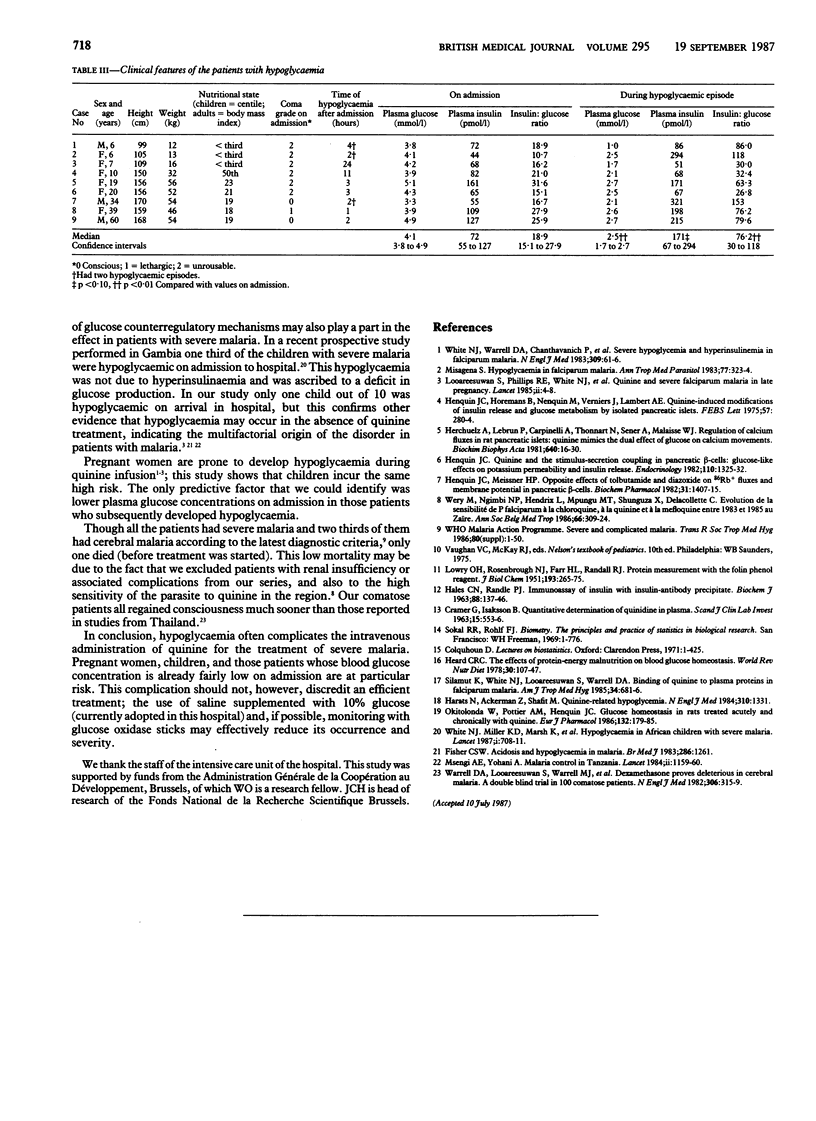
Abstract
Changes in plasma glucose and insulin concentrations were monitored over 24 hours in 28 African patients receiving quinine intravenously in an average dose of 8.5 mg base/kg over one hour eight hourly for severe malaria. The patients (nine children and 19 adults) were moderately undernourished; none was pregnant or had renal insufficiency. Plasma insulin concentrations rose during the infusion and then declined. Plasma glucose concentrations were decreased at two, three, and four hours after the start of the infusion. Insulin: glucose ratios were raised between half an hour and two hours after the start of the infusion. The three infusions of quinine increased plasma insulin concentrations in a similar way. In nine patients, including four children, plasma glucose concentrations fell below 2.8 mmol/l on one or two occasions. At the time of the hypoglycaemia plasma insulin concentrations were inappropriately high as shown by a consistent and often considerable increase in the insulin:glucose ratio. Hypoglycaemia that may pass unnoticed in comatose patients is thus a common complication of treating severe malaria with quinine, in particular in children. Its high incidence calls for attentive monitoring and preventive measures.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAMER G., ISAKSSON B. QUANTITATIVE DETERMINATION OF QUINIDINE IN PLASMA. Scand J Clin Lab Invest. 1963;15:553–556. doi: 10.1080/00365516309079786. [DOI] [PubMed] [Google Scholar]
- Daffos F., Forestier F., Grangeot-Keros L., Capella Pavlovsky M., Lebon P., Chartier M., Pillot J. Prenatal diagnosis of congenital rubella. Lancet. 1984 Jul 7;2(8393):1–3. doi: 10.1016/s0140-6736(84)91993-7. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harats N., Ackerman Z., Shalit M. Quinine-related hypoglycemia. N Engl J Med. 1984 May 17;310(20):1331–1331. doi: 10.1056/NEJM198405173102018. [DOI] [PubMed] [Google Scholar]
- Heard C. R. The effects of protein-energy malnutrition on blood glucose homeostasis. World Rev Nutr Diet. 1978;30:107–147. doi: 10.1159/000401238. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Horemans B., Nenquin M., Verniers J., Lambert A. E. Quinine-induced modifications of insulin release and glucose metabolism by isolated pancreatic islets. FEBS Lett. 1975 Oct 1;57(3):280–284. doi: 10.1016/0014-5793(75)80317-6. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Quinine and the stimulus-secretion coupling in pancreatic beta-cells: glucose-like effects on potassium permeability and insulin release. Endocrinology. 1982 Apr;110(4):1325–1332. doi: 10.1210/endo-110-4-1325. [DOI] [PubMed] [Google Scholar]
- Herchuelz A., Lebrun P., Carpinelli A., Thonnart N., Sener A., Malaisse W. J. Regulation of calcium fluxes in rat pancreatic islets. Quinine mimics the dual effect of glucose on calcium movements. Biochim Biophys Acta. 1981 Jan 8;640(1):16–30. doi: 10.1016/0005-2736(81)90528-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Migasena S. Hypoglycaemia in falciparum malaria. Ann Trop Med Parasitol. 1983 Jun;77(3):323–324. doi: 10.1080/00034983.1983.11811716. [DOI] [PubMed] [Google Scholar]
- Okitolonda W., Pottier A. M., Henquin J. C. Glucose homeostasis in rats treated acutely and chronically with quinine. Eur J Pharmacol. 1986 Dec 16;132(2-3):179–185. doi: 10.1016/0014-2999(86)90603-5. [DOI] [PubMed] [Google Scholar]
- Silamut K., White N. J., Looareesuwan S., Warrell D. A. Binding of quinine to plasma proteins in falciparum malaria. Am J Trop Med Hyg. 1985 Jul;34(4):681–686. doi: 10.4269/ajtmh.1985.34.681. [DOI] [PubMed] [Google Scholar]
- Wery M., Ngimbi N. P., Hendrix L., Mpungu M. T., Shunguza, Delacollette C. Evolution de la sensibilité de P. falciparum à la chloroquine, à la quinine et la méfloquine entre 1983 et 1985 au Zaïre. Ann Soc Belg Med Trop. 1986;66(4):309–324. [PubMed] [Google Scholar]
- White N. J., Miller K. D., Marsh K., Berry C. D., Turner R. C., Williamson D. H., Brown J. Hypoglycaemia in African children with severe malaria. Lancet. 1987 Mar 28;1(8535):708–711. doi: 10.1016/s0140-6736(87)90354-0. [DOI] [PubMed] [Google Scholar]
- White N. J., Warrell D. A., Chanthavanich P., Looareesuwan S., Warrell M. J., Krishna S., Williamson D. H., Turner R. C. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983 Jul 14;309(2):61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]


