Abstract
Background
Clinical exome and genome sequencing has transformed the diagnostic workup of patients with genetic disorders. The extensive body of evidence supporting the application of this clinical genomics approach in pediatric patients stands in stark contrast to the relative paucity of evidence for its use in the adult population. Here, we describe the largest cohort to date of adult patients who underwent clinical exome and genome sequencing for suspected genetic diagnoses.
Methods
A total of 2763 adult patients (2529 families) from all regions of Saudi Arabia are included in this cohort (2202 exomes, and 561 genomes).
Results
The diagnostic rate is 38.9% spanning 535 Mendelian genes and revealing clinical diagnostic errors in 38% of patients with positive reports. Structured feedback using C-GUIDE demonstrates clinical utility in 90% of positive cases. Consistent with the highly consanguineous nature of the local population, the majority (61%) of diagnosed phenotypes are recessive (94.6% homozygous) and founder variants account for 85% (414/487) of these variants. The same population characteristic has also led to the encounter of extremely rare, even novel recessive disorders including a highly penetrant novel RNF43-related hemochromatosis, NFXL1-related syndrome of hyperlaxity, short stature, and kidney disease, as well as autosomal recessive forms of typically dominant disorders. Multilocus phenotypes are observed in 5% of cases although only 26.7% of these are caused by two recessive variants. That 70% of molecular diagnoses encountered in our cohort are typically described in pediatric patients allowed us to observe highly unusual clinical presentations in the adult population. This delayed diagnosis also represents a missed opportunity for effective treatment in many instances and we note the availability of treatment for 26% of diagnosed conditions. Of particular interest are patients with monogenic disorders that could be overlooked as common multifactorial adult diseases (e.g., diabetes, dyslipidemia, stroke, chronic kidney disease, and dementia). Finally, we note the opportunities of deploying adult clinical genomics in an underrepresented population where 45.5% (373/819) of encountered variants are completely absent in gnomAD.
Conclusions
Our results illustrate numerous benefits of a clinical genomics approach in adult medicine and argue for a broader implementation than currently practiced.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-025-01529-2.
Keywords: Exome, Reflex genome, Clinical utility, Underrepresented population, Rare diseases, Founder variants, Mendelian phenocopies, Novel allelic disorders, Novel disease genes
Background
Clinical exome and genome sequencing (CES/CGS) has become an integral component of diagnostic protocols in patients with suspected genetic disorders, owing to its comprehensive nature and declining cost. Notwithstanding the well-recognized access inequalities related to racial, geographic, and socioeconomic factors, the application of CES/CGS in pediatric populations has been extensively documented, demonstrating diagnostic yields up to 70% [1–3]. The observation that many (up to 80% [4]) of these children carried erroneous diagnostic labels prior to CES/CGS exemplifies the central role of this new diagnostic method in precision pediatrics. The established clinical utility of this approach is not limited to improved patient management but also extends to reproductive planning among family members [5]. Additionally, the cost-effectiveness of CES/CGS in clinical practice has been well documented [6]. This growing body of evidence has prompted the American College of Medical Genetics and Genomics (ACMG) to advocate for the use of CES/CGS as a first-tier diagnostic tool in children with congenital anomalies, developmental delays, or intellectual disabilities (ID) [7]. This endorsement by a major professional society has been a major factor in facilitating coverage of CES/CGS in pediatric medicine, which has expanded recently to include rapid CES/CGS for NICU and PICU patients [8].
The abundance of data on pediatric applications of CES/CGS stands in stark contrast to the notable paucity of literature addressing their use in adult patients. In a recent systematic review, only four out of 161 published CES/CGS studies were dedicated to adult patients [5]. The first of these four studies involved CES on 486 adult patients with a diverse range of suspected genetic disorders and reported a 17% yield [9]. A surprisingly identical yield was reported in a 2020 study comprising 1190 adults who underwent CGS with similarly diverse indications [10]. Two studies in 2021 focused on neurological indications in adult patients (CES on 427 in one and CGS on 100 in the second) revealed a diagnostic yield of 18–27% [11, 12]. In addition to these four studies, there are only a few more recent studies that postdate the systematic review by Chung et al. Walsh et al. described in 2022 their experience with CES in 250 consecutive patients from an adult genetics department and reported a 29% diagnostic yield [13]. On the other hand, Wallis et al. implemented CGS and achieved a 32% diagnostic yield on 50 undiagnosed adult patients in their 2024 study [14]. An interesting preprint describes the use of CES in ICU adult patients with a diagnostic yield of 25% [15]. All these studies with the exception of one from Korea are from countries where European ancestry represents a majority (the USA, Europe, and Australia), which highlights the need to generate evidence from more diverse backgrounds. Additionally, while the impact of consanguinity on the landscape of genetic diagnoses is well established for pediatric patients, similar data for adult patients are largely lacking. This is particularly important when one considers the potential of enhanced homozygosity to unmask very rare or even novel autosomal recessive diseases that are especially challenging to diagnose clinically. The same phenomenon can also reveal autosomal recessive forms of common adult-onset diseases, and this may influence future recommendations for genetic investigation of these diseases.
Here, we describe our experience from a single major diagnostic laboratory serving all regions of Saudi Arabia, a country of 34 million inhabitants with high rates of consanguinity. The widespread coverage of CES/CGS in patients who receive their care through the public healthcare system presents an opportunity to collect much needed data on how CES/CGS is being used to deliver precision medicine in the adult population. In addition to patterns we identified through this analysis that are likely generalizable to other countries, we note the influence of consanguinity on many aspects of adult genomic medicine in the local population.
Methods
Human subjects
All patients included in this analysis were at least 18 years of age at the time of CES/CGS and the cohort spans the period of 2016 to 2024. Saudi Arabia has a two-tier healthcare system: public and private. All citizens are eligible to free public healthcare, which guarantees access to CES/CGS, and this can be ordered by any attending physician irrespective of their specialty.
CES/CGS was requested as a clinical test and part of routine healthcare. In addition to the standard CES/CGS consent, only patients who also signed the optional “research use” of their data were included in this analysis (a copy of the consent form used can be downloaded at https://www.centogene.com/downloads.html). This study was approved by IRB with waiver of consent (KFSHRC RAC# 2230016) for aggregated data. Written informed consent was obtained from all participants and/or their legal guardians for the publication of possible identifiable clinical details and identifiable facial images, in accordance with IRB-approved protocols (KFSHRC RAC# 2080006 and KAIMRC RC19/120/R). Demographics and clinical phenotypes were collected from the requisition forms. Previously established categories for phenotypes [13] were utilized with the following modifications: immune disorders and reproductive disorders were added as new categories and two subcategories (epilepsy and leukodystrophy) were added under neurological [13].
CES/CGS and variant interpretation
CES/CGS was performed by the same commercial lab (Centogene, Rostock, Germany) for all patients. The technical details of exome and genome sequencing and variant calling (including CNVs) have been described elsewhere [16, 17]. Briefly, a proprietary dry blood spot card (CentoCard) was used to collect blood samples from patients. Genomic DNA was isolated from the CentoCard with QIASymphony using a magnetic bead-based method (Qiagen), with an acceptance criterion of minimum 3 ng/µl. After fragmentation of genomic DNA by sonication, Illumina adapters were ligated to generate fragments for subsequent sequencing on the HiSeqX platform (Illumina, Inc., San Diego, CA, USA) to yield an average coverage depth of more than 30X. For exome sequencing, Twist Human Core Exome Plus, the Nextera Rapid Capture Exome Kit (Illumina, San Diego, CA), or the SureSelect Human All Exon kit (Agilent, Santa Clara, CA) were used for the enrichment, followed by 150 paired-end protocol. Exome sequencing achieved an average depth of 119X. Data analysis, including alignment to the hg19 human reference genome (Genome Reference Consortium GRCh37), variant calling, and annotation is performed using a previously described validated in-house pipeline [16]. Variants with insufficient quality scores were confirmed via Sanger sequencing according to previously published established criteria [18].
A previously described semi-automated filtering strategy for the SNV and small indels was used [17]. Briefly, variants fulfilling the following criteria were selected for further evaluation: (1) previously classified in Centogene’s Bio/Databank [19]; (2) variants with minor allele frequency (MAF) < 1% in gnomAD and Centogene’s Bio/Databank of > 24,000 “unaffected” adults with available CES/CGS data, and in silico predictions of high/moderate impact on protein function; (3) all variants described as disease-causing by external databases (HGMD and ClinVar); (4) variants with adaptive boosting (ADA) and random forest (RF) scores from dbscSNV19 > 0.6, consistent with predictions of abnormal splicing; (5) exclusion of variants previously classified as (likely) benign. For CNV selection, only CNVs with < 2% MAF affecting > 2 exons were considered. CNVs were selected in the manner described by Almeida and colleagues [17]. While homozygous variants did not usually require confirmation by an orthogonal method, heterozygous CNVs were always confirmed by qPCR, multiplex ligation-dependent probe amplification (MLPA), or chromosomal microarray (CMA).
The selected variants were then evaluated for pathogenicity and causality and classified into five classes (P, LP, VUS, LB, B), according to the ACMG guidelines for variant classification [20]. The phenotypes of the patients, suspected clinical diagnosis by ordering physician, and results from other lab tests (if provided) were taken into consideration for final variant classification and reporting. The final reports were issued as follows: (i) positive, for patients with P/LP variant(s) explaining the phenotype(s) with the correct zygosity; (ii) ambiguous, for cases with VUS compatible with the clinical phenotype (at least partially) or single P/LP heterozygous variant in a gene linked to an autosomal recessive phenotype; (iii) negative, for cases with no relevant variant identified. The ACMG-recommended genes for reporting of secondary findings were updated as per the most recent version at the time of analysis.
C-GUIDE
We used C-GUIDE (Clinician-reported Genetic testing Utility InDEx) score as a validated tool to measure the clinical utility of genetic testing from the perspective of clinicians. It assesses various aspects such as understanding diagnosis and prognosis, informing medical management, awareness and actionability of reproductive and health risks for patients and family members, and patient and family well-being [21].
Results
General characteristics of the study cohort
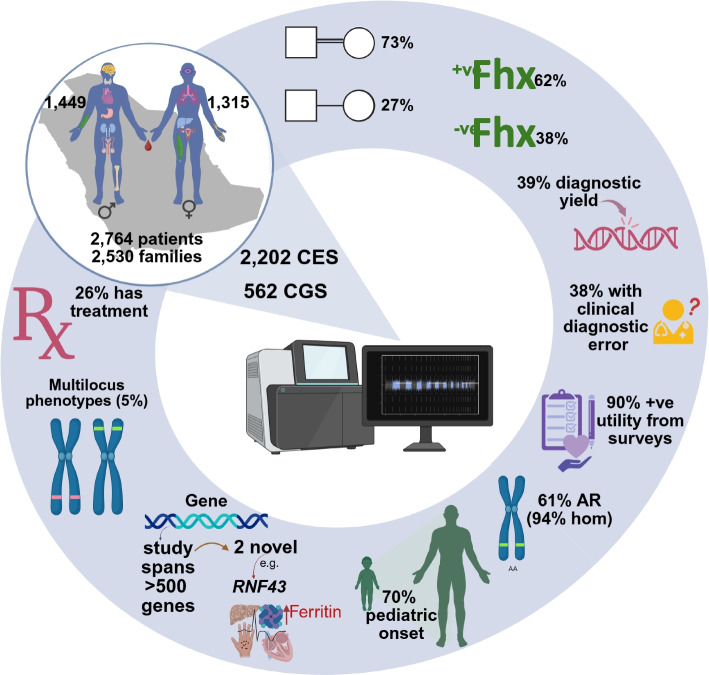
Our cohort comprises 2763 adult patients (after removal of duplicates, see below) who underwent CES (2202), or reflex CGS (561). Their age distribution ranges from 18 to 88 years (Fig. 1 and Additional file 1: Table S1). The ratio of males to females is 1.1:1 (1449 and 1314). Family history is only documented for 73% (n = 2009) of the cohort and is positive in 62% (n = 1245). A positive family history was defined based on the availability of information indicating that one or more family members had a similar or overlapping phenotype. In the overwhelming majority of cases (99.7%, n = 2755), adult patients underwent CES/CGS because of a clinical phenotype (including abnormal laboratory findings in the absence of clinical features). However, in 8 cases (0.3%), there was no such phenotype, and the only indication was a positive family history (adults who were tested for carrier screening or as part of a trio CES/CGS because of an affected child were excluded unless the adult had a positive phenotype). Six duplicates were identified representing patients who were independently tested under different MRNs by different institutions. For each of these duplicates, only the more recent CES/CGS test is retained in this analysis.
Fig. 1.
Schematic representation of the study design and outcome. Adult patients from Saudi Arabia (aged 18 and older) underwent clinical exome and genome sequencing because of phenotypic presentation (all organ systems are represented). Diagnostic yield was ~ 40%. Due to the high prevalence of consanguinity, there is enrichment of autosomal recessive disorders (the majority of which are homozygous). There was ~ 90% reported positive clinical utility from the 155 surveys collected from 23 physicians. The majority of the phenotypes are pediatric onset and 26% of them have treatment available. Fhx: family history, AR: autosomal recessive, Hom: homozygous. The figure was created with https://BioRender.com
The diagnostic yield of CES/CGS across a wide range of clinical indications for CES/CGS in adult patients
While all 27 phenotypic categories and subcategories are represented in our cohort, some are clearly overrepresented, e.g., multisystem (34%), compared to others, e.g., non-syndromic intellectual disability (0.07%) (Table 1 and Fig. 2). Surprisingly, a large majority (70%) of tested adults had features indicative of developmental processes consistent with pediatric onset, e.g., intellectual disability, facial dysmorphism, and congenital malformations (Additional file 1: Table S1). The overall diagnostic yield of CES/CGS was 38.9%. There was 20 percentage point difference in the yield of CES versus reflex CGS (43.1 vs. 22.5%). Of 561 adults who underwent CGS after having received a non-diagnostic CES or other genetic test (hence the term reflex CGS), 126 did receive positive reports. Additional file 2: Table S2 explains the reason why prior genetic testing had missed the molecular diagnosis for each of the positive reflex CGS cases. In the majority (116 out of 126) of positive reflex CGS, the variant could have been identified by exome sequencing. This supports the conclusion we made in a previous study that in most instances, the diagnostic gap in exome and genome sequencing is due to interpretation rather than detection challenges [22].
Table 1.
Phenotypic categories. This table compares the diagnostic yield across various phenotypic categories, demonstrating that the yield is influenced by the type of clinical presentation, with the highest yield observed in patients with syndromic intellectual disability and the lowest in those with nonspecific phenotypes
| Disorder | Positive | Total | Positive rate |
|---|---|---|---|
| Syndromic-ID | 5 | 7 | 0.71429 |
| Ocular | 172 | 243 | 0.70782 |
| Ear, nose, throat | 18 | 33 | 0.54545 |
| Dermatological | 38 | 70 | 0.54286 |
| Leukodystrophies | 8 | 15 | 0.53333 |
| Skeletal | 26 | 58 | 0.44828 |
| Multisystem | 394 | 950 | 0.41474 |
| Dementia | 6 | 15 | 0.40000 |
| Gastrointestinal | 10 | 25 | 0.40000 |
| Pulmonary | 8 | 20 | 0.40000 |
| Metabolic | 12 | 32 | 0.37500 |
| Muscle | 89 | 240 | 0.37083 |
| Neuropathy | 8 | 22 | 0.36364 |
| Hematological | 61 | 168 | 0.36310 |
| Ataxia | 35 | 97 | 0.36082 |
| Renal | 29 | 81 | 0.35802 |
| Neuro-other (with movement disorder) | 66 | 199 | 0.33166 |
| Dystonia | 6 | 19 | 0.31579 |
| Hereditary spastic paraplegia | 5 | 16 | 0.31250 |
| Cardiovascular | 16 | 54 | 0.29630 |
| Endocrine | 13 | 55 | 0.23636 |
| Immune disorders | 3 | 13 | 0.23077 |
| Reproductive | 15 | 97 | 0.15464 |
| Cancer | 21 | 141 | 0.14894 |
| Epilepsy | 3 | 32 | 0.09375 |
| Others | 3 | 54 | 0.05556 |
| Nonsyndromic-ID | 0 | 2 | 0.00000 |
Fig. 2.
Distribution of phenotypic categories. This figure shows all 27 categories and highlights notable disparities in their diagnostic yield
We set out to test the following as factors that could potentially influence the diagnostic yield of CES/CGS:
1- Family history: the diagnostic yield of CES/CGS was 47% (592/1245) in those with documented positive family history for the condition being tested and 33% (252/764) in those without (p < 0.001).
2- Consanguinity: there was a clear correlation between reported consanguinity and the presence of an autosomal recessive cause of the phenotype in consanguineous vs. nonconsanguineous patients (455/597 (76%) vs. 86/232 (37.1%); p < 0.001) and having a positive report in general (597/1291 (46.2%) vs. 232/742 (31.3%); p < 0.001).
3- Pediatric vs. adult-onset disorders: we tested the hypothesis that phenotypes with pediatric onset are more likely to be genetic in origin. We found that the diagnostic yield in these phenotypes is indeed higher compared to those with adult onset (51 vs. 23%; p < 0.001).
4- Phenotypic categories: we also tested if the average diagnostic yield of 38.9% is influenced by the phenotypic categories. As shown in Table 1 and Fig. 2, the yield was indeed highly variable, being highest in those presenting with syndromic intellectual disability (71%) and lowest in those presenting with “other” categories, i.e., nonspecific phenotypes (5%).
5- Specific differential diagnosis: a specific clinical diagnostic label was provided for only 1174 cases (Additional file 1: Table S1). However, when provided, it did not predict a positive result (459/1174 vs. 615/1590, p = 0.92).
Unique aspects of adult presentation
The observation that 70% (n = 305) of molecular diagnoses in our cohort are typically associated with pediatric diseases gave us an opportunity to examine how their adult presentation could add to our understanding of their phenotypic spectrum across age groups. One aspect we explored was facial appearance of conditions known to be associated with facial dysmorphism (Fig. 3). For some disorders, the adult patients in our cohort represent the first examples of adults diagnosed with these disorders to our knowledge, e.g., GPD1-related transient infantile hypertriglyceridemia (OMIM #614480) (Additional file 1: Table S1). Sometimes, the phenotype reflected an unusually mild adult-onset presentation such as CLN5-related adult-onset cerebellar ataxia and macular degeneration [23] (Fig. 3), ACAD9-related non-syndromic optic atrophy [24], and DMD-related cardiomyopathy (OMIM #302045) without skeletal muscle involvement which are strikingly different from the established severe and pediatric onset CLN5-related ceroid lipofuscinosis (OMIM #256731), ACAD9-related mitochondrial complex I deficiency (OMIM #611126), and DMD-related muscular dystrophy (OMIM #310200), respectively. In other cases, the adult patient was completely asymptomatic for the condition diagnosed by CES/CGS; e.g., case 192LO9459 with homozygous CAPN3:NM_000070.2:c.1466G > A:p.(Arg489Gln) was tested for male infertility but did not have muscle weakness. Table 2 provides detailed description of the available evidence supporting the pathogenesis of variants associated with apparent non-penetrance. We acknowledge that the observed non-penetrance may reflect lack of pathogenicity of the respective variant despite our best effort to follow the ACMG criteria. A fourth aspect of adult presentations concerns the impact on fertility, which is an adult-specific biological function. For example, case 172LO1175 with MANBA-related mannosidosis (OMIM #248510), case 178LO3214 with DARS1-related HBSL (OMIM #615281) and EMC10-related NEDDFAS (OMIM #619264) presented with non-obstructive azoospermia; case 167LO1365 with ALDH4A1-related hyperprolinemia (OMIM #239510) presented with male infertility; and case 157LO3983 with WT1-related Denys-Drash syndrome (OMIM #194080) and case 186LO6363 with DCAF17-related WSS (OMIM #241080) who both presented with primary amenorrhea. Additional atypical presentations that may or may not represent long-term consequences of the underlying disorder include retinal and pancreatic involvement in SLC66A1-related cystinosis [38], central and peripheral demyelinating disease in PRF1-related hemophagocytosis (OMIM #603553), severe CNS involvement in SH3TC2-related Charcot-Marie-Tooth disease (OMIM #601596), severe heart involvement in GRHPR-related hyperoxaluria (OMIM #260000), behavioral changes and dysmyelination in PDCD10-related cerebral cavernous malformation (OMIM #603285) (Fig. 3), diabetes and cardiomyopathy in PCNT-related Seckel syndrome (OMIM #210720), and pancytopenia and chronic kidney disease in ABCA1-related Tangier disease (OMIM #205400) (Additional file 1: Table S1). For additional examples of radiological findings from the cohort, please refer to Additional file 3: Figure S1. Some presentations were sufficiently different to justify their characterization as potentially novel allelic disorders. For example, three siblings who shared the same pathogenic variant in PLCE1 presented with severe retinal disease and retinal detachment, in addition to the expected renal phenotype [39]. We also highlight two sisters who presented with autosomal dominant RAD50-related primary ovarian insufficiency [40] rather than autosomal recessive RAD50-related Nijmegen chromosomal breakage syndrome (Additional file 1: Table S1). Finally, we highlight a number of common adult-onset diseases that were found to be monogenic on CES/CGS and testing was typically prompted by positive family history, e.g., AGPAT2-related metabolic syndrome (OMIM #608594), CLCN5-related chronic kidney disease (OMIM #310468), LDHD-related gout (OMIM #245450), PAX4-related diabetes (OMIM #125853), LDLR-related dyslipidemia (OMIM #143890), ABCA7-related Alzheimer disease (OMIM #608907), and NOTCH3-related stroke (OMIM #125310). These do not include homozygous APO4E-related Alzheimer disease (observed in 4 of 28 Alzheimer disease patients in our cohort), which we did not consider to be a Mendelian form of Alzheimer disease.
Fig. 3.
Examples of clinical and radiographic features of adult patients with confirmed genetic diagnoses. A Facial photographs of a patient with splice variant c.169 + 1G > A in MGP gene show the typical presentation of Keutel syndrome with midface hypoplasia. B Facial photograph of a patient with Koolen-De Vries syndrome, who has a frameshift variant c.2642_2654del p.Gln881Profs*14 in KANSL1 gene. C Facial photograph of a patient with Marfan syndrome with a missense variant c.5866 T > C p.Cys1956Arg in FBN1 gene shows malar hypoplasia and myopia. D Scalp image of a patient with alopecia caused by a pathogenic nonsense variant NM_001793.5:c.747C > A p.(Tyr249*) in CDH3 gene, which is associated with autosomal recessive congenital hypotrichosis with juvenile macular dystrophy. E Lower extremity photograph of a patient with congenital ichthyosis, displaying severe scaling and hyperkeratosis; the genetic analysis revealed compound heterozygous pathogenic variants NM_173483.3:c.1303C > T (p.(His435Tyr) and c.177C > G (p.(Phe59Leu) in the CYP4F22 gene. F Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) showing global thinning in both eyes with reduced RNFL thickness, particularly in the temporal and inferior quadrants, consistent with optic atrophy in a patient with homozygous missense variant c.1586C > T p.(Ala529Val) in AFG3L2 gene. G Mouth photograph of a patient with autosomal recessive dentin dysplasia, type I, harboring a homozygous deletion of approximately 589 Kb encompassing the entire SMOC2 gene. The photograph shows missing teeth, microdontia, and misshapen dentition. H The corresponding panoramic dental X-ray for the same patient in G, which further illustrates the dental anomalies consistent with the clinical findings. I Brain MRI (sagittal (Ii) and axial (Iii)) showing cerebellar atrophy in a patient with missense variant c.562 T > C p.(Phe188Leu) in CLN5 gene, which represents a novel allelic disorder. J Axial MRI image showing cavernous malformations with multiple cerebral lesions. A recent hemorrhage is evident in the right parietal cavernous malformation. Genetic analysis identified a pathogenic 475 kb deletion in the 3q26.1 region encompassing the PDCD10 gene, confirming the diagnosis of autosomal dominant cerebral cavernous malformations type 3. K Chest X-rays showing Pre-OP (left) and Post-OP (right) cardiomyopathy in a patient with homozygous likely pathogenic missense variant c.574G > A p.(Glu192Lys) in TPM1 gene. L Pedigree shows the pseudodominant inheritance pattern of autosomal recessive retinitis pigmentosa type 14. The index patient (ID: 141LO0350) was found to be homozygous for the nonsense pathogenic variant (NM_003322.4:c.901C > T p.(Gln301*)) in the TULP1 gene
Table 2.
Instances of non-penetrance. List of patients who lack the clinical phenotypes predicted by P/LP variants identified by CES/CGS even after careful reverse phenotyping
| ID | Patient phenotype | Gene | NM | Zygosity | Type and classification | OMIM phenotype | Evidence of pathogenicity | Result of reverse phenotyping |
|---|---|---|---|---|---|---|---|---|
| 192LO9459 | Hearing impairment—Male infertility—Chronic sinusitis—Reduced sperm motility—Abnormal sperm morphology | CAPN3 | NM_000070.2:c.1466G > A;p.(Arg489Gln) | Homozygous | Missense Pathogenic (class 1) | Muscular dystrophy, limb-girdle, autosomal recessive 1 (253,600) AR | This variant has been published several times before (PMIDs: 10,330,340, 39,411,402, 31,589,614, 38,523,675, 32,668,095, 14,578,192, 38,324,470, 30,919,934). It is classified as pathogenic based on the criteria: PP5_Strong, PM1_Moderate, PM5_Moderate, PP3_Moderate, PM2_Supporting | Lack of clinical muscle involvement |
| 123LO2080 | Brain atrophy—Cerebellar atrophy—Colpocephaly—Diabetes mellitus—Hypoplasia of the corpus callosum—Increased size of nasopharyngeal adenoids—Intellectual disability—Long nose—Microcephaly—Short philtrum | COL1A1 | NM_000088.3:c.2010del;p.(Gly671Alafs*95) | Heterozygous | Frameshift Pathogenic (class 1) | Osteogenesis imperfecta type I | This variant has been published several times before (PMIDs: 11,317,364, 26,863,094, 32,860,008, 33,939,306, 35,611,912, 31,239,369, 37,270,749, 37,270,749). It has been classified as pathogenic based on the criteria: PVS1_Very Strong, PP5_Very Strong, PM2_Supporting, PMID: 11,317,364 | Lack of clinical and radiological bone involvement |
| 163LO7560 | Abnormality of the eye—Blindness—Congenital onset—Retinal degeneration—Visual impairment | RNASEH2A | NM_006397.2:c.557G > A;p.(Arg186Gln) | Homozygous | Missense Pathogenic (class 2) | Aicardi-Goutieres syndrome | This variant has been published several times (PMIDs: 24,300,241, 29,239,743, 38,976,295, 36,937,954, 38,178,268, 36,705,819, 38,909,119, 36,065,636, 31,130,284 and 34,374,989). It has been classified as pathogenic based on the following criteria: PP5_Very Strong, PP3_Strong, PM5_Moderate and PM2_Supporting | Lack of neurological involvement |
Burden of genetic disorders in the population
The top five molecular diagnoses in our cohort are ABCA4-related retinal disease (OMIM #248200), NF1-related neurofibromatosis (OMIM #162200), HBB-related sickle cell disease (OMIM #603903), USH2A-related retinal disease (OMIM #276901), and PKD1-related polycystic kidney disease (OMIM #173900) (see Table 3 for the top 60 disorders by frequency). Because our cohort, despite being drawn from all parts of the country, may not be necessarily representative of the actual burden of these diseases, we resorted to a previously published method of estimating disease burden for autosomal recessive conditions based on the corresponding allele frequency [22]. The resulting ranking of diseases by their estimated burden is shown in Additional file 4: Table S3 that lists the predicted top 75 diseases, which reassuringly include 33 of the top 41 observed recessive diseases in Table 3. This argues that our cohort is likely representative of the local population.
Table 3.
Top 60 molecular diagnoses by frequency. This table displays the frequency distribution of the top 60 molecular diagnoses in our cohort
| Gene | OMIM phenotype | Phenotype MIM number | Mode of inheritance | No. of cases* |
|---|---|---|---|---|
| ABCA4 | Stargardt disease 1 | 248200 | AR | 21 |
| NF1 | Neurofibromatosis, type 1 | 162200 | AD | 20 |
| HBB | Sickle cell disease | 603903 | AR | 12 |
| USH2A | Usher syndrome, type 2A | 276901 | AR | 12 |
| PKD1 | Polycystic kidney disease 1 | 173900 | AD | 11 |
| HBA2 | Thalassemia, alpha- | 604131 | AR | 9 |
| TTN | Congenital myopathy 5 with cardiomyopathy | 611705 | AR | 9 |
| G6PD | Anemia, congenital, nonspherocytic hemolytic, 1, G6PD deficient | 300908 | XL | 8 |
| COL1A1 | Osteogenesis imperfecta, type I | 166200 | AD | 8 |
| COL4A5 | Alport syndrome 1, X-linked | 301050 | XLD | 8 |
| DCAF17 | Woodhouse-Sakati syndrome | 241080 | AR | 8 |
| DYSF | Muscular dystrophy, limb-girdle, autosomal recessive 2 | 253601 | AR | 8 |
| RP1 | Retinitis pigmentosa 1 | 180100 | AR | 8 |
| SORD | Neuronopathy, distal hereditary motor, autosomal recessive 8 | 618912 | AR | 8 |
| BEST1 | Bestrophinopathy, autosomal recessive | 611809 | AR | 7 |
| CBS | Thrombosis, hyperhomocysteinemic | 236200 | AR | 7 |
| CRB1 | Retinitis pigmentosa-12 | 600105 | AR | 7 |
| IMPG2 | Retinitis pigmentosa 56 | 613581 | AR | 7 |
| RSPH9 | Ciliary dyskinesia, primary, 12 | 612650 | AR | 7 |
| CYP1B1 | Glaucoma 3 A, primary open angle, congenital, juvenile, or adult onset | 231300 | AR | 6 |
| ADAT3 | Neurodevelopmental disorder with brain abnormalities, poor growth, and dysmorphic facies | 615286 | AR | 6 |
| ATP7B | Wilson disease | 277900 | AR | 6 |
| CAPN3 | Muscular dystrophy, limb-girdle, autosomal recessive 1 | 253600 | AR | 6 |
| COL4A4 | Hematuria, familial benign, 1 | 141200 | AD | 6 |
| EYS | Retinitis pigmentosa 25 | 602772 | AR | 6 |
| FBN1 | Marfan syndrome | 154700 | AD | 6 |
| UGT1A1 | Crigler-Najjar syndrome, type I | 218800 | AR | 6 |
| KCNV2 | Retinal cone dystrophy 3B | 610356 | AR | 6 |
| MYO7A | Usher syndrome, type 1B | 276900 | AR | 6 |
| SPG11 | Spastic paraplegia 11, autosomal recessive | 604360 | AR | 6 |
| CFTR | Cystic fibrosis | 219700 | AR | 5 |
| CNGB1 | Retinitis pigmentosa 45 | 613767 | AR | 5 |
| CYP2U1 | Spastic paraplegia 56, autosomal recessive | 615030 | AR | 5 |
| CYP4F22 | Ichthyosis, congenital, autosomal recessive 5 | 604777 | AR | 5 |
| MT-ND4 | Leber hereditary optic neuroretinopathy | 500001 | MT | 5 |
| NOTCH3 | Cerebral arteriopathy with subcortical infarcts and leukoencephalopathy 1 | 125310 | AD | 5 |
| OPA1 | Optic atrophy 1 | 165500 | AD | 5 |
| PROM1 | Cone-rod dystrophy 12 | 612657 | AR | 5 |
| SOD1 | Amyotrophic lateral sclerosis 1 | 105400 | AD | 5 |
| TULP1 | Retinitis pigmentosa 14 | 600132 | AR | 5 |
| VHL | von Hippel-Lindau syndrome | 193300 | AD | 5 |
| ANO5 | Muscular dystrophy, limb-girdle, autosomal recessive 12 | 611307 | AR | 4 |
| APC | Adenomatous polyposis coli | 175100 | AD | 4 |
| CA2 | Osteopetrosis, autosomal recessive 3, with renal tubular acidosis | 259730 | AR | 4 |
| CYP21A2 | Hyperandrogenism, nonclassic type, due to 21-hydroxylase deficiency | 201910 | AR | 4 |
| DNAH11 | Ciliary dyskinesia, primary, 7, with or without situs inversus | 611884 | AR | 4 |
| IRF6 | {Orofacial cleft 6} | 608864 | AD | 4 |
| GNE | Nonaka myopathy | 605820 | AR | 4 |
| LDLR | Hypercholesterolemia, familial, 1 | 143890 | AD | 4 |
| MC4R | Obesity (BMIQ20) | 618406 | AR | 4 |
| MERTK | Retinitis pigmentosa 38 | 613862 | AR | 4 |
| MPL | Amegakaryocytic thrombocytopenia, congenital, 1 | 604498 | AR | 4 |
| MPV17 | Charcot-Marie-Tooth disease, axonal, type 2EE | 618400 | AR | 4 |
| OCA2 | Albinism, oculocutaneous, type II | 203200 | AR | 4 |
| PAX2 | Glomerulosclerosis, focal segmental, 7 | 616002 | AD | 4 |
| PTPN11 | Noonan syndrome 1 | 163950 | AD | 4 |
| SCN4A | Hypokalemic periodic paralysis, type 2 | 613345 | AD | 4 |
| SCN5A | Cardiomyopathy, dilated, 1E | 601154 | AD | 4 |
| SETX | Spinocerebellar ataxia, autosomal recessive, with axonal neuropathy 2 | 606002 | AR | 4 |
| SGCA | Muscular dystrophy, limb-girdle, autosomal recessive 3 | 608099 | AR | 4 |
*CMP het are counted only once
Spectrum of molecular findings
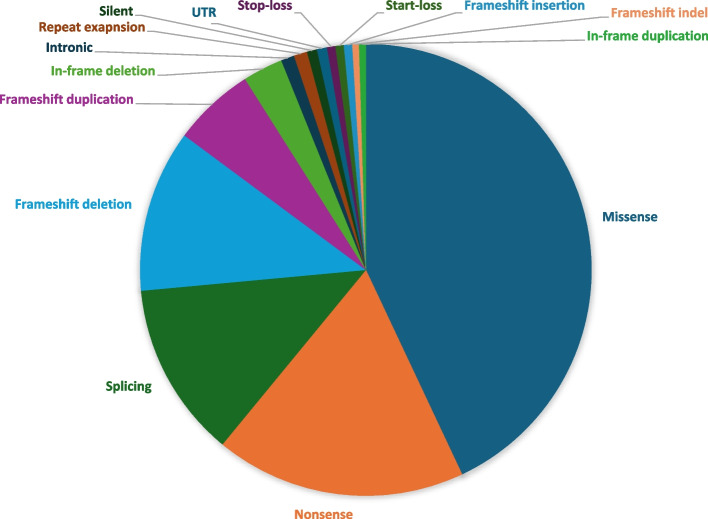
The diagnostic molecular findings in our cohort comprise 829 SNVs, 8 repeat expansions, and 90 CNVs spanning 535 genes (without counting CNVs). SNVs accounted for the majority (~ 90%) of variants in positive reports (see Fig. 4 for breakdown of classes: UTR, missense, nonsense, startloss, stoploss, splicing, frameshift indels, inframe indels, and repeat expansions). CNVs accounted for ~ 10% of positive reports and they ranged in size from 120 bp to entire chromosomes, e.g., Klinefelter syndrome. Additional file 5: Table S4 lists all cases with CNVs and SVs. The majority of candidate variants in positive reports (61%, 698/1142) were autosomal recessive, and of these, the overwhelming majority (94.6%, 660/698) were homozygous. In the 39 cases with compound heterozygous variants, at least one of the two variants was a founder variant in the majority (n = 35, 89.7%). In general, founder variants accounted for 49.9% (414/829) of all SNVs. Of all the variants reported in positive cases, the candidate variant was autosomal dominant (de novo status could not be always evaluated) in 31.6% (361/1142), while X-linked and mitochondrial variants were observed in 5.9% (67/1142) and 1.4% (16/1142) of cases, respectively. Of the 919 unique SNVs we encountered in this cohort, 33% (n = 303) have not been previously published while the remaining have corresponding HGMD identifiers linking them to their original published reports (Additional file 6: Table S5). Homozygous variants in genes typically linked to autosomal dominant conditions were observed in some cases, which usually represented bona fide recessive forms of the same disease, e.g., SYNE1-related Emery-Dreifus muscular dystrophy (OMIM #612998) in 185LO6214, AFG3L2-related optic atrophy (OMIM #618977) in 183LO3607 (Fig. 3), and OPA1-related Optic atrophy plus syndrome (OMIM #125250) in 124LO7729, or a much more severe version of the disease, e.g., MAPRE2-related congenital symmetric circumferential skin creases syndrome (MIM #616734) in 130LO6674 (Additional file 1: Table S1). In other instances, the observed homozygosity was for a known dominant variant that was inherited from both parents resulting in a more pronounced phenotype, e.g., TPM1-related cardiomyopathy (OMIM #611878) in 167LO7958 (Fig. 3). In contrast, homozygosity for dominantly acting repeat expansion in ATXN8OS/ATXN8 in case 176LO5597 and homozygosity for dominantly acting NM_000435.2:c.1790G > C p.Cys597Ser variant in NOTCH3 were not associated with a more severe phenotype consistent with the known toxic gain of function mechanism of these diseases [25, 26]. We have also encountered pseudodominance where one parent is homozygous for a recessive condition and the other parent is a carrier for the same condition due to consanguinity resulting in what appears to be a “vertical” transmission of an otherwise recessive condition (see Fig. 3 for an example).
Fig. 4.
Breakdown of diagnostic molecular variant classes. This figure summarizes the molecular findings in our cohort and further delineates them into various classes
The high consanguinity rate in the study cohort made it also possible to encounter extremely rare and even novel recessive disorders. Table 4 lists cases from this cohort supporting candidate gene-disease links that had been proposed based on single reports or not yet established in OMIM, e.g., DHX32-related retinal degeneration, MCM8-related primary ovarian insufficiency, MPIG6B-related myelofibrosis, MYH14-related myopathy, C19orf12-spastic paraplegia, KCNK4-related NDD with facial dysmorphism, and RBP3-related retinal degeneration. In other instances, the disease is listed in OMIM but with a question mark indicating the tentative nature of the link, typically because it is based on a single family. Table 4 also lists cases in our cohort that provide support to such tentative links such as CIBAR1 (FAM92A)-related polydactyly (see also Supplemental Clinical Material). On the other hand, we propose two genes as candidates for novel gene-disease links that have not been reported previously. RNF43 is a gene we propose to have a novel link to hemochromatosis. We were able to identify a founder LOF variant c.1403C > G(p.Ser468*) in 15 patients (13 families) all of whom had tested negative for HFE as a potential cause of their unexplained hemochromatosis. Similarly, we propose NFXL1 as a candidate gene for an apparently novel syndrome comprising joint hyperlaxity with or without short stature and renal disease. Six patients (5 families) with this phenotype were found to be homozygous for the founder LOF NFXL1 variant c.1617del;p.(Cys539Trpfs*64), while two patients (2 families) were homozygous for another founder LOF NFXL1 variant NM_001278623.1:c.2041A > T p.(Lys681*). Additional file 8: Supplemental Clinical Report describes the phenotypic features of all these patients, some of whom are not part of the original cohort and were added after they were identified by querying our internal database. This same founder phenomenon has allowed us to successfully upgrade (where multiple unrelated individuals share the phenotype of the index) 37 variants and downgrade (where multiple unrelated individuals lack the phenotype of the index) 112 variants that we encountered in our cohort (Additional file 7: Table S6).
Table 4.
This table lists cases from our cohort that support candidate gene-disease associations proposed in the literature. It includes gene-disease links that had been proposed based on single reports or not yet established in OMIM as well as cases reinforcing tentative OMIM associations (indicated by a question mark).
| Case ID | HPO Phenotypes | OMIM | PMID | Gene | Variant |
| 175LO6913 | Methylmalonic acidemia - Abnormal cerebral white matter morphology - Abnormal spinal cord morphology - Brain imaging abnormality - Decreased circulating vitamin B12 concentration - Difficulty walking - Dysarthria - Lower limb muscle weakness - Lower limb spasticity - Progressive spastic paraparesis |
Spastic paraplegia 9B, autosomal recessive (based on a single publication) |
PMID: 26026163 | ALDH18A1 | NM_001323413.1:c.1073C>T;p.(Pro358Leu) - Homozygous - Missense Upgraded to Likely pathogenic |
| 178LO0879 | Inability to walk - Intellectual disability - Spasticity | ?Spastic paraplegia 43, autosomal recessive | PMID: 23857908 | C19orf12 | NM_001031726.2:c.157G>A;p.(Gly53Arg) - Homozygous - Missense Likely pathogenic (class 2) |
| 163LO7450 | Abnormal hand morphology - Abnormal jaw morphology - Abnormal nasal bridge morphology - Abnormal tongue morphology - High palate - Horizontal eyebrow - Hypokalemia - Low-set ears - Muscle weakness - Narrow palate - Obesity - Pointed chin - Polydactyly - Prominent nasal bridge - Reduced visual acuity - Short foot - Small hand - Spastic paraparesis - Tall chin | ?Polydactyly, postaxial, type A9 | PMID: 30395363 | CIBAR1 | NM_145269.4:c.478C>T;p.(Arg160*) - Homozygous - Nonsense Affecting protein function (class 1P) |
| 143LO3954 | Anxiety; Attention deficit hyperactivity disorder; Autistic behavior; Intellectual disability - borderline; Intellectual disability - moderate | NA | PMID: 40330149 | CSMD1 | NM_033225.5:c.1004_1005delCT;p.(Ser335Cysfs*12) - Heterozygous - Frameshift Upgraded to Likely pathogenic |
| 187LO6113 | Abnormal light- and dark-adapted electroretinogram - Abnormal retinal morphology - Abnormality of retinal pigmentation - Nyctalopia - Retinal dystrophy - Rod-cone dystrophy - Visual impairment | NA | PMID: 29320387 | DHX32 | NM_018180.3:c.1756dup;p.(Val586Glyfs*5) - Homozygous - Frameshift Uncertain effect on protein function (class 3P) |
| 178LO8102 | Abnormality of vision - Nyctalopia - Nystagmus - Photophobia - Reduced visual acuity - Retinal dystrophy - Rod-cone dystrophy | NA | PMID: 26355662, PMID: 31130284 | DNAJC17 | NM_018163.2:c.681G>A;p.(=) - Homozygous - Silent Likely pathogenic (class 2) |
| 183LO3286 | Abnormality of speech or vocalization - Delayed speech and language development - Hyperactivity | NA | PMID: 37433783 | EZH1 | NM_001321079.1:c.2109T>G;p.(Tyr703*) - Homozygous - Nonsense Upgraded to Likely pathogenic |
| 153LO4988 | Abnormal bone marrow cell morphology - Abnormal granulocyte morphology - Abnormal megakaryocyte morphology - Abnormality of neutrophils - Increased micromegakaryocyte count - Leukopenia - Megakaryocyte dysplasia - Monocytosis - Pancytopenia - Thrombocytopenia | NA | 10.1182/blood-2019-130142 | FANCG |
NM_004629.1:c.769C>G;p.(Arg257Gly) - Homozygous - Missense Upgraded to Likely pathogenic. multiple pathogenic mosaic copy number variations arr[GRCh37] 5q15q35.3(92994855_180715096)x1[0.5] arr[GRCh37] 1q21.1q44(146660142_249224684)x3[0.5] arr[GRCh37] 3q26.2q29(169218685_197851444)x3[0.5] arr[GRCh37] 21q22.13q22.3(38211999_48093361)x3[0.5] - Heterozygous Duplication and Heterozygous deletion |
| 159LO1779 | Abnormal upper lip morphology - Astigmatism - Low-set ears - Nyctalopia - Oligohydramnios - Reduced visual acuity - Secondary amenorrhea - Short philtrum - Specific learning disability | NA | PMID: 39572588 | GPATCH11 | NM_174931.3: c.211A>T;p.(Lys71*)- Homozygous – Nonsense Likely pathogenic (class 2) |
| 156LO3123 | Parkinsonism - Rigidity | NA | PMID: 40056900 | ITSN1 |
NM_003024.2:c.1715T>G; p.(Leu572*)- Heterozygous – Nonsense Likely pathogenic (class 2) |
| 184LO6973 | Bilateral tonic-clonic seizure - Coarse facial features - Cognitive impairment - Gingival overgrowth - Hirsutism - Intellectual disability - Seizure - Specific learning disability |
Facial dysmorphism, hypertrichosis, epilepsy, intellectual/developmental delay, and gingival overgrowth syndrome (based on a single publication) |
PMID: 30290154 | KCNK4 | NM_001317090.1:c.698C>T;p.(Pro233Leu) - Heterozygous - Missense Likely pathogenic (class 2) |
| 172LO1016 | Abnormal ovarian morphology - Hypoplasia of the uterus - Primary amenorrhea | ?Premature ovarian failure 10 | PMID: 28863940, PMID: 27573988 | MCM8 | NM_001281521.1:c.482A>C;p.(His161Pro) - Homozygous - Missense Likely pathogenic (class 2) |
| 140LO2157 | Female infertility - Premature ovarian insufficiency | ?Premature ovarian failure 10 | PMID: 28863940, PMID: 27573988 | MCM8 | NM_001281521.1:c.486+1G>A - Homozygous - Substitution Upgraded to Likely pathogenic |
| 173LO6128 | Hematological neoplasm - Myelodysplasia - Pancytopenia | ?Thrombocytopenia, anemia, and myelofibrosis | PMID: 29898956 | MPIG6B | NM_138272.2:c.149dup; p.(Ala52Glyfs*128) - Homozygous - Frameshift Likely Pathogenic (class 2) |
| 174LO1837 | Distal muscle weakness - Weakness of muscles of respiration | ?Peripheral neuropathy, myopathy, hoarseness, and hearing loss | PMID: 21480433, PMID:27875632, PMID:31231018 | MYH14 | NM_001145809.1:c.2921G>T;p.(Arg974Leu) - Heterozygous - Missense Pathogenic (class 1) |
| 165LO1356 | Abdominal pain - Anemia - Diarrhea - Hemolytic anemia - Immunodeficiency - Leukocytosis - Neutrophilia - Recurrent bacterial infections - Recurrent cutaneous abscess formation - Recurrent fever | ?RAS-associated autoimmune lymphoproliferative syndrome type IV, somatic | PMID: 21063026 | NRAS | NM_002524.3:c.38G>T;p.(Gly13Val) - 24% of 192 NGS reads - Missense Pathogenic (class 1) |
| 169LO9100 | Abnormal bleeding - Abnormal platelet aggregation - Impaired platelet aggregation | ?Bleeding disorder, platelet-type, 18 | PMID: 24958846 | RASGRP2 | NM_001098670.1:c.71_73+30del - Homozygous - In-frame Likely Pathogenic (class 2) |
| 187LO7238 | Abnormal cerebral white matter morphology - Brain imaging abnormality - Headache - Hyperintensity of cerebral white matter on MRI - Hyperlipidemia - Hypertension - Migraine - Multifocal hyperintensity of cerebral white matter on MRI - Rod-cone dystrophy | ?Retinitis pigmentosa 66 | PMID: 19074801, PMID: 21067480 | RBP3 | NM_002900.2:c.1162C>T;p.Arg388* - Homozygous - Nonsense Likely pathogenic (class 2) |
| 170LO0122 | Abnormal ileum morphology, Crohn’s disease, Jaundice, Unconjugated hyperbilirubinemia | NA | PMID: 24945726 | SLCO3A1 | NM_013272.3:c.1553A>G;p.(Glu518Gly) - Homozygous - Missense Uncertain effect on protein function (class 3P) |
| 124LO2324 | Giant cell granuloma of mandible - Seizure - Small for gestational age | Neurodevelopmental disorder with seizures and gingival overgrowth (based on a single publication) | PMID: 32623794 | TBC1D2B | NM_144572.1:c.570dup;p.(Val191Cysfs*26) - Homozygous - Frameshift Upgraded to Likely pathogenic |
| 163LO1126 | Ataxia - Cerebral atrophy - Chorea | ?Spinocerebellar ataxia, autosomal recessive, with axonal neuropathy | PMID: 12244316, PMID: 31182267 | TDP1 | NM_001008744.1:c.1478A>G;p.(His493Arg) - Homozygous - Missense Upgraded to Likely pathogenic |
| 192LO5298 | Abnormality of blood and blood-forming tissues - Thrombocytopenia - Neutropenia - Pancytopenia - Metabolic acidosis - Fever - Immunodeficiency - Recurrent lower respiratory tract infections - Elevated hepatic transaminase - Poor appetite - Hypofibrinogenemia - Hemophagocytosis - Decreased hemoglobin concentration |
Immunodeficiency 109 with lymphoproliferation (based on a single publication) |
PMID: 30872117, PMID37644014 | TNFRSF9 | NM_001561.5:c.325G>A;p.(Gly109Se) - Homozygous - Missense Upgraded to Likely pathogenic |
| 173LO1390 | Abnormal retinal morphology - Myopia - Nyctalopia - Photophobia - Reduced visual acuity - Rod-cone dystrophy | Cone-rod dystrophy 19 (based on a single publication) | PMID: 30054919 | TTLL5 | NM_015072.4: c.1039T>C;p.(Phe347Leu) - Homozygous - Missense Uncertain significance (class 3) |
| 187LO9693 | Lower limb muscle weakness - Memory impairment - Muscle weakness - Progressive proximal muscle weakness - Proximal muscle weakness in lower limbs - Proximal muscle weakness in upper limbs - Upper limb muscle weakness | NA | PMID: 28192369, PMID: 27790088 | UNC13A | NM_001080421.2:c.3215+3G>C;p.(?) - Heterozygous - Splicing Upgraded to Likely pathogenic |
| 154LO1809 | Abnormality of the nose - Absent speech - Ataxia - Bilateral single transverse palmar creases - Broad forehead - Bulbous nose - Deeply set eye - Depressed nasal bridge - Developmental regression - Global developmental delay - Leukodystrophy - Microdontia - Mild global developmental delay - Round face - Seizure - Synophrys - Thick upper lip vermilion - Widely spaced teeth | NA | PMID: 31231135 | WDR91 | NM_014149.3:c.1000C>T;p.(Gln334*) - Homozygous - Nonsense Likely affecting protein function (class 2P) |
Multilocus phenotypes resulting from the coexistence of two or more Mendelian diseases were encountered in 5% (n = 56) of positive cases (Additional file 9: Table S7). In 15 (26.7%) of such cases, the multilocus phenotype resulted from two recessive variants while the rest involved various other combinations (Additional file 9: Table S7). Mosaicism was rare and was encountered in only 8 (0.74%) positive cases and was mostly related to clonal hematopoiesis of indeterminate potential (CHIP) involving JAK2, SF3B1, and ASXL1.
Clinical utility of CES/CGS in adult medicine
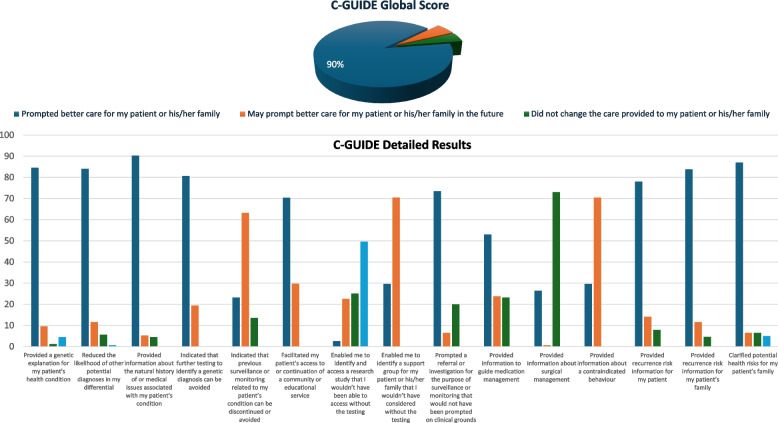
At the diagnostic level, we found that 38% of patients had the wrong diagnostic label in the requisition form (287 out of 457 for whom a diagnostic label was provided had molecular confirmation of the diagnostic label). However, this should be interpreted with caution because there are 717 cases whose diagnostic label could not be verified because the CES/CGS report was non-diagnostic. By interviewing 23 clinicians and applying the C-GUIDE to 155 positive reports, we found a very high score (90%) of clinical utility in positive results (Fig. 5). Please refer to the Additional file 10: Supplemental C-GUIDE Material for a detailed overview of the questions included in the questionnaire. To further highlight the clinical significance of genomic testing, physicians shared case examples that highlighted the clinical value of genomic testing in guiding management. For instance, the identification of X-linked dominant Alport syndrome type 1 (OMIM #301,050) in a patient who presented with hearing loss has led to early nephrology referral, routine blood pressure monitoring, and avoidance of nephrotoxic medications, ultimately minimizing renal complications. In another patient with RTEL1-related dyskeratosis congenita (OMIM #616,373), early diagnosis helped avert overtreatment for hyperpigmentation and prompted hematology evaluation due to risk of bone marrow failure. Another patient misdiagnosed with multiple sclerosis was correctly identified with spastic paraplegia type 9B (OMIM #616,586), an autosomal recessive disorder, shifting management to focus on spasticity rather than demyelination. These cases underscore the clinical utility of genomic testing in delivering personalized, efficient, and anticipatory care.
Fig. 5.
Clinical utility of positive genetic testing results as assessed by C-GUIDE. This figure displays the breakdown of C-GUIDE scores across various domains, including diagnosis/prognosis understanding, medical management, reproductive, and health risk awareness. The panel corresponds to the answers provided for each category in the same order in which they appear in the standard questionnaire (see Supplemental C-GUIDE Material)
To compensate for the lack of follow-up clinical data, we used a previously published compendium of treatable genetic disorders that is regularly updated online (https://www.rx-genes.com/) to identify molecular diagnoses that are likely to result in a treatment recommendation [27]. Remarkably, 26% of positive reports involved at least one disease for which a treatment recommendation exists. The predominance of molecular diagnoses associated with pediatric-onset conditions suggests that numerous opportunities for otherwise highly effective interventions have been missed, e.g., phenylketonuria. This analysis excludes ACMG recommended list of genes for reporting secondary findings. The consent to provide secondary findings was provided by most (n = 2166 (78.4%)) of participants, and of those, at least one ACMG secondary finding was identified in 2.35% (n = 51) (Additional file 1: Table S1). From a reproductive counseling perspective, we note that nearly half (n = 1320, 47.8%) of participants were found to have at least one pathogenic recessive variant in the heterozygous state (one participant carried six variants; and up to five variants were carried by five participants) (Additional file 1: Table S1).
Discussion
To our knowledge, this is the largest adult-focused CES/CGS cohort to date. The fact that it comes from an underrepresented population group makes it even more relevant for evidence generation supporting a CES/CGS-powered precision medicine approach to adult medicine in diverse settings. In view of the overrepresentation of pediatrics in clinical genomics literature, attempts at exploring the role of clinical genomics in adult medicine such as this reveal that this imbalance should not be simplistically attributed to biological reasons. Indeed, these studies uncover an alarming degree of underappreciation among adult medicine practitioners of the share of monogenic diseases in their practice. That many such diseases appear to receive the wrong diagnostic label, as revealed by our study, underlines the value of disease-agnostic clinical genomics enabled by CES/CGS.
Our cohort highlights several aspects that make the practice of adult clinical genomics distinct from its pediatric counterpart. Apart from the obvious and expected enrichment for adult-onset genetic disorders, their potential overlap with common (multifactorial) diseases can be a challenge. It is well known that for each of these common diseases, there are monogenic forms that can easily evade detection although their relative contribution varies widely. For example, one CES study found that > 9% of chronic kidney disease in adults can be traced to monogenic conditions [28]. On the other hand, < 0.5% of diabetes appears to be monogenic [29]. Although our cohort cannot be used to estimate the contribution of monogenic forms of common diseases, we note the relatively high yield when cases are selected based on positive family history even in the presence of traditional risk factors. Another aspect worth highlighting about adult clinical genomics is the need to view it as a continuum of its pediatric counterpart. For example, most of the newly described developmental syndromes were described in children and are, therefore, largely unknown to adult medicine practitioners. Cohorts such as ours not only increase awareness of these conditions in the adult population, but also “complete” the phenotypes originally described in pediatrics. The evolution of these phenotypes in adult patients can take many forms as we have shown in this study, sometimes making the disease unrecognizable when compared to its pediatric form. The lack of a clear molecular diagnosis in the majority of our cohort is a common finding among cohorts of clinical exome and genome sequencing. As shown in our analysis, even a follow-up genome after a negative exome result only partially addresses this diagnostic gap. In previous work, we investigated many potential reasons and concluded that they are mostly related to interpretation rather than detection [29–31]. In addition, there is a factor worth highlighting in the setting of adult genome sequencing specifically, that is, the potential for non-genetic etiology. Indeed, adults are more likely to accumulate sufficient exposure to non-genetic risk factors to cause phenotypes as compared to their pediatric counterpart.
Our study population with its enrichment for consanguinity offers several added benefits when compared to similar studies in other populations. The power of consanguinity to render extremely rare deleterious variants homozygous is known to increase the burden of autosomal recessive diseases. This explains the preponderance of these diseases in our cohort, which was an opportunity to observe clinical phenotypes that would be very difficult to encounter otherwise. In addition to extremely rare disorders that have previously been described, we highlight two novel ones. For example, RNF43 is a gene that we propose to be an important cause of hemochromatosis in our local population. RNF43 is an E3 ubiquitin ligase that has been shown to dampen WNT signaling [32], which upregulates the expression of DMT1, a major player in intestinal iron absorption. It is tempting to hypothesize that loss of RNF43, therefore, may lead to upregulation of DMT1, a possibility we plan to pursue in future work. Interestingly, the knockout mouse for Rnf43 (https://www.informatics.jax.org/allele/MGI:6403354) has hyperchromasia (high hemoglobin concentration) and there is a Finnish missense variant that is strongly associated with iron deficiency anemia, which may reflect a gain of function effect [33]. The biological basis of the proposed link between NFXL1 and the syndrome of short stature, joint laxity, and renal disease is less clear although we note that short long bones are listed in the phenotype of Nfxl1 knockout mouse (https://www.informatics.jax.org/allele/MGI:4432670). While the human genetics supporting the link between NFXL1 and the observed novel syndrome is compelling, we note the scarcity of data that support biological plausibility. For example, the exact function of NFXL1 remains poorly understood and there are no published mouse models of NFXL1 deficiency. The surprising number of families we identified for these two novel conditions highlights the opportunities of uncovering medically relevant founder variants in the local population as discussed in previous work [34].
Clinical utility has emerged as a key determinant of broader adoptions of healthcare innovations, CES/CGS included. In the pilot phase of the 100,000 UK Project involving 1612 adult probands, the authors highlighted that referring physicians predicted an impact on management in 25% of positive cases and only reported no benefit in 0.2% [35]. Here, we used a validated instrument to objectively measure clinical utility and found a much higher percentage of 90% albeit in the small fraction that was sampled, a limitation we acknowledge. One potential reason for this discrepancy is the preponderance of autosomal recessive diseases in our cohort. Unlike de novo dominant conditions, which tended to be the more common category in the 100,000 UK Project, autosomal recessive conditions are inherently more amenable to preventive strategies, which is an important component of clinical utility as shown previously [36, 37]. Another potential reason is that CES/CGS was typically the first non-karyotype genetic test patients in our cohort were exposed to. In other words, more familiar diagnoses with available treatment options, which would have been picked up by more traditional genetic tests in the UK cohort, were more likely to turn up in our cohort.
There are other limitations we acknowledge in this study. Our cohort was established based on retrospective review of cases, a design that may limit the generalizability of the patterns we observed. One unique challenge in adult genomic medicine is the difficulty in obtaining parental samples for trio analysis compared to pediatrics. While the predominance of homozygous causal variants in our cohort may have minimized the impact of this limitation, it is worth noting that 58% of the “ambiguous” diagnoses involved heterozygous variants that could have potentially been properly classified by parental testing. Indeed, we note that only 1.9% of the 293 trio exomes/genomes in our cohort were reported to have heterozygous variants of uncertain significance, while the corresponding percentage among the 2524 solo exomes/genomes was 4.47%. The competence of adult patients to provide informed consent can be compromised by many genetic neurological conditions, and this may complicate the wider adoption of genomic sequencing in the adult population. Fortunately for us, all adults in this study were accompanied by relatives who provided the consent on their behalf when necessary. Many of the ambiguous reports could have been upgraded or downgraded if segregation data were available for some families, which is another limitation. Despite these limitations, we believe that the large sample size, the diversity of indications, the high rates of consanguinity, the underrepresentation of the local population, and the consistent reporting procedure by the same lab are noteworthy strengths.
Conclusions
We show in a large nationwide cohort from a single testing laboratory that CES/CGS is a powerful tool of precision medicine in adult patients. The added benefits we gained from the special structure of the study population suggest that CES/CGS in adult medicine is not only applicable in diverse settings but such settings can also inform its deployment in novel ways. We emphasize the remarkably high clinical utility revealed by our analysis as well as the new knowledge offered by the discovery of novel aspects about gene-disease links.
Supplementary Information
Additional file 1: Table S1: Comprehensive cohort overview. This table lists the detailed characteristics of the entire study cohort. The table is organized to provide an in-depth look at the demographic, clinical, and molecular findings. This supplementary table enables readers to understand the distribution and variability of the data that underpin the study’s analyses and conclusions.
Additional file 2: Table S2: Reasons for missed molecular diagnoses by prior genetic testing in positive reflex CGS cases. This table details the specific technical or interpretative limitations that led to prior genetic testing missing the molecular diagnosis in each case, as later identified by reflex genomic sequencing (CGS).
Additional file 3: Figure S1: Radiological imaging findings. This figure presents a series of radiological images from our adult patient cohort, highlighting key structural features and anomalies that support the clinical and genetic findings of the study.
Additional file 4: Table S3: Ranking of diseases by estimated burden. This table presents diseases ranked according to their estimated burden within the cohort based on allele frequency data in the local population.
Additional file 5: Table S4: List of all cases with CNVs and SVs. This table presents all cases with CNVs and SVs among our cohort.
Additional file 6: Table S5: HGMD-reported SNVs. This table lists the subset of SNVs from our cohort that have corresponding HGMD identifiers.
Additional file 7: Table S6: Reclassification of variants using the founder phenomenon. This table provides a summary of the variants that were reclassified based on the founder phenomenon, with some variants being upgraded and others downgraded. It offers justification for these reclassifications.
Additional file 8: Supplemental Clinical Report: This report provides clinical information on each case along with the rationale supporting their designation as new candidate gene-disease links.
Additional file 9: Table S7: Multilocus phenotypes. This table summarizes cases where patients exhibit multilocus phenotypes due to the coexistence of two or more Mendelian disorders. It categorizes the combinations based on their inheritance patterns.
Additional file 10: Supplemental C-GUIDE (Clinician-reported Genetic testing Utility InDEx) Material: This document presents the previously validated questionnaire (C-GUIDE) designed to evaluate the clinical utility of genomic testing. The questionnaire explores multiple dimensions related to the application of genomic testing in clinical settings. It assesses key aspects such as understanding diagnosis and prognosis, informing medical management, awareness and actionability of reproductive and health risks for patients and family members.
Acknowledgements
We thank patients and their families. We thank Dr. Ahmed Attar, and Dr. Ashraf Warsi for their contribution. The authors gratefully acknowledge the partial funding support provided by the King Salman Center for Disability Research through the Excellence Research Center (Grant No. KSCDR-ERC-2024-02). We also thank the Centogene team for providing molecular diagnostic reports.
Abdulaziz Baazeem46, Abdulrahman Alsultan10, Abdulrahman AlTahan47, AbdulRahman Hummadi48, Ahmed AlBadawi1, Ali AlAsmari19, Amaal Aldakheel49, Ali Awaji25, Bader Alghamdi50, Basma Zahid49, Dalal K. Bubshait39, Dia Ali Arafah Mohammed51, Elham Bagrayn52, Firdous Abdulwahab45, Hussein A Algahtani53, Iram A Alluhaydan3, Jameela A. Kari54, Mahmoud Alhajji55, Mai Labani49, Mais O. Hashem45, Moayed Aljack1, Mohamed Alzawahmah49, Mohammed A Mahnashi56, Mohammed Ali Tohary25, Mohd Elsunni24, Mona A Fouda57, Nizar Algarni58, Nouriya Abbas Al-Sannaa7, Ohoud Alzahrani36, Omar Abu Yousef45, Omnia Ahmed Abdulaty2,3, Saeed Bohlega59, Sameer Abdullah60, Saud Abu-Harbesh61, Wael Alqarawi62,63, Yousef Housawi5, Zainab AlArfaj64.
46Department of General Surgery, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia.
47Specialized Neurology Complex and King Saud University, Riyadh, Saudi Arabia.
48Endocrine and Diabetes Center, Ministry of Health, Jazan, Saudia Arabia.
49King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
50Respirology, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
51Maternity and Children Hospital, Ministry of Health, Makkah, Saudi Arabia.
52Fellow at Prince Sultan Military Medical City, Riyadh, Saudia Arabia.
53Aseer Central Hospital, Abha, Aseer region, Saudi Arabia.
54Pediatric Nephrology Center of Excellence and Pediatric Nephrology Unit, Pediatric Department, Faculty of Medicine, King Abdulaziz University Hospital, King Abdulaziz University, Jeddah, Saudi Arabia.
55Almoosa Specialist Hospital Dhahran Rd, Al Mubarraz 36,342, Saudi Arabia.
56King Fahad Central Hospital, Jazan, Saudi Arabia.
57Department of Medicine, College of Medicine, King Saud University, and King Saud University Medical City, Riyadh, Saudi Arabia.
58Department of Orthopedic Surgery, College of Medicine, King Saud University, Riyadh, Saudia Arabia.
59Department of Neurosciences (MBC-76), King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
60Royal Commission Health Services, Medical Affairs, Riyadh, Saudia Arabia.
61National blood & Cancer Center, King Abdullah Branch Road, Almughrizat, Riyadh, Saudi Arabia.
62Department of Cardiac Sciences, College of medicine, King Saud University, Riyadh, Saudi Arabia.
63The National Center for Sudden Death, Riyadh, Saudi Arabia.
64King Fahad University Hospital, Alkhobar, Saudi Arabia.
Authors’ contributions
K.B., H.H., B.A., WB, T.B., F.S.K.: collected and analyzed data and wrote the manuscript. M.A., A.A., W.E., T.A., F.A., A.A., F.A., M.A., S.M., A.A., F.A., H.A., M.A., E.A.F., A.M., R.A., M., B.A., N.S.S., S.A., M.A., A.T., G.G., F.H., F.A., H.A., A.A., R.B., M.A.A., A.A., A.S.A., A.A., R.A.S., A.A., E.D., A.A., D.A., B.S.A., A.S., M.A., W.S.M., Z.A.A., M.A., A.B., A.A., A.A., A.H., A.A., A.A., A.A., A.A., B.A., B.Z., D.K.B., D.A.M., E.B., F.A., H.A.A., I.A.A., J.K., M.A., M.L., M.O.H., M.A., M.A., M.A.M., M.A.T., M.E., M.A.F., N.A., N.A.A., O.A., O.A.Y., O.A.A., S.B., S.A., S.A., W.A., Y.H., Z.A., L.A.: collected and analyzed data. K.B., H.H., B.A., M.A., A.A., W.E., T.A., F.A., A.A., F.A., M.A., S.M., A.A., F.A., H.A., M.A., E.A.F., A.M., R.A., B.A., N.S.S., S.A., M.A., A.T., G.G., F.H., F.A., H.A., A.A., R.B., M.A.A., A.A., A.S.A., A.A., R.A.S., A.A., E.D., A.A., D.A., B.S.A., A.S., M.A., W.S.M., Z.A.A., M.A., W.A., A.B., A.A., A.A., A.H., A.A., A.A., A.A., A.A., B.A., B.Z., D.K.B., D.A.M., E.B., F.A., H.A.A., I.A.A., J.K., M.A., M.L., M.O.H., M.A., M.A., M.A.M., M.A.T., M.E., M.A.F., N.A., N.A.A., O.A., O.A.Y., O.A.A., S.B., S.A., S.A., W.A., Y.H., Z.A., L.A, T.B., F.S.K: reviewed the manuscript and approved its submitted version.
Funding
This work was funded in part by King Salman Center for Disability Research through Excellence Research Center No. KSCDR-ERC-2024–02.
Data availability
All data supporting the findings described in this paper are available in the article, its supplemental information, and from the lead contact upon request. Due to local privacy laws and privileged human information, all requests for whole-exome sequencing and whole-genome sequencing data are subject to prior approval from the local Institutional Review Board, which can be reached at ora@kfshrc.edu.sa with an expected time frame for response of 2 months.
Declarations
Ethics approval and consent to participate
This study adheres to the principles of the Declaration of Helsinki (1996) and was approved by the IRB with a waiver of consent by the Ethics Committee at the Office of Research Affairs, King Faisal Specialist Hospital, and Research Center (KFSHRC RAC# 2230016) for the use of aggregated data. Ethical approval was also granted by Ministry of National Guard Health Affairs (MNG-HA) Institutional Review Board (KAIMRC RC19/120/R).
Consent for publication
Written informed consent was obtained from participants and/or their legal guardians for the publication of possible identifiable clinical details and identifiable facial images, in accordance with IRB-approved protocols (KFSHRC RAC# 2080006 and KAIMRC RC19/120/R).
Competing interests
KB, HH, BA, AA, MA, WB and FSA are paid employees and TB is a paid consultant of Lifera Omics.
Footnotes
Publisher’s Note
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Fowzan S. Alkuraya, Email: falkuraya@liferaomics.com.sa
Saudi Adult Genomics Group:
Abdulaziz Baazeem, Abdulrahman Alsultan, Abdulrahman AlTahan, AbdulRahman Hummadi, Ahmed AlBadawi, Ali AlAsmari, Amaal Aldakheel, Ali Awaji, Bader Alghamdi, Basma Zahid, Dalal K. Bubshait, Dia Ali Arafah Mohammed, Elham Bagrayn, Firdous Abdulwahab, Hussein A Algahtani, Iram A Alluhaydan, Jameela A. Kari, Mahmoud Alhajji, Mai Labani, Mais O. Hashem, Moayed Aljack, Mohamed Alzawahmah, Mohammed A Mahnashi, Mohammed Ali Tohary, Mohd Elsunni, Mona A Fouda, Nizar Algarni, Nouriya Abbas Al-Sannaa, Ohoud Alzahrani, Omar Abu Yousef, Omnia Ahmed Abdulaty, Saeed Bohlega, Sameer Abdullah, Saud Abu-Harbesh, Wael Alqarawi, Yousef Housawi, and Zainab AlArfaj
References
- 1.Shickh S, Mighton C, Uleryk E, Pechlivanoglou P, Bombard Y. The clinical utility of exome and genome sequencing across clinical indications: a systematic review. Hum Genet. 2021;140(10):1403–16. [DOI] [PubMed] [Google Scholar]
- 2.Guo F, Liu R, Pan Y, Collins C, Bean L, Ma Z, et al. Evidence from 2100 index cases supports genome sequencing as a first-tier genetic test. Genet Med. 2024;26(1):100995. [DOI] [PubMed] [Google Scholar]
- 3.Wright CF, Campbell P, Eberhardt RY, Aitken S, Perrett D, Brent S, et al. Genomic diagnosis of rare pediatric disease in the United Kingdom and Ireland. N Engl J Med. 2023;388(17):1559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monies D, Abouelhoda M, Assoum M, Moghrabi N, Rafiullah R, Almontashiri N, et al. Lessons learned from large-scale, first-tier clinical exome sequencing in a highly consanguineous population. Am J Hum Genet. 2019;104(6):1182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung CCY, Hue SPY, Ng NYT, Doong PHL, Hong Kong Genome Project, Chu ATW, et al. Meta-analysis of the diagnostic and clinical utility of exome and genome sequencing in pediatric and adult patients with rare diseases across diverse populations. Genet Med Off J Am Coll Med Genet. 2023;25(9):100896. [DOI] [PubMed] [Google Scholar]
- 6.Ewans LJ, Minoche AE, Schofield D, Shrestha R, Puttick C, Zhu Y, et al. Whole exome and genome sequencing in mendelian disorders: a diagnostic and health economic analysis. Eur J Hum Genet. 2022;30(10):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manickam K, McClain MR, Demmer LA, Biswas S, Kearney HM, Malinowski J, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23(11):2029–37. [DOI] [PubMed] [Google Scholar]
- 8.Jobanputra V, Schroeder B, Rehm HL, Shen W, Spiteri E, Nakouzi G, et al. Advancing access to genome sequencing for rare genetic disorders: recent progress and call to action. NPJ Genomic Med. 2024;9(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posey JE, Rosenfeld JA, James RA, Bainbridge M, Niu Z, Wang X, et al. Molecular diagnostic experience of whole-exome sequencing in adult patients. Genet Med. 2016;18(7):678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou YCC, Yu HC, Martin R, Cirulli ET, Schenker-Ahmed NM, Hicks M, et al. Precision medicine integrating whole-genome sequencing, comprehensive metabolomics, and advanced imaging. Proc Natl Acad Sci U S A. 2020;117(6):3053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo MH, Bardakjian TM, Brzozowski MR, Scherer SS, Quinn C, Elman L, et al. Temporal trends and yield of clinical diagnostic genetic testing in adult neurology. Am J Med Genet A. 2021;185(10):2922–8. [DOI] [PubMed] [Google Scholar]
- 12.Sainio MT, Aaltio J, Hyttinen V, Kortelainen M, Ojanen S, Paetau A, et al. Effectiveness of clinical exome sequencing in adult patients with difficult-to-diagnose neurological disorders. Acta Neurol Scand. 2022;145(1):63–72. [DOI] [PubMed] [Google Scholar]
- 13.Walsh M, West K, Taylor JA, Thompson BA, Hopkins A, Sexton A, et al. Real world outcomes and implementation pathways of exome sequencing in an adult genetic department. Genet Med Off J Am Coll Med Genet. 2022;24(7):1536–44. [DOI] [PubMed] [Google Scholar]
- 14.Wallis M, Bodek SD, Munro J, Rafehi H, Bennett MF, Ye Z, et al. Experience of the first adult-focussed undiagnosed disease program in Australia (AHA-UDP): solving rare and puzzling genetic disorders is ageless. Orphanet J Rare Dis. 2024;19(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold J, Kripke CM, Regeneron Genetics Center, Penn Medicine BioBank, Drivas TG. Universal exome sequencing in critically ill adults: a diagnostic yield of 25% and race-based disparities in access to genetic testing. MedRxiv Prepr Serv Health Sci. 2024;2024.03.11.24304088.
- 16.Trujillano D, Bertoli-Avella AM, Kumar Kandaswamy K, Weiss ME, Köster J, Marais A, et al. Clinical exome sequencing: results from 2819 samples reflecting 1000 families. Eur J Hum Genet EJHG. 2017;25(2):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida LS, Pereira C, Aanicai R, Schröder S, Bochinski T, Kaune A, et al. An integrated multiomic approach as an excellent tool for the diagnosis of metabolic diseases: our first 3720 patients. Eur J Hum Genet EJHG. 2022;30(9):1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer P, Kandaswamy KK, Weiss MER, Paknia O, Werber M, Bertoli-Avella AM, et al. Development of an evidence-based algorithm that optimizes sensitivity and specificity in ES-based diagnostics of a clinically heterogeneous patient population. Genet Med. 2019;21(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trujillano D, Oprea GE, Schmitz Y, Bertoli-Avella AM, Abou Jamra R, Rolfs A. A comprehensive global genotype-phenotype database for rare diseases. Mol Genet Genomic Med. 2017;5(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayeems RZ, Luca S, Ungar WJ, Venkataramanan V, Tsiplova K, Bashir NS, et al. The clinician-reported genetic testing utility index (C-GUIDE): preliminary evidence of validity and reliability. Genet Med. 2022;24(2):430–8. [DOI] [PubMed] [Google Scholar]
- 22.Abouelhoda M, Sobahy T, El-Kalioby M, Patel N, Shamseldin H, Monies D, et al. Clinical genomics can facilitate countrywide estimation of autosomal recessive disease burden. Genet Med. 2016;18(12):1244–9. [DOI] [PubMed] [Google Scholar]
- 23.Magliyah MS, Geuer S, Alsalamah AK, Lenzner S, Drasdo M, Schatz P. Association of the recurrent rare variant c.415T>C p.Phe139Leu in CLN5 with a recessively inherited macular dystrophy. JAMA Ophthalmol. 2021;139(3):339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gueguen N, Piarroux J, Sarzi E, Benkirane M, Manes G, Delettre C, et al. Optic neuropathy linked to ACAD9 pathogenic variants: a potentially riboflavin-responsive disorder? Mitochondrion. 2021;1(59):169–74. [DOI] [PubMed] [Google Scholar]
- 25.Iruzubieta P, Alves CAPF, Al Shamsi AM, ElGhazali G, Zaki MS, Pinelli L, et al. Clinical and neuroradiological spectrum of biallelic variants in NOTCH3. EBioMedicine. 2024;107:105297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, et al. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5(8):e1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bick D, Bick SL, Dimmock DP, Fowler TA, Caulfield MJ, Scott RH. An online compendium of treatable genetic disorders. Am J Med Genet C Semin Med Genet. 2021;187(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallawaarachchi AC, Lundie B, Hort Y, Schonrock N, Senum SR, Gayevskiy V, et al. Genomic diagnostics in polycystic kidney disease: an assessment of real-world use of whole-genome sequencing. Eur J Hum Genet. 2021;29(5):760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shamseldin HE, Maddirevula S, Faqeih E, Ibrahim N, Hashem M, Shaheen R, et al. Increasing the sensitivity of clinical exome sequencing through improved filtration strategy. Genet Med. 2017;19(5):593–8. [DOI] [PubMed] [Google Scholar]
- 30.AlAbdi L, Maddirevula S, Shamseldin HE, Khouj E, Helaby R, Hamid H, et al. Diagnostic implications of pitfalls in causal variant identification based on 4577 molecularly characterized families. Nat Commun. 2023;14(1):5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddirevula S, Kuwahara H, Ewida N, Shamseldin HE, Patel N, Alzahrani F, et al. Analysis of transcript-deleterious variants in Mendelian disorders: implications for RNA-based diagnostics. Genome Biol. 2020;21(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukiyama T. New insights in ubiquitin-dependent Wnt receptor regulation in tumorigenesis. In Vitro Cell Dev Biol Anim. 2024;60(5):449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toivonen J, Allara E, FinnGen, Castrén J, di Angelantonio E, Arvas M. The value of genetic data from 665,460 individuals in managing iron deficiency anaemia and suitability to donate blood. Vox Sang. 2024;119(1):34–42. [DOI] [PubMed] [Google Scholar]
- 34.AlAbdi L, Maddirevula S, Aljamal B, Hamid H, Almulhim A, Hashem MO, et al. Arab founder variants: contributions to clinical genomics and precision medicine. Med N Y N. 2025;6(3):100528. [DOI] [PubMed] [Google Scholar]
- 35.100,000 Genomes Project Pilot Investigators, Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, et al. 100,000 genomes pilot on rare-disease diagnosis in health care - preliminary report. N Engl J Med. 2021;385(20):1868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monies D, Goljan E, Rapid Exome Consortium, Assoum M, Albreacan M, Binhumaid F, et al. The clinical utility of rapid exome sequencing in a consanguineous population. Genome Med. 2023;15(1):44. [DOI] [PMC free article] [PubMed]
- 37.Baz DS, Baz D, Alrwuili F, Aldowaish A, Shamseldin HE, Elhomoudi A, et al. Clinical exome sequencing by general pediatricians: high clinical utility and no evidence of inappropriate testing. Front Pediatr. 2024;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talya, Millo Antonio, Rivera Alexey, Obolensky Devora, Marks-Ohana Mingchu, Xu Yumei, Li Enosh, Wilhelm Prakadeeswari, Gopalakrishnan Menachem, Gross Boris, Rosin Mor, Hanany Andrew, Webster Anna Maria, Tracewska Robert K., Koenekoop Rui, Chen Gavin, Arno Ora, Schueler-Furman Susanne, Roosing Eyal, Banin Dror, Sharon (2022) Identification of autosomal recessive novel genes and retinal phenotypes in members of the solute carrier (SLC) superfamily Genetics in Medicine 24(7) 1523-1535. 10.1016/j.gim.2022.03.020. [DOI] [PubMed]
- 39.Thibaud S, Boutin David G, Charteris Aman, Chandra Susan, Campbell Caroline, Hayward Archie, Campbell Priyanka, Nandakumar David, Hinds Michelle, Agee Babak, Alipanahi Adam, Auton Robert K, Bell Katarzyna, Bryc Sarah L, Elson Pierre, Fontanillas Nicholas A, Furlotte Barry, Hicks Karen E, Huber Ethan M, Jewett Yunxuan, Jiang Aaron, Kleinman Keng-Han, Lin Nadia K, Litterman Matthew H, McIntyre Kimberly F, McManus Joanna L, Mountain Elizabeth S, Noblin Carrie A M, Northover Steven J, Pitts G David, Poznik J Fah, Sathirapongsasuti Janie F, Shelton Suyash, Shringarpure Chao, Tian Joyce Y, Tung Vladimir, Vacic Xin, Wang Catherine H, Wilson Danny, Mitry Veronique, Vitart (2020) (2020) (2019) Insights into the genetic basis of retinal detachment Abstract Human Molecular Genetics 29(4) 689-702. 10.1093/hmg/ddz294. [DOI] [PMC free article] [PubMed]
- 40.Yuefang, Liu Zhen, Lin Junyi, Yan Xi, Zhang Ming-Han, Tong (2024) A Rad50 -null mutation in mouse germ cells causes reduced DSB formation abnormal DSB end resection and complete loss of germ cells ABSTRACT Development 151(8). 10.1242/dev.202312. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Comprehensive cohort overview. This table lists the detailed characteristics of the entire study cohort. The table is organized to provide an in-depth look at the demographic, clinical, and molecular findings. This supplementary table enables readers to understand the distribution and variability of the data that underpin the study’s analyses and conclusions.
Additional file 2: Table S2: Reasons for missed molecular diagnoses by prior genetic testing in positive reflex CGS cases. This table details the specific technical or interpretative limitations that led to prior genetic testing missing the molecular diagnosis in each case, as later identified by reflex genomic sequencing (CGS).
Additional file 3: Figure S1: Radiological imaging findings. This figure presents a series of radiological images from our adult patient cohort, highlighting key structural features and anomalies that support the clinical and genetic findings of the study.
Additional file 4: Table S3: Ranking of diseases by estimated burden. This table presents diseases ranked according to their estimated burden within the cohort based on allele frequency data in the local population.
Additional file 5: Table S4: List of all cases with CNVs and SVs. This table presents all cases with CNVs and SVs among our cohort.
Additional file 6: Table S5: HGMD-reported SNVs. This table lists the subset of SNVs from our cohort that have corresponding HGMD identifiers.
Additional file 7: Table S6: Reclassification of variants using the founder phenomenon. This table provides a summary of the variants that were reclassified based on the founder phenomenon, with some variants being upgraded and others downgraded. It offers justification for these reclassifications.
Additional file 8: Supplemental Clinical Report: This report provides clinical information on each case along with the rationale supporting their designation as new candidate gene-disease links.
Additional file 9: Table S7: Multilocus phenotypes. This table summarizes cases where patients exhibit multilocus phenotypes due to the coexistence of two or more Mendelian disorders. It categorizes the combinations based on their inheritance patterns.
Additional file 10: Supplemental C-GUIDE (Clinician-reported Genetic testing Utility InDEx) Material: This document presents the previously validated questionnaire (C-GUIDE) designed to evaluate the clinical utility of genomic testing. The questionnaire explores multiple dimensions related to the application of genomic testing in clinical settings. It assesses key aspects such as understanding diagnosis and prognosis, informing medical management, awareness and actionability of reproductive and health risks for patients and family members.
Data Availability Statement
All data supporting the findings described in this paper are available in the article, its supplemental information, and from the lead contact upon request. Due to local privacy laws and privileged human information, all requests for whole-exome sequencing and whole-genome sequencing data are subject to prior approval from the local Institutional Review Board, which can be reached at ora@kfshrc.edu.sa with an expected time frame for response of 2 months.