trimester pain or bleeding in the hemodynamically stable patient in the emergency department (ED). β-hCG = β human chorionic gonadotropin, US = ultrasonography, IUP = intrauterine pregnancy, EP = ectopic pregnancy.
Treatment
Expectant management
Ectopic pregnancy can resolve spontaneously through regression or tubal abortion. However, about 90% of women with ectopic pregnancy and serum β-hCG levels greater than 2000 IU/L require operative intervention owing to increasing symptoms or tubal rupture.3,4 Tubal rupture can also occur when serum β-hCG levels are low or declining, or both.45 Expectant management should be offered only when transvaginal ultrasonography fails to show the location of the gestational sac and the serum levels of β-hCG and progesterone are low and declining. Because of the possibility of tubal rupture, these patients must be carefully monitored until the serum β-hCG concentration falls below 15 IU/L; at this point almost all ectopic pregnancies resolve spontaneously, without rupture.
Surgical management
Surgical management of ectopic pregnancy should be reserved for patients who refuse or have contraindications to medical treatment, those in whom medical treatment has failed and those who are hemodynamically unstable.
Three randomized studies have demonstrated that, compared with laparotomy, laparoscopic treatment of ectopic pregnancy is associated with lower cost, shorter hospital stay, less operative time, less blood loss, less analgesic requirement and faster recovery.46,47,48 Patients randomly assigned to laparoscopy also had fewer adhesions than patients treated with laparotomy (19% v. 64%).49
Tube-sparing salpingostomy (in which the gestational sac is removed, without the tube, through a 1-cm-long incision on the tubal wall) is preferred to salpingectomy (removal of the tube), as the former is less invasive but has comparable rates of subsequent fertility and ectopic pregnancy.50,51,52 However, 8% of patients have persistent ectopic pregnancy after salpingostomy.50 Follow-up determinations are required until β-hCG is undetectable. Regardless of the type of surgery, contralateral tubal abnormalities predispose the patient to recurrent ectopic pregnancy. In a retrospective study of 276 women with ectopic pregnancy, the cumulative rates of spontaneous intrauterine pregnancy over 7 year were 89% after conservative surgery and 66% after radical surgery.50 There was no significant difference in the risk of repeat ectopic pregnancy (17% after conservative surgery and 16% after radical surgery).
In summary, salpingostomy is preferred, particularly for women who wish to have another pregnancy. Salpingectomy may be necessary for women with uncontrolled bleeding, recurrent ectopic pregnancy in the same tube, a severely damaged tube or a tubal gestational sac greater than 5 cm in diameter.53
Medical treatment
Methotrexate (MTX), a folic acid antagonist, inhibits DNA synthesis in actively dividing cells, including trophoblasts. Administered to properly selected patients, it has a success rate of up to 94%.53 The success in ectopic pregnancy depends mainly on β-hCG concentration: a meta-analysis of data for 1327 women with ectopic pregnancy treated with MTX showed that resolution was inversely associated with β-hCG level, and that increasing levels were significantly correlated with treatment failure. Fetal cardiac activity was also associated with MTX treatment failure. However, tubal diameter, a measure of fetal size, is unrelated to outcome.54
The criteria for MTX treatment of ectopic pregnancy are as follows:
Hemodynamic stability.
Ability and willingness of the patient to comply with post-treatment monitoring.
Pretreatment serum β-hCG concentration less than 5000 IU/L.
Absence of ultrasound evidence of fetal cardiac activity.
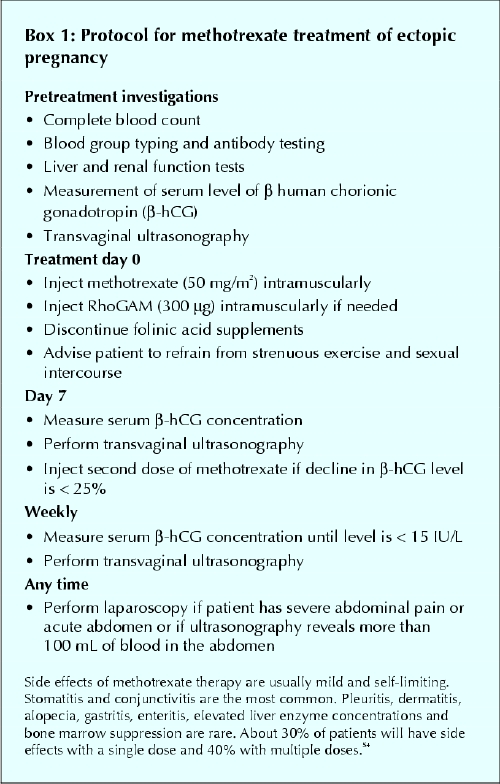
Our protocol for using MTX in the management of ectopic pregnancy is shown in Box 1.
Box 1.
The overall success rate is greater with multiple-dose MTX therapy than with single-dose therapy (93% v. 88%); however, single-dose therapy is less expensive, has a lower rate of side effects (29% v. 48%), requires less intensive monitoring, does not require rescue with folinic acid and is effective for most women.54 The 2 regimens have not been directly compared in randomized trials. Patients in whom laparoscopy may be challenging (including those with many previous laparotomies and scarring) may have a better outcome with MTX treatment. In the presence of relative contraindications, such as high serum β-hCG levels (≥ 5000 IU/L) and the presence of fetal cardiac activity, multiple-dose treatment should be considered.
Patients treated with MTX should be followed closely. The serum β-hCG concentration should be measured weekly. An increased level is uncommon 3 to 4 d after MTX administration. Patients may experience abdominal pain from tubal abortion or tubal distention due to hematoma formation. Severe abdominal pain, however, can be a sign of actual or impending tubal rupture. If the serum β-hCG concentration has not declined by at least 25% 1 week after MTX administration, a second dose should be given. In general, a second dose is needed in 15% to 20% of patients.54,55 Only 1% of patients need more than 2 doses.54 The time for the serum β-hCG concentration to decline to less than 15 IU/L is 33.6 days on average but may be up to 109 days.55
Surgical versus medical treatment
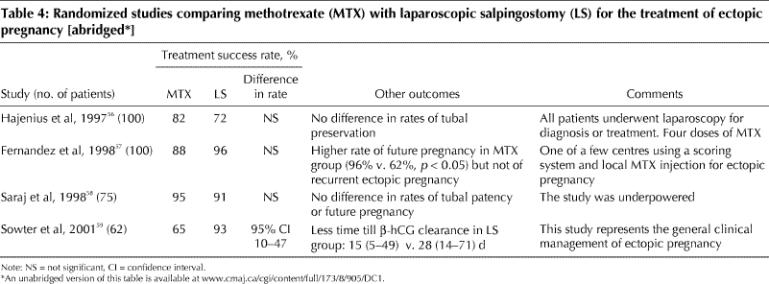
Several randomized studies found that MTX treatment in selected patients with ectopic pregnancy was as effective as laparoscopic treatment (Table 4).56,57,58,59 The 2 treatments were also equally effective in tubal preservation; however, the β-hCG concentration declined more quickly after laparoscopic surgery.56 After MTX treatment the health-related quality of life may diminish, possibly owing to both long-term persistence of the ectopic pregnancy and the long treatment course. There were more physical symptoms after 2 days and 2 weeks in those given MTX, although symptoms were increased in both treatment groups. However, because of the noninvasive nature of MTX treatment, most patients are willing to cope with this short-term burden.60 MTX treatment is less expensive than laparoscopic surgery,59,60 although in one study this was true only if the initial serum β-hCG level was less than 1500 IU/L.60
Table 4
Conclusions
Ectopic pregnancy is a common and serious problem, with a significant morbidity rate and the potential for maternal death. Many patients have no documented risk factors and no physical indications of ectopic pregnancy. Ultrasonography (either formal or ED-based) is the initial investigation that should be done in an ED patient with 1st-trimester bleeding or pain; indeterminate results may be clarified by measurement (single or serial) of the serum β-hCG and progesterone concentrations. Expert consultation with radiologists and gynecologists is recommended whenever ectopic pregnancy is suspected.
Management is dictated by the clinical presentation, serum β-hCG levels and transvaginal ultrasound findings. MTX, as a single intramuscular injection, can be given to women who are hemodynamically stable and compliant and have an initial serum β-hCG concentration of less than 5000 IU/L and no ultrasound evidence of fetal cardiac activity. Patients who do not meet these criteria should be treated surgically, in most cases by laparoscopy. Surgical treatment is particularly appropriate for women who are hemodynamically unstable or unlikely to be compliant with post-treatment monitoring and those who do not have immediate access to medical care. The choice of treatment should be guided by the patient's preference, after a detailed discussion about monitoring, outcome, risks, and benefits of the 2 approaches.
Supplementary Material
Footnotes
This article has been peer reviewed.
Contributors: Heather Murray and Trevor Bardell reviewed the relevant literature and wrote the first draft of the diagnosis section of the manuscript. Hanadi Baakdah and Togas Tulandi reviewed the relevant literature and wrote the first draft of the treatment section of the manuscript. All of the authors provided revisions and approved the final content of the manuscript.
Competing interests: None declared.
Correspondence to: Dr. Heather Murray, Department of Emergency Medicine, Queen's University, Kingston General Hospital, 76 Stuart St., Kingston ON K7L 2V7; fax 613 548-1374; hm9@post.queensu.ca
References
- 1.Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy — United States, 1970–1989. MMWR CDC Surveill Summ 1993;42:73-85. [PubMed]
- 2.Ectopic pregnancy — United States, 1990–1992. MMWR Morb Mortal Wkly Rep 1995;44:46-8. [PubMed]
- 3.Shalev E, Peleg D, Tsabari A, Romano S, Bustan M. Spontaneous resolution of ectopic tubal pregnancy: natural history. Fertil Steril 1995;63:15-9. [DOI] [PubMed]
- 4.Elson J, Tailor A, Banerjee S, Salim R, Hillaby K, Jurkovic D. Expectant management of tubal ectopic pregnancy: prediction of successful outcome using decision tree analysis. Ultrasound Obstet Gynecol 2004;23:552-6. [DOI] [PubMed]
- 5.Turner LA, Cyr M, Kinch RA, Liston R, Kramer MS, Fair M, et al.; Maternal Mortality and Morbidity Study Group of the Canadian Perinatal Surveillance System. Under-reporting of maternal mortality in Canada: a question of definition. Chronic Dis Can 2002;23:22-30. [PubMed]
- 6.Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med 1993;329:1174-81. Comments in N Engl J Med 1994;330:712-3. [DOI] [PubMed]
- 7.Barnhart K, Mennuti MT, Benjamin I, Jacobson S, Goodman D, Coutifaris C. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol 1994;84:1010-5. [PubMed]
- 8.Buckley RG, King KJ, Disney JD, Gorman JD, Klausen JH. History and physical examination to estimate the risk of ectopic pregnancy: validation of a clinical prediction model. Ann Emerg Med 1999;34:589-94. Comment in Ann Emerg Med 1999;34:664-7. [DOI] [PubMed]
- 9.Dart RG, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med 1999;33:283-90. [DOI] [PubMed]
- 10.Durham B, Lane B, Burbridge L, Balasubramaniam S. Pelvic ultrasound performed by emergency physicians for the detection of ectopic pregnancy in complicated first-trimester pregnancies. Ann Emerg Med 1997;29:338-47. [DOI] [PubMed]
- 11.Spandorfer SD, Barnhart KT. Role of previous ectopic pregnancy in altering the presentation of suspected ectopic pregnancy. J Reprod Med 2003;48:133-6. [PubMed]
- 12.Mateer JR, Valley VT, Aiman EJ, Phelan MB, Thoma ME, Kefer MP. Outcome analysis of a protocol including bedside endovaginal sonography in patients at risk for ectopic pregnancy. Ann Emerg Med 1996;27:283-9. [DOI] [PubMed]
- 13.Sauer MV, Rodi IA. Utility of an algorithm to diagnose ectopic pregnancy. Int J Gynaecol Obstet 1990;31:29-34. [DOI] [PubMed]
- 14.Stovall TG, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med 1990;19:1098-103. [DOI] [PubMed]
- 15.Xiao GH, Chen DJ, Sun XF, She RQ, Mai YM. Abdominal pregnancy: full-term viable baby. Eur J Obstet Gynecol Reprod Biol 2005;118:117-8. [DOI] [PubMed]
- 16.Ramachandran K, Kirk P. Massive hemorrhage in a previously undiagnosed abdominal pregnancy presenting for elective cesarean delivery. Can J Anaesth 2004;51:57-61. [DOI] [PubMed]
- 17.Ankum WM, Mol BW, van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril 1996;65:1093-9. Comment in Fertil Steril 1997;67:791-2. [PubMed]
- 18.Mol BW, Ankum WM, Bossuyt PM, van der Veen F. Contraception and the risk of ectopic pregnancy: a meta-analysis. Contraception 1995;52:337-41. [DOI] [PubMed]
- 19.Cacciatore B, Stenman UH, Ylostalo P. Early screening for ectopic pregnancy in high-risk symptom-free women. Lancet 1994;343:517-8. [DOI] [PubMed]
- 20.Mol BW, Hajenius PJ, Engelsbel S, Ankum WM, van der Veen F, Hemrika DJ, et al. Can noninvasive diagnostic tools predict tubal rupture or active bleeding in patients with tubal pregnancy? Fertil Steril 1999;71:167-73. [DOI] [PubMed]
- 21.Mol BW, Hajenius PJ, Engelsbel S, Ankum WM, van der Veen F, Hemrika DJ, et al. Should patients who are suspected of having an ectopic pregnancy undergo physical examination? Fertil Steril 1999;71:155-7. [DOI] [PubMed]
- 22.Brennan DF. Ectopic pregnancy — Part I: Clinical and laboratory diagnosis. Acad Emerg Med 1995;2:1081-9. [DOI] [PubMed]
- 23.Kaplan BC, Dart RG, Moskos M, Kuligowska E, Chun B, Adel Hamid M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med 1996;28:10-7. Comment in Ann Emerg Med 1997;29:295-6. [DOI] [PubMed]
- 24.Kohn MA, Kerr K, Malkevich D, O'Neil N, Kerr MJ, Kaplan BC. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med 2003;10:119-26. [DOI] [PubMed]
- 25.Saxon D, Falcone T, Mascha EJ, Marino T, Yao M, Tulandi T. A study of ruptured tubal ectopic pregnancy. Obstet Gynecol 1997;90:46-9. Comment in Obstet Gynecol 1997;90:866-7. [DOI] [PubMed]
- 26.Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol 1981;58:162-6. [PubMed]
- 27.Dart RG, Mitterando J, Dart LM. Rate of change of serial beta-human chorionic gonadotropin values as a predictor of ectopic pregnancy in patients with indeterminate transvaginal ultrasound findings. Ann Emerg Med 1999;34:703-10. [DOI] [PubMed]
- 28.Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol 2004;104:50-5. [DOI] [PubMed]
- 29.Mol BW, Lijmer JG, Ankum WM, van der Veen F, Bossuyt PM. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: a meta-analysis. Hum Reprod 1998;13:3220-7. [DOI] [PubMed]
- 30.Buckley RG, King KJ, Disney JD, Riffenburgh RH, Gorman JD, Klausen JH. Serum progesterone testing to predict ectopic pregnancy in symptomatic first-trimester patients. Ann Emerg Med 2000;36:95-100. [DOI] [PubMed]
- 31.Dart R, Ramanujam P, Dart L. Progesterone as a predictor of ectopic pregnancy when the ultrasound is indeterminate. Am J Emerg Med 2002;20:575-9. [DOI] [PubMed]
- 32.Albayram F, Hamper UM. First-trimester obstetric emergencies: spectrum of sonographic findings. J Clin Ultrasound 2002;30:161-77. [DOI] [PubMed]
- 33.Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol 1981;58:156-61. Comment in Obstet Gynecol 2003;102:672. [PubMed]
- 34.Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: Is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology 1997;205:569-73. [DOI] [PubMed]
- 35.Barnhart KT, Simhan H, Kamelle SA. Diagnostic accuracy of ultrasound above and below the beta-hCG discriminatory zone. Obstet Gynecol 1999;94:583-7. Comment in Obstet Gynecol 2000;95:475-6. [DOI] [PubMed]
- 36.Braffman BH, Coleman BG, Ramchandani P, Arger PH, Nodine CF, Dinsmore BJ, et al. Emergency department screening for ectopic pregnancy: a prospective US study. Radiology 1994;190:797-802. [DOI] [PubMed]
- 37.Kadar N, Bohrer M, Kemmann E, Shelden R. The discriminatory human chorionic gonadotropin zone for endovaginal sonography: a prospective, randomized study. Fertil Steril 1994;61:1016-20. Comment in Fertil Steril 1995;63:683-4. [DOI] [PubMed]
- 38.Habana A, Dokras A, Giraldo JL, Jones EE. Cornual heterotopic pregnancy: contemporary management options. Am J Obstet Gynecol 2000;182:1264-70. Comment in Am J Obstet Gynecol 2001;185:522. [DOI] [PubMed]
- 39.Tal J, Haddad S, Gordon N, Timor-Tritsch I. Heterotopic pregnancy after ovulation induction and assisted reproductive technologies: a literature review from 1971 to 1993. Fertil Steril 1996;66:1-12. [DOI] [PubMed]
- 40.Mertz HL, Yalcinkaya TM. Early diagnosis of ectopic pregnancy. Does use of a strict algorithm decrease the incidence of tubal rupture? J Reprod Med 2001;46:29-33. [PubMed]
- 41.Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: How often is the study diagnostic? Ann Emerg Med 1997;30:135-40. Comment in Ann Emerg Med 1997; 30: 206-9. [DOI] [PubMed]
- 42.Mateer JR, Aiman EJ, Brown MH, Olson DW. Ultrasonographic examination by emergency physicians of patients at risk for ectopic pregnancy. Acad Emerg Med 1995;2:867-73. [DOI] [PubMed]
- 43.Mateer J, Plummer D, Heller M, Olson D, Jehle D, Overton D, et al. Model curriculum for physician training in emergency ultrasonography. Ann Emerg Med 1994;23:95-102. [DOI] [PubMed]
- 44.Shih CH. Effect of emergency physician-performed pelvic sonography on length of stay in the emergency department [discussion 352]. Ann Emerg Med 1997;29:348-51. [DOI] [PubMed]
- 45.Tulandi T, Hemmings R, Khalifa F. Rupture of ectopic pregnancy in women with low and declining serum beta-human chorionic gonadotropin concentrations. Fertil Steril 1991;56:786-7. [DOI] [PubMed]
- 46.Lundorff P, Hahlin M, Kallfelt B, Thorburn J, Lindblom B. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril 1991;55:911-5. [DOI] [PubMed]
- 47.Murphy AA, Nager CW, Wujek JJ, Kettel LM, Torp VA, Chin HG. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: a prospective trial. Fertil Steril 1992;57:1180-5. [DOI] [PubMed]
- 48.Vermesh M, Silva PD, Rosen GF, Stein AL, Fossum GT, Sauer MV. Management of unruptured ectopic gestation by linear salpingostomy: a prospective, randomized clinical trial of laparoscopy versus laparotomy. Obstet Gynecol 1989;73(3 pt 1):400-4. Comment in Obstet Gynecol 1989;74:282-3. [PubMed]
- 49.Lundorff P, Thorburn J, Hahlin M, Kallfelt B, Lindblom B. Laparoscopic surgery in ectopic pregnancy. A randomized trial versus laparotomy. Acta Obstet Gynecol Scand 1991;70:343-8. [DOI] [PubMed]
- 50.Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG 2003;110:765-70. Comment in BJOG 2004;111:635-6. [PubMed]
- 51.Dubuisson JB, Morice P, Chapron C, De Gayffier A, Mouelhi T. Salpingectomy — the laparoscopic surgical choice for ectopic pregnancy. Hum Reprod 1996;11:1199-203. [DOI] [PubMed]
- 52.Fernandez H, Marchal L, Vincent Y. Fertility after radical surgery for tubal pregnancy. Fertil Steril 1998;70:680-6. [DOI] [PubMed]
- 53.Yao M, Tulandi T. Current status of surgical and nonsurgical management of ectopic pregnancy. Fertil Steril 1997;67:421-33. Comment in Fertil Steril 1997;68:945-7. [DOI] [PubMed]
- 54.Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-84. [DOI] [PubMed]
- 55.Lipscomb GH, Bran D, McCord ML, Portera JC, Ling FW. Analysis of three hundred fifteen ectopic pregnancies treated with single-dose methotrexate. Am J Obstet Gynecol 1998;178:1354-8. [DOI] [PubMed]
- 56.Hajenius PJ, Engelsbel S, Mol BW, van der Veen F, Ankum WM, Bossuyt PM, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet 1997;350:774-9. Comments in Lancet 1997;350:1554-5. [DOI] [PubMed]
- 57.Fernandez H, Yves Vincent SC, Pauthier S, Audibert F, Frydman R. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod 1998;13:3239-43. [DOI] [PubMed]
- 58.Saraj AJ, Wilcox JG, Najmabadi S, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-94. [DOI] [PubMed]
- 59.Sowter MC, Farquhar CM, Petrie KJ, Gudex G. A randomised trial comparing single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured tubal pregnancy. BJOG 2001;108:192-203. [DOI] [PubMed]
- 60.Nieuwkerk PT, Hajenius PJ, Ankum WM, van der Veen F, Wijker W, Bossuyt PM. Systemic methotrexate therapy versus laparoscopic salpingostomy in patients with tubal pregnancy. Part I. Impact on patients' health-related quality of life. Fertil Steril 1998;70:511-7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


