Abstract
Regulatory T cells (Tregs) are a specialized subset of CD4+ T cells essential for the maintenance of immune homeostasis and prevention of autoimmunity. Treg lineage and functions are programmed by the X-chromosome encoded transcription factor forkhead box P3 (FOXP3). In humans, multiple FOXP3 isoforms are generated through alternative splicing. A full-length isoform containing all coding exons (FOXP3-FL) and a version lacking the second exon (FOXP3-ΔE2) are the predominant FOXP3 isoforms. Additionally, there are two minor isoforms lacking either exon 7 (FOXP3-ΔE7) and both exons 2 and 7 (FOXP3-ΔE2ΔE7). Although healthy humans express approximately equal levels of the FOXP3-FL and FOXP3-ΔE2 isoforms, sole expression of FOXP3-ΔE2 results in the development of a systemic autoimmune disease that resembles immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. These clinical observations strongly suggest functional defects in suppression by Tregs programmed by the FOXP3-ΔE2 isoform. Work from the past two decades has provided phenotypic and functional evidence of differences between Tregs programmed by the FOXP3-FL, FOXP3-ΔE2, and FOXP3-ΔE7 isoforms. In this review, we discuss the discovery of the FOXP3 isoforms, differences in the phenotype and function of Tregs programmed by different FOXP3 isoforms, and the role that these isoforms are known to play in autoimmunity.
Keywords: IPEX syndrome, regulatory T cells, transcription factors
Splice variants of human FOXP3
Graphical Abstract
Graphical Abstract.
Introduction
Regulatory T cells (Tregs) play an essential role in the maintenance of immune homeostasis and self-tolerance. Tregs normally prevent aberrant inflammatory responses by suppressing reactivity toward self (1), commensal (2), dietary (3), and environmental (4) antigens through several distinct mechanisms. Although general Treg biology has been reviewed elsewhere in greater detail (5–7), we will provide a summary here. First, Tregs can suppress target cells through direct cell–cell contact mediated mechanisms, such as CTLA-4-mediated removal of CD80 or CD86 from target antigen-presenting cells (8, 9). Second, Tregs secrete inhibitory cytokines such as IL-10, IL-35, and TGF-β which leads to broad suppression of target cells, including suppression of protein translation and proliferation (10–12). Finally, Tregs can drive cytokine sequestration through the expression of cytokine receptors such as CD25 (the high-affinity IL-2Rα chain) which preferentially uptakes IL-2 and suppresses target T cell proliferation (13). These primary Treg functions rely on lineage programming by the X-chromosome encoded gene forkhead box P3 (FOXP3) (14). This review will focus on Treg subsets defined by the expression of different isoforms of FOXP3 and the role that these isoforms are known to play in autoimmunity.
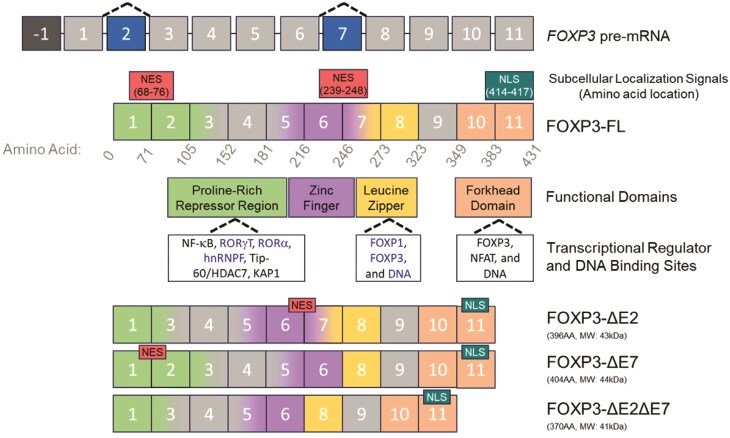
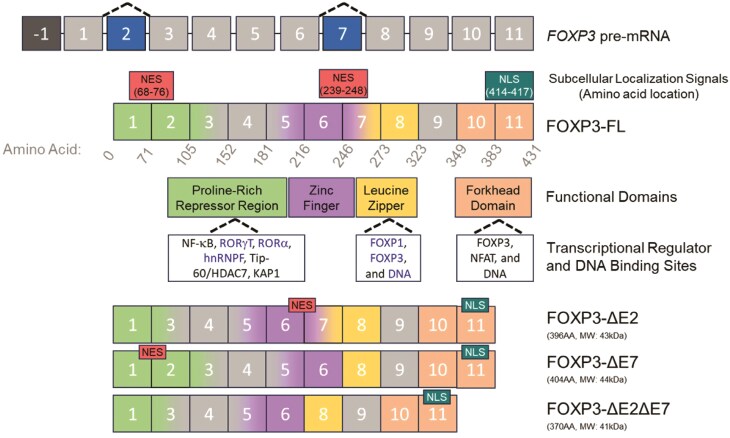
FOXP3 includes one non-coding exon and 11 coding exons which are referred to as exon -1 and exons 1–11, respectively. FOXP3 has a high degree of conservation between species, including 86.5% amino acid sequence homology between human and murine Foxp3. Additionally, human and murine FOXP3 have conserved functional domains, including an N-terminal proline-rich region, a zinc finger, a leucine zipper, and a forkhead domain (15). Although rodents are only known to express the full-length isoform of Foxp3 (15, 16), humans produce several alternatively spliced isoforms of the FOXP3 gene (17). The two predominantly expressed isoforms are a full-length version containing all 11 coding exons (FOXP3-FL) and a shorter version lacking exon 2 (FOXP3-ΔE2), and the two minor isoforms are a version lacking exon 7 (FOXP3-ΔE7) as well as a version lacking exons 2 and 7 (FOXP3-ΔE2ΔE7) (Fig. 1). Although the precise functional differences between the FOXP3 isoforms remain unclear, individuals with sole FOXP3-ΔE2 or FOXP3-ΔE7 expression develop lethal autoimmunity (18–20). These observations imply isoform-specific functions of FOXP3 with important consequences for human health and disease.
Figure 1.
Molecular features of FOXP3 and its alternatively spliced isoforms. The human FOXP3 gene is located on the X chromosome (GRCH38/hg38 chrX: 49,250,438-49,254,710 [including UTRs]) and is made up of one non-coding exon and 11 coding exons. The full-length FOXP3 isoform (FOXP3-FL) contains two nuclear export signals (NES) and one nuclear localization signal (NLS) as well as several distinct functional domains including a proline-rich repressor region, a zinc finger, a leucine zipper, and a forkhead domain. The FOXP3 protein binds to transcriptional regulators and DNA at several sites. Known binding partners of FOXP3 exon 2 include RORγt, RORα, and hnRNPF while FOXP3 exon 7 binding partners include FOXP1, FOXP3, and DNA. The three identified alternatively spliced isoforms of FOXP3 lack exon 2 (FOXP3-ΔE2), exon 7 (FOXP3-ΔE7), or both 2 and 7 (FOXP3-ΔE2ΔE7) are also shown. These isoforms have differences in subcellular localization signals and transcriptional regulator binding sites, suggesting putative functional differences that may contribute to disease.
The discovery of FOXP3 and isoforms of FOXP3
The characterization of systemic autoimmune syndromes in humans and mice preceded the discovery of FOXP3. In humans, FOXP3 deficiency causes immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome, also previously known as X-linked autoimmunity–allergic dysregulation (XLAAD) syndrome. This multisystem disease presents early in life—with symptoms including diabetes mellitus, diarrhea, nephropathy, and severe atopic dermatitis (eczema)—and can result in premature mortality in the absence of a bone marrow transplantation (21). The first case study of human IPEX syndrome was reported in 1982, describing 17 individuals with severe autoimmunity among related infant males (22). In late 2000, Chatila et al. identified a mutation in the FOXP3 gene (known as JM2 at the time) as the cause of IPEX syndrome in humans (23–26).
A systemic autoimmune disease similar in phenotype to human IPEX syndrome exists in the scurfy mouse line. In 1949 at Oak Ridge National Laboratory, investigators identified a spontaneously occurring mutation in a female mouse which led to the establishment of the scurfy mouse line. Bill Russell et al. reported on the scurfy mouse line in a 1959 publication as the first evidence of a sex-linked gene in mice, characterized by lethality in hemizygous males shortly after weaning (27). However, comprehensive immunophenotyping of these mice did not occur for another 30 years. In the 1990 the Russell Group and others published several studies that described the immune phenotype of scurfy mice as a CD4+ T cell-dependent autoimmune syndrome with increased white blood cell counts, chronic external skin inflammation, and colitis that was not improved by housing in pathogen-free conditions (28–31). Later work determined that the scurfy phenotype could develop by transferring scurfy T cells into T-cell-deficient mice but failed to induce disease when scurfy T cells were transferred into mice with endogenous CD4+ T cells (29).
The causative gene for the mutation that drives autoimmunity in scurfy mice (Foxp3) was ultimately molecularly cloned in 2001 (32). Further work confirmed that the murine Foxp3 gene encoded a transcription factor that was primarily expressed in CD4+CD25+ regulatory T cells, and expression of Foxp3 in naïve CD4+ T cells generates regulatory cells that restrain T-cell activation to prevent autoimmunity (14, 32–35). The connection between the scurfy mouse model and human IPEX syndrome occurred in the early 2000s with the discovery of a common deficiency in FOXP3 expression as the driver of both human and murine disease (23–25, 32, 36).
Analysis of FOXP3 gene mutations in humans and mice has been instrumental in our understanding of FOXP3 function. Autoimmunity in scurfy mice is driven by a frameshift mutation in the Foxp3 gene which leads to a truncated gene product that lacks the carboxy-terminal forkhead domain of the protein (32). IPEX patients have mutations in FOXP3 that span the entire coding region, allowing a systematic functional analysis of the FOXP3 protein. To date, IPEX syndrome in humans has been associated with over 70 mutations that impact the isoform usage, expression, DNA-binding domain structure, protein dimerization, and/or transcriptional function of FOXP3 (37). Notably, patients with mutations in other immune genes (STAT5b, STAT1, STAT3, IL2RA, CTLA4, LRBA, TTC7A, TTC37, and DOCK8) also develop a severe autoimmune disease that resembles IPEX syndrome due to overlapping roles that these genes play in regulating FOXP3 expression and/or Treg activity (37, 38).
The discovery of alternatively spliced isoforms of human FOXP3 occurred shortly after the FOXP3 gene was linked to Treg activity. Western blot analyses of FOXP3 reported a consistent band pattern which suggested the presence of several FOXP3 isoforms (39–41). In 2005, Allan et al. provided the first evidence of alternative splicing of FOXP3 in human Tregs. The first isoform contained all 11 coding exons and corresponded to the FOXP3-FL isoform. The second isoform, identified by its smaller protein size on a western blot, lacked exon 2 and corresponded to the FOXP3-ΔE2 isoform (17). Although healthy humans have an expression of both the FOXP3-FL and FOXP3-ΔE2 isoforms, sole expression of the FOXP3-ΔE2 isoform has been linked to the development of an IPEX-like disease (18, 38). Several different point deletions have been identified in FOXP3 exon 2 which cause a frameshift in the FOXP3-FL reading frame, resulting in the absence of functional FOXP3-FL. However, when alternative splicing occurs to remove FOXP3 exon 2, functional FOXP3-ΔE2 protein is generated but fails to suppress systemic autoimmunity. Thus, sole FOXP3-ΔE2 expression can promote autoimmunity and demonstrates defects in suppression by Tregs programmed by the FOXP3-ΔE2 isoform.
Reports of additional isoforms of FOXP3 lacking exon 7 emerged in the 2000s. In 2006, an isoform of FOXP3 lacking both exons 2 and 7 (FOXP3-ΔE2ΔE7) was first reported in human PBMCs (42), and in 2010, Kaur et al. provided the first report of a FOXP3 isoform lacking exon 7 (FOXP3-ΔE7) among freshly isolated human CD4+CD25+ cells (43). These minor isoforms only make up about 1%–3% of total FOXP3 RNA in CD4+CD25+ cells from humans (42, 44, 45). Two mutations (p.L250del and p.E251del) in exon 7 of FOXP3 have been identified in patients with IPEX syndrome, demonstrating the importance of FOXP3 exon 7 to the overall suppressive function of FOXP3 (46, 47).
Functional differences between FOXP3-FL and FOXP3-ΔE2 isoforms
Distinct functional domains present in exon 2 of FOXP3 drive functional differences between the FOXP3-FL and FOXP3-ΔE2 isoforms. Previous work has demonstrated that exon 2 of FOXP3 contains binding sites for the TH17-associated transcription factors RORα and RORγt via an LxxLL motif (48, 49). FOXP3 binding to RORα reduces expression of RORα target genes, including IL-17 and IL-22, and FOXP3 binding to RORγt reduces expression of RORγt target genes, including IL-17. Notably, the FOXP3 forkhead domain also contributes to the suppression of TH17-signature genes through DNA binding-dependent mechanisms (49). Thus, FOXP3 suppresses TH17-signature gene expression directly through binding to RORγt and indirectly through binding to DNA and functioning as a transcription suppressor. FOXP3 exon 2 also interacts with the RNA-binding protein heterogeneous nuclear ribonucleoprotein F (hnRNPF) which results in suppression of hnRNPF-driven alternative splicing (50). Therefore, in addition to its role in promoting or suppressing target gene expression, FOXP3 can also play a role in the regulation of alternative splicing of other target genes. The absence of the hnRNPF binding site in the FOXP3-ΔE2 isoform suggests putative differences in the regulation of alternative splicing of hnRNPF—and potentially other yet-to-be-identified splicing regulators—targets between the FOXP3-FL and FOXP3-ΔE2 isoforms.
In addition to protein-binding domains, FOXP3 also contains distinct functional domains which influence subcellular localization. Amino acids 414RKKR417 in the forkhead domain, encoded by FOXP3 exon 11, form a nuclear import signal (51), and Magg et al. demonstrated the presence of two nuclear export signals at the exons 1/2 and 6/7 boundaries corresponding to amino acids 68QLQLPTLPL76 and 239VQSLEQQLVL248, respectively (52). These nuclear export signals are therefore absent in the FOXP3-ΔE2 and FOXP3-ΔE7 isoforms. Correspondingly, FOXP3-ΔE2 and FOXP3-ΔE7 isoforms have moderately increased nuclear localization in comparison to FOXP3-FL while the FOXP3-ΔE2ΔE7 isoform has almost complete nuclear localization. Interestingly, transduction of CD4+ T cells with a FOXP3 construct containing mutated nuclear export signals altered gene expression patterns and enhanced suppression of CD4+ conventional T cells while transduction with the FOXP3-ΔE2ΔE7 isoform failed to generate a cell capable of suppressing CD4+ T cell proliferation. These results indicate that both subcellular localization of FOXP3 and the unique functional domains present within exons 2 and 7 provide important contributions to FOXP3 functions and subsequent Treg suppressive capabilities.
Studies of human cells have demonstrated phenotypic and functional differences in Tregs programmed by the FOXP3-FL, FOXP3-ΔE2, or both isoforms. In one case study, investigators compared FOXP3-FL and FOXP3-ΔE2 Tregs from a female heterozygous carrier for the c.305delT mutation which leads to sole expression of the FOXP3-ΔE2 isoform in ~50% of Tregs generated (53). FOXP3-ΔE2 Tregs were out-competed by FOXP3-FL expressing Tregs, with a ratio of 4:1 (FOXP3-FL: FOXP3-ΔE2) Tregs among PBMCs and had lower cell surface expression levels of CD25. In a separate clinical investigation, authors used mass cytometry to phenotype CD4+ T cells from patients with antineutrophil cytoplasmic antibody-associated vasculitis vs. healthy controls and found in both populations a cell subset which was FOXP3 exon 1+ exon 2− (putative FOXP3-ΔE2 Tregs) (54). These FOXP3-ΔE2 Tregs had a CD25lo, CD127+, and Helios− phenotype in comparison to FOXP3 exon 2+ Tregs. Phenotypic studies of natural FOXP3-ΔE2 Tregs have been complicated by difficulties in identifying FOXP3+ exon 2− cells in healthy individuals although advancements in single-cell profiling technologies like mass cytometry and spectral cytometry may lead to breakthroughs in phenotyping these cells.
Other studies have interrogated the functional differences between FOXP3-FL and FOXP3-ΔE2 Tregs using in vitro approaches. In one such study, investigators transduced human CD4+ T cells with either FOXP3-FL or FOXP3-ΔE2 constructs and found that both FOXP3-FL and FOXP3-ΔE2 Tregs suppress conventional CD4+ T cell proliferation in vitro. However, investigators did observe that FOXP3-ΔE2 Tregs have higher expression levels of the TGF-β release-associated molecule Glycoprotein A repetitions predominant (GARP) (55). In a separate study, Sato et al. used CRISPR/Cas9 to knock out endogenous FOXP3 in human CD4+ T cells and subsequently transduced cells with FOXP3-FL, FOXP3-ΔE2, or both isoforms (56). Co-transduction with both FOXP3-FL and FOXP3-ΔE2 constructs generated the highest percentage of cells expressing CD25 and enhanced conventional CD4+ T cell proliferation suppression in vitro in comparison to solely FOXP3-FL or FOXP3-ΔE2 transduced cells. Although these studies provided important first lines of evidence for the functional differences between FOXP3-FL and FOXP3-ΔE2 Tregs, further work is required to comprehensively understand the defects in suppression by FOXP3-ΔE2 Tregs which result in autoimmunity.
The recent development of two mouse models to study Tregs programmed by the Foxp3-FL and Foxp3-ΔE2 isoforms provided further evidence of the phenotypic and functional differences between Foxp3-FL and Foxp3-ΔE2 Tregs. Although mice do not naturally express the Foxp3-ΔE2 isoform, human and mouse Foxp3 exon 2 shares 94% amino acid sequence homology, suggesting an important conserved function within Foxp3 exon 2 that could be usefully modeled by a Foxp3 exon 2 knockout mouse. The first mouse model developed for the study of Foxp3-ΔE2 Tregs used CRISPR-Cas9 gene editing technology to delete the second coding exon of the murine Foxp3 gene (18). Using this model, Du et al. demonstrated that mice with Tregs solely expressing the Foxp3-ΔE2 isoform lose self-tolerance immediately after weaning in the absence of overt challenge. This loss of self-tolerance included spontaneous lymphadenopathy characterized by increased activation of CD4+ T cells (18). Foxp3-ΔE2 Tregs in this model had a cell-intrinsic reduction in cell surface expression levels of key regulatory molecules, including CD25 and CTLA-4. Deficiencies in these key Treg molecules associated with reduced proportions of Foxp3-ΔE2: Foxp3-FL Tregs in Foxp3-FL/Foxp3-ΔE2 heterozygous female mice, potentially due to Foxp3-FL Tregs outcompeting Foxp3-ΔE2 Tregs.
Du et al. further observed chronically active germinal center structures and increased production of double-stranded DNA-specific autoantibodies in Foxp3-ΔE2 mice (18). Further studies from our group have confirmed that Foxp3-ΔE2 mice exhibit increased germinal center activity and express substantially higher titers of IgE class antibodies that target skin autoantigens. The production of skin-specific IgE autoantibodies and germinal center activity is further increased after the skin is treated with moderate doses of ultraviolet B light (Domeier et al., submitted for publication). Elevated IgE antibody titers and increased keratin-specific antibodies have been detected in Foxp3-deficient scurfy mice and IPEX patients (57). Overall, this suggests that the signaling mechanisms that involve the second coding exon of Foxp3 regulate IgE antibody production, germinal center activity, and skin-specific autoantibody production. This autoimmune germinal center phenotype is likely mediated by defects in T follicular regulatory cell activity, but further work will be required to confirm this hypothesis.
A second mouse model, independently developed for the study of Foxp3-ΔE2 Tregs, yielded similar findings, including spontaneous lymphadenopathy and decreased IL-2 signaling sensitivity in Foxp3-ΔE2 Tregs (58). Gu et al. also used AlphaFold2 to predict structural differences between the murine Foxp3-FL and Foxp3-ΔE2 isoforms which suggested a potential increase in forkhead domain accessibility in the Foxp3-ΔE2 isoform. A luciferase assay provided evidence that the Foxp3-ΔE2 isoform has increased DNA binding in comparison to the Foxp3-FL isoform, suggesting potential differences in transcriptional activation or repression between the two isoforms. Taken together, these data provide evidence that Tregs programmed by the Foxp3-ΔE2 isoform have a deficiency in the expression of key regulatory molecules which may drive defects in the maintenance of self-tolerance.
Functional differences between FOXP3-FL, FOXP3-ΔE7, and FOXP3-ΔE2ΔE7 isoforms
Recent work has provided evidence for functional differences between the FOXP3-FL isoform and isoforms lacking FOXP3 exon 7 (FOXP3-ΔE7 and FOXP3-ΔE2ΔE7). FOXP3 exon 7 encodes a portion of the leucine zipper domain which is involved in FOXP3 homodimerization (51, 59). This domain is also required for heterodimer formation between FOXP3 and FOXP1 or NFAT, suggesting that FOXP3 exon 7 is critical for FOXP3-mediated transcriptional regulation in Tregs (51, 59, 60). Mutations in the leucine zipper domain, such as deletion of glutamic acid (ΔE250), result in a loss of FOXP3 homodimerization as well as diminished Treg suppression of cytokine production by target TH1 and TH2 cells (61). Interestingly, in transduced CD4+ T cells, the FOXP3-ΔE2ΔE7 isoform has been reported to retain FOXP3 homodimerization capabilities although these cells lack the capacity to suppress conventional CD4+ T cells (44).
Studies of human Tregs programmed by the FOXP3-FL vs. FOXP3-ΔE7 or FOXP3-ΔE2ΔE7 isoforms demonstrate defects in suppressive capabilities by FOXP3 exon 7 deficient Tregs. CD4+ T cells transduced with the FOXP3-ΔE2ΔE7 isoform fail to upregulate key Treg molecules such as CD25 and CTLA-4 and fail to suppress proliferation of target conventional CD4+ T cells in vitro (44). In line with these observations, a novel mouse model with sole Foxp3-ΔE2ΔE7 expression phenocopied the scurfy mouse model (62), showing the critical role of Foxp3 exon 7 in the programming of functionally suppressive Tregs. FOXP3-ΔE2ΔE7 Tregs generated using morpholino antisense oligonucleotides have increased production of IL-2 and IL-17 (45), suggesting a potential role for FOXP3-ΔE2ΔE7 Tregs in the promotion of TH17 responses. These studies highlight the critical importance of the region encoded by FOXP3 exon 7 to the programming of functionally suppressive Tregs.
Regulation of FOXP3 isoform usage
Although little remains known about what controls FOXP3 alternative splicing, limited evidence suggests that TCR stimulation, cell localization, metabolism, and cytokine signaling may impact FOXP3 isoform usage. Stimulation of natural Tregs, FOXP3− effector CD4+ T cells and naïve CD4+ T cells in vitro induces expression of FOXP3-FL and FOXP3-ΔE2, demonstrating a role for signaling downstream of TCR activation in the induction of FOXP3 alternative splicing (17, 63, 64). In a study of human infants, Kröger et al. found increased FOXP3-FL:FOXP3-ΔE2 ratios in thymus vs. peripheral blood samples from human infants (64). This finding provides evidence that the FOXP3-FL isoform is favored upon initial thymic Treg induction with increased incidence of FOXP3 alternative splicing peripherally. De Rosa et al. showed that inhibition of glycolysis during stimulation of conventional CD4+ T cells in the presence of low-density αCD3/αCD28 beads generates induced Tregs which favor the expression of the FOXP3-ΔE2 isoform (65). These cells had reduced expression levels of CD25, PD-1, CD71, and CTLA-4 along with reduced CD4+ T cell suppression. Thus, Tregs induced in an environment that suppresses glycolysis may favor the generation of FOXP3-ΔE2 Tregs with a reduced suppressive capacity. Finally, treatment of activated human CD4+CD25+CD127lo cells with IL-1β in vitro increased excision of FOXP3 exon 7, suggesting a role for inflammatory signaling in favoring of FOXP3 exon 7 isoform usage (66).
Alternative splicing of FOXP3 to generate the FOXP3-FL and FOXP3-ΔE2 isoforms is known to occur in several mammalian species, including humans (17), non-human primates (67), domestic house cats (68), and domestic canines (our data, not published). In contrast, only the Foxp3-FL isoform has been observed in rodents. Future work could interrogate the differences between rodents and other mammalian species to more comprehensively identify the factors which regulate and promote alternative splicing of FOXP3 isoforms.
FOXP3 isoforms and autoimmunity
The crucial role of FOXP3 in the maintenance of self-tolerance is clearly demonstrated by IPEX syndrome in humans and the scurfy mouse line, which both develop rapid and lethal autoimmunity in the absence of functional FOXP3 (23–25, 32, 36). Later identification and characterization of two primary truncation isoforms of FOXP3 in humans (FOXP3-ΔE2 and FOXP3-ΔE2ΔE7) suggested that variable expression of these isoforms could modulate the regulatory activity of these Tregs (15), and further clinical studies have demonstrated an association between aberrant FOXP3 isoform expression and autoimmunity (69–72).
Four distinct frameshift mutations in the second coding exon of the FOXP3 gene (c.227delT, c232_233delAT, c.239delC and c.303_304delTT) have been identified in patients with IPEX syndrome (18, 38, 73, 74). These frameshift mutations cause a truncated amino acid sequence in the full-length isoform of the FOXP3 protein but are not predicted to impact the protein sequence of the FOXP3-ΔE2 isoform (which lacks the mutated coding exon) (18, 38, 73). Although these patients had CD4+ FOXP3+ cells present—as programmed by the FOXP3-ΔE2 isoform—patients with the c.227delT mutation had a significant reduction in the proportion of circulating Tregs, reduced surface expression of CD25 on Tregs, and increased expression of CD45RO on circulating T cells (73). Individuals with these mutations also developed multi-organ autoimmunity, including enteropathy, thyroiditis, autoimmune diabetes, eczema, hemolytic anemia, immune thrombocytopenic purpura, autoimmune neutropenia, and/or nephropathy within the first 6 months after birth (18). Thus, sole expression of the FOXP3-ΔE2 isoform in humans can drive the development of multisystem autoimmunity which resembles IPEX syndrome.
Alterations in the ratios of FOXP3 isoform expression are also associated with the development of several different autoimmune diseases. Elevated expression of the FOXP3-ΔE2 isoform has been reported in PBMCs from patients with antineutrophil cytoplasmic antibody-associated vasculitis (69), Hashimoto’s thyroiditis (70), giant cell arteritis (71), and multiple sclerosis (72). Furthermore, in patients who underwent carotid endarterectomy, increased FOXP3-ΔE2 isoform expression in plaques was associated with higher atherosclerotic plaque stability (55). Notably, patients with stable and unstable plaques had similar FOXP3-ΔE2 isoform expression levels, suggesting potential differences in the localization of FOXP3-ΔE2 expressing Tregs in tissue vs. systemically. Expression of the FOXP3-ΔE2 isoform is also increased in CD4+ T-cells derived from the small intestine of patients with celiac disease (75). Conversely, expression of the FOXP3-ΔE2 isoform among circulating PBMCs was reduced or unchanged in other inflammatory conditions, including systemic lupus erythematosus and rheumatoid arthritis (76, 77). Similarly, no difference in FOXP3-FL vs. FOXP3-ΔE2 isoform usage was observed in human intestinal tissue from patients with ulcerative colitis or Crohn’s disease (78). Altogether, these studies provide evidence that FOXP3 isoform ratios are altered in patients with autoimmunity in a disease-specific manner.
Previous work to interrogate FOXP3 isoform expression levels in patients with autoimmunity has been limited due to prior technical capabilities. Many of these studies focused on global changes in FOXP3-ΔE2 isoform expression within whole tissues or total PBMCs, resulting in a paucity of data on the single-cell level. Additionally, much of this work used primers spanning the exons 1–3 junction to identify the FOXP3-ΔE2 isoform. However, sequence similarity in the 3ʹ end of FOXP3 exons 1 and 2 results in predicted cross-reactivity of primers designed to target the exons 1–3 junction with the exons 1 and 2 junctions, potentially resulting in amplification of both FOXP3-FL and FOXP3-ΔE2 isoforms. Recent developments in single-cell profiling techniques such as scRNA-seq and high dimensional spectral flow cytometry may provide new opportunities to identify and profile subsets of Tregs programmed by the FOXP3-FL and/or FOXP3-ΔE2 isoforms in a more robust manner, also potentially resolving published discrepancies in the role of the FOXP3-ΔE2 isoform in protecting against or contributing to autoimmunity.
A limited number of clinical studies have associated FOXP3 exon 7 deficient isoforms with the development of autoimmune diseases other than IPEX syndrome. One study showed that patients with Crohn’s disease express increased transcripts of FOXP3 lacking exon 7 (45). Another clinical investigation found that patients with rheumatoid arthritis, but not healthy controls, had detectable levels of the FOXP3-ΔE2ΔE7 isoform in synovial fluid and peripheral blood (79). Thus, FOXP3 isoforms lacking exon 7 may also contribute to the promotion of autoimmunity.
Conclusions
The Treg lineage-programming transcription factor FOXP3 has multiple alternatively spliced isoforms in humans. Although healthy humans express all isoforms, sole or elevated expression levels of the FOXP3-ΔE2, FOXP3-ΔE7, or FOXP3-ΔE2ΔE7 isoforms are associated with the development of autoimmunity. Future work is needed to understand the normal functions of the alternatively spliced isoforms of FOXP3, the factors which control alternative splicing of FOXP3, and the mechanisms by which the exons 2 and 7 deficient isoforms contribute to autoimmunity.
Contributor Information
Kristin N Weinstein, Center for Fundamental Immunology, Benaroya Research Institute, Seattle, WA, 98101, USA; Department of Immunology, University of Washington, Seattle, WA, USA.
Phillip P Domeier, Center for Fundamental Immunology, Benaroya Research Institute, Seattle, WA, 98101, USA.
Steven F Ziegler, Center for Fundamental Immunology, Benaroya Research Institute, Seattle, WA, 98101, USA.
Conflict of interest statement: The authors declare no conflict of interest.
Funding
This work was supported by funding from the National Institutes of Allergy and Infectious Disease (NIAID) [grant numbers R21 AI178426 and R21 AI152444] to S.F.Z., NIAID grant number F32 AI154787 to P.P.D., and the National Science Foundation Graduate Research Fellowship Grant No. DGE-2140004 to K.N.W.
References
- 1. Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000;101:455–8. https://doi.org/ 10.1016/s0092-8674(00)80856-9 [DOI] [PubMed] [Google Scholar]
- 2. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013;504:451–5. https://doi.org/ 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Izikson L, Liu L, et al. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol 2001;167:4245–53. https://doi.org/ 10.4049/jimmunol.167.8.4245 [DOI] [PubMed] [Google Scholar]
- 4. Thornton CA, Upham JW, Wikström ME, et al. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol 2004;173:3084–92. https://doi.org/ 10.4049/jimmunol.173.5.3084 [DOI] [PubMed] [Google Scholar]
- 5. Santosh Nirmala S, Kayani K, Gliwiński M, et al. Beyond FOXP3: a 20-year journey unravelling human regulatory T-cell heterogeneity. Front Immunol 2023;14:1321228. https://doi.org/ 10.3389/fimmu.2023.1321228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt A, Oberle N, Krammer PH.. Molecular mechanisms of Treg-mediated T cell suppression. Front Immunol 2012;3:51. https://doi.org/ 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shevyrev D, Tereshchenko V.. Treg heterogeneity, function, and homeostasis. Front Immunol 2019;10:3100. https://doi.org/ 10.3389/fimmu.2019.03100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–5. https://doi.org/ 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- 9. Tekguc M, Wing JB, Osaki M, et al. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci USA 2021;118:e20239118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;2007:7169–450. [DOI] [PubMed] [Google Scholar]
- 11. Collison LW, Pillai MR, Chaturvedi V, et al. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol 2009;182:6121–8. https://doi.org/ 10.4049/jimmunol.0803646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. So L, Obata-Ninomiya K, Hu A, et al. Regulatory T cells suppress CD4+ effector T cell activation by controlling protein synthesis. J Exp Med 2023;220:e20221676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol 2016;17:1322–33. https://doi.org/ 10.1038/ni.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hori S, Nomura T, Sakaguchi S.. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. https://doi.org/ 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 15. Mailer RKW. Alternative splicing of FOXP3-virtue and vice. Front Immunol 2018;9:530. https://doi.org/ 10.3389/fimmu.2018.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol 2006;24:209–26. https://doi.org/ 10.1146/annurev.immunol.24.021605.090547 [DOI] [PubMed] [Google Scholar]
- 17. Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 2005;115:3276–84. https://doi.org/ 10.1172/JCI24685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du J, Wang Q, Yang S, et al. FOXP3 exon 2 controls T(reg) stability and autoimmunity. Sci Immunol 2022;7:eabo5407. https://doi.org/ 10.1126/sciimmunol.abo5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magg T, Wiebking V, Conca R, et al. IPEX due to an exon 7 skipping FOXP3 mutation with autoimmune diabetes mellitus cured by selective T. Clin Immunol 2018;191:52–8. https://doi.org/ 10.1016/j.clim.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 20. Mailer RK. IPEX as a consequence of alternatively spliced FOXP3. Front Pediatr 2020;8:594375. https://doi.org/ 10.3389/fped.2020.594375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Q, Liu X, Zhang Y, et al. Molecular feature and therapeutic perspectives of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. J Genet Genomics 2020;47:17–26. https://doi.org/ 10.1016/j.jgg.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 22. Powell BR, Buist NR, Stenzel P.. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr 1982;100:731–7. https://doi.org/ 10.1016/s0022-3476(82)80573-8 [DOI] [PubMed] [Google Scholar]
- 23. Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 2000;106:R75–81. https://doi.org/ 10.1172/JCI11679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennett CL, Brunkow ME, Ramsdell F, et al. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA-->AAUGAA) leads to the IPEX syndrome. Immunogenetics 2001;53:435–9. https://doi.org/ 10.1007/s002510100358 [DOI] [PubMed] [Google Scholar]
- 25. Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001;27:20–1. https://doi.org/ 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- 26. Bennett CL, Ochs HD.. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr 2001;13:533–8. https://doi.org/ 10.1097/00008480-200112000-00007 [DOI] [PubMed] [Google Scholar]
- 27. Russell WL, Russell LB, Gower JS.. Exceptional inheritance of a sex-linked gene in the mouse explained on the basis that the X/O sex-chromosome constitution is female. Proc Natl Acad Sci U S A 1959;45:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyon MF, Peters J, Glenister PH, et al. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott–Aldrich syndrome. Proc Natl Acad Sci U S A 1990;87:2433–7. https://doi.org/ 10.1073/pnas.87.7.2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blair PJ, Bultman SJ, Haas JC, et al. CD4+CD8– T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J Immunol 1994;153:3764–74. [PubMed] [Google Scholar]
- 30. Godfrey VL, Wilkinson JE, Rinchik EM, et al. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 1991;88:5528–32. https://doi.org/ 10.1073/pnas.88.13.5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Godfrey VL, Wilkinson JE, Russell LB.. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 1991;138:1379–87. [PMC free article] [PubMed] [Google Scholar]
- 32. Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001;27:68–73. https://doi.org/ 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- 33. Schubert LA, Jeffery E, Zhang Y, et al. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem 2001;276:37672–9. https://doi.org/ 10.1074/jbc.M104521200 [DOI] [PubMed] [Google Scholar]
- 34. Fontenot JD, Gavin MA, Rudensky AY.. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6. https://doi.org/ 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 35. Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003;4:337–42. https://doi.org/ 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- 36. Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001;27:18–20. https://doi.org/ 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- 37. Ben-Skowronek I. IPEX syndrome: genetics and treatment options. Genes (Basel) 2021;12:323. https://doi.org/ 10.3390/genes12030323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gambineri E, Ciullini Mannurita S, Hagin D, et al. Clinical, immunological, and molecular heterogeneity of 173 patients with the phenotype of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Front Immunol 2018;9:2411. https://doi.org/ 10.3389/fimmu.2018.02411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest 2003;112:1437–43. https://doi.org/ 10.1172/JCI19441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 2004;16:1643–56. https://doi.org/ 10.1093/intimm/dxh165 [DOI] [PubMed] [Google Scholar]
- 41. Scotto L, Naiyer AJ, Galluzzo S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28- T suppressor cells. Hum Immunol 2004;65:1297–306. https://doi.org/ 10.1016/j.humimm.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 42. Smith EL, Finney HM, Nesbitt AM, et al. Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation. Immunology 2006;119:203–11. https://doi.org/ 10.1111/j.1365-2567.2006.02425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaur G, Goodall JC, Jarvis LB, et al. Characterisation of Foxp3 splice variants in human CD4+ and CD8+ T cells—identification of Foxp3Δ7 in human regulatory T cells. Mol Immunol 2010;48:321–32. https://doi.org/ 10.1016/j.molimm.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 44. Mailer RK, Falk K, Rotzschke O.. Absence of leucine zipper in the natural FOXP3Delta2Delta7 isoform does not affect dimerization but abrogates suppressive capacity. PLoS One 2009;4:e6104. https://doi.org/ 10.1371/journal.pone.0006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mailer RK, Joly AL, Liu S, et al. IL-1beta promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep 2015;5:14674. https://doi.org/ 10.1038/srep14674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li B, Samanta A, Song X, et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int Immunol 2007;19:825–35. https://doi.org/ 10.1093/intimm/dxm043 [DOI] [PubMed] [Google Scholar]
- 47. Song X, Li B, Xiao Y, et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep 2012;1:665–75. https://doi.org/ 10.1016/j.celrep.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du J, Huang C, Zhou B, et al. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol 2008;180:4785–92. https://doi.org/ 10.4049/jimmunol.180.7.4785 [DOI] [PubMed] [Google Scholar]
- 49. Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008;453:236–40. https://doi.org/ 10.1038/nature06878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du J, Wang Q, Ziegler SF, et al. FOXP3 interacts with hnRNPF to modulate pre-mRNA alternative splicing. J Biol Chem 2018;293:10235–44. https://doi.org/ 10.1074/jbc.RA117.001349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lopes JE, Torgerson TR, Schubert LA, et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol 2006;177:3133–42. https://doi.org/ 10.4049/jimmunol.177.5.3133 [DOI] [PubMed] [Google Scholar]
- 52. Magg T, Mannert J, Ellwart JW, et al. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur J Immunol 2012;42:1627–38. https://doi.org/ 10.1002/eji.201141838 [DOI] [PubMed] [Google Scholar]
- 53. Seitz C, Joly AL, Fang F, et al. The FOXP3 full-length isoform controls the lineage-stability of CD4. Clin Immunol 2022;237:108957. https://doi.org/ 10.1016/j.clim.2022.108957 [DOI] [PubMed] [Google Scholar]
- 54. Agosto-Burgos C, Wu EY, Iannone MA, et al. The frequency of Treg subsets distinguishes disease activity in ANCA vasculitis. Clin Transl Immunol 2022;11:e1428. https://doi.org/ 10.1002/cti2.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joly AL, Seitz C, Liu S, et al. Alternative splicing of FOXP3 controls regulatory T cell effector functions and is associated with human atherosclerotic plaque stability. Circ Res 2018;122:1385–94. https://doi.org/ 10.1161/CIRCRESAHA.117.312340 [DOI] [PubMed] [Google Scholar]
- 56. Sato Y, Liu J, Lee E, et al. Co-expression of FOXP3FL and FOXP3Δ2 isoforms is required for optimal Treg-like cell phenotypes and suppressive function. Front Immunol 2021;12:752394. https://doi.org/ 10.3389/fimmu.2021.752394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huter EN, Natarajan K, Torgerson TR, et al. Autoantibodies in scurfy mice and IPEX patients recognize keratin 14. J Invest Dermatol 2010;130:1391–9. https://doi.org/ 10.1038/jid.2010.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gu Q, Zhao X, Guo J, et al. The splicing isoform Foxp3Delta2 differentially regulates tTreg and pTreg homeostasis. Cell Rep 2023;42:112877. https://doi.org/ 10.1016/j.celrep.2023.112877 [DOI] [PubMed] [Google Scholar]
- 59. Wang B, Lin D, Li C, et al. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem 2003;278:24259–68. https://doi.org/ 10.1074/jbc.M207174200 [DOI] [PubMed] [Google Scholar]
- 60. Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 2006;126:375–87. https://doi.org/ 10.1016/j.cell.2006.05.042 [DOI] [PubMed] [Google Scholar]
- 61. Chae WJ, Henegariu O, Lee SK, et al. The mutant leucine-zipper domain impairs both dimerization and suppressive function of Foxp3 in T cells. Proc Natl Acad Sci U S A 2006;103:9631–6. https://doi.org/ 10.1073/pnas.0600225103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Joly AL, Liu S, Dahlberg CI, et al. Foxp3 lacking exons 2 and 7 is unable to confer suppressive ability to regulatory T cells in vivo. J Autoimmun 2015;63:23–30. https://doi.org/ 10.1016/j.jaut.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 63. Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 2007;19:345–54. https://doi.org/ 10.1093/intimm/dxm014 [DOI] [PubMed] [Google Scholar]
- 64. Kröger B, Spohn M, Mengel M, et al. Expression of full-length FOXP3 exceeds other isoforms in thymus and stimulated CD4 + T cells. J Clin Immunol 2024;44:114. https://doi.org/ 10.1007/s10875-024-01715-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Rosa V, Galgani M, Porcellini A, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol 2015;16:1174–84. https://doi.org/ 10.1038/ni.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mailer RK, Joly AL, Liu S, et al. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep 2015;5:14674. https://doi.org/ 10.1038/srep14674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coleman CA, Muller-Trutwin MC, Apetrei C, et al. T regulatory cells: aid or hindrance in the clearance of disease? J Cell Mol Med 2007;11:1291–325. https://doi.org/ 10.1111/j.1582-4934.2007.00087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lankford S, Petty C, LaVoy A, et al. Cloning of feline FOXP3 and detection of expression in CD4+CD25+ regulatory T cells. Vet Immunol Immunopathol 2008;122:159–66. https://doi.org/ 10.1016/j.vetimm.2007.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Free ME, Bunch DO, McGregor JA, et al. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum 2013;65:1922–33. https://doi.org/ 10.1002/art.37959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kristensen B, Hegedus L, Madsen HO, et al. Altered balance between self-reactive T helper (Th)17 cells and Th10 cells and between full-length forkhead box protein 3 (FoxP3) and FoxP3 splice variants in Hashimoto’s thyroiditis. Clin Exp Immunol 2015;180:58–69. https://doi.org/ 10.1111/cei.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miyabe C, Miyabe Y, Strle K, et al. An expanded population of pathogenic regulatory T cells in giant cell arteritis is abrogated by IL-6 blockade therapy. Ann Rheum Dis 2017;76:898–905. https://doi.org/ 10.1136/annrheumdis-2016-210070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sambucci M, Gargano F, De Rosa V, et al. FoxP3 isoforms and PD-1 expression by T regulatory cells in multiple sclerosis. Sci Rep 2018;8:3674. https://doi.org/ 10.1038/s41598-018-21861-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fuchizawa T, Adachi Y, Ito Y, et al. Developmental changes of FOXP3-expressing CD4+CD25+ regulatory T cells and their impairment in patients with FOXP3 gene mutations. Clin Immunol 2007;125:237–46. https://doi.org/ 10.1016/j.clim.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 74. Kobayashi I, Shiari R, Yamada M, et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX). J Med Genet 2001;38:874–6. https://doi.org/ 10.1136/jmg.38.12.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Serena G, Yan S, Camhi S, et al. Proinflammatory cytokine interferon-gamma and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin Exp Immunol 2017;187:490–506. https://doi.org/ 10.1111/cei.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin SC, Chen KH, Lin CH, et al. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest 2007;37:987–96. https://doi.org/ 10.1111/j.1365-2362.2007.01882.x [DOI] [PubMed] [Google Scholar]
- 77. Suzuki K, Setoyama Y, Yoshimoto K, et al. Decreased mRNA expression of two FOXP3 isoforms in peripheral blood mononuclear cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Immunopathol Pharmacol 2011;24:7–14. https://doi.org/ 10.1177/039463201102400102 [DOI] [PubMed] [Google Scholar]
- 78. Lord JD, Valliant-Saunders K, Hahn H, et al. Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2. Dig Dis Sci 2012;57:2846–55. https://doi.org/ 10.1007/s10620-012-2292-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ryder LR, Bartels EM, Woetmann A, et al. FoxP3 mRNA splice forms in synovial CD4+ T cells in rheumatoid arthritis and psoriatic arthritis. APMIS 2012;120:387–96. https://doi.org/ 10.1111/j.1600-0463.2011.02848.x [DOI] [PubMed] [Google Scholar]