Abstract
Background and Objectives
We characterised an Ag-Chitosan-NaF product (ClearDefense), marketed for off-label use in caries arrest in children, as an alternative to 38% silver diamine fluoride (SDF). The label lists 0.2% Ag+ and 1.36% F−. No child efficacy or safety studies are reported.
Methods
We followed U.S. National Institute of Standards and Technology (NIST) guidelines to describe the composition and stability of the product and assess its safety for children.
Results
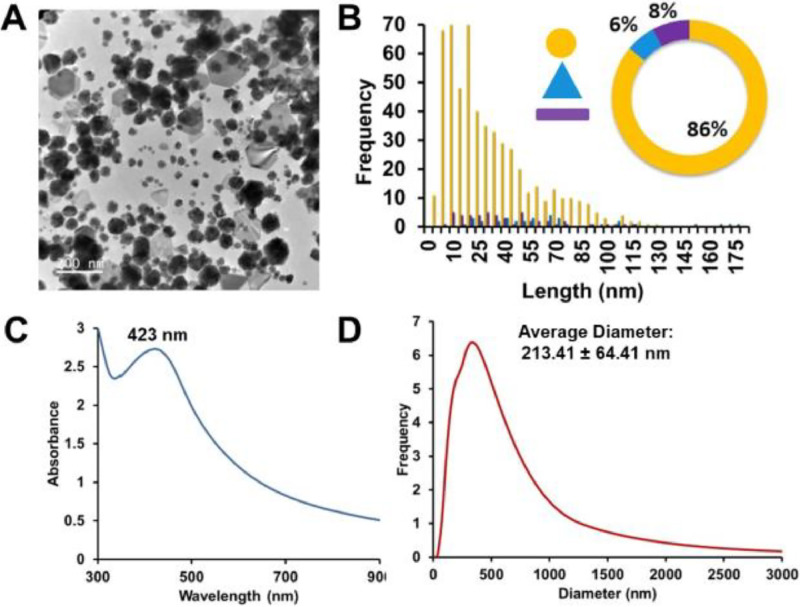
The product features heterogeneous AgNPs: 86% irregular spherical (32 ± 26 nm), 6% triangular (101 ± 100 nm), and 8% rod-shaped (42 ± 28 nm length, 16 ± 10 nm width). Ag-Chitosan-NaF exhibits agglomeration with Chitosan and NaF, and stability studies reveal an increase in Ag+ ion concentration upon exposure to air, raising concerns about dosage variations. Toxicity assessment reports a Margin of Exposure (MOE) for preschool children of 8-20 mg/kg, based on application amount and a 100X correction factor.
Clinical Significance
This Ag-Chitosan-NaF product requires refinement to meet the standards for formulation, purity, and safety for paediatric off-label use.
Conclusions
ClearDefense differs from the FDA-approved 38% SDF, raising concerns about consistency and paediatric suitability. A thorough reassessment by the manufacturer is needed to meet paediatric clinical standards.
Key words: Anti-bacterial agents, Pharmacology, Paediatric dentistry, Adverse effects, Metal nanoparticles, Chemistry, Nanotechnology silver, Adverse effect, Silver
Introduction
Nanomaterials are utilised in a wide range of applications,1, 2, 3 including drug delivery imaging,4, 5 sensing,6, 7 regenerative medicine,8, 9 and, more recently, in dentistry.10, 11, 12, 13, 14, 15 Several investigative groups have advocated for the use of silver nanoparticles (AgNPs) in combination with sodium fluoride (NaF) as a treatment for dental caries.16, 17, 18, 19, 20 Despite the promise of this application, only a handful of these formulations have undergone limited human clinical studies,21, 22, 23, 24, 25, 26, 27, 28 and none have been reported to comply with American standards for paediatric applications. Neither do they appear to meet the National Institute of Standards and Technology or any other guidelines for quality assurance of formulation and purity.29, 30
An examination of the literature on nanosilver particle-sodium fluoride (NSF) formulations highlights several methodological considerations, both in vitro and in vivo. The range of testing approaches appears narrower than those typically employed in nanomaterials chemistry, which may affect the interpretability and reproducibility of findings. In particular, there is limited discussion regarding the long-term stability of these formulations in suspension media, including aspects such as particle agglomeration and silver ion (Ag⁺) release. Santos et al. (2014), as cited in Targino et al., describe the NSF formulation as a yellow solution stable for up to 3 years.26,31 However, supporting data for this claim, including detailed analysis of Ag⁺ ion dissolution kinetics in biological environments, are not provided in the original or subsequent publications.26,31, 32, 33 Given that nanoparticle stability, including agglomeration and ion release, can significantly influence biological interactions, uptake, biodistribution, and toxicity, further clarification in this area would strengthen the evidence base.34, 35, 36 Studies on the antimicrobial efficacy of NSF against cariogenic bacteria report silver nanoparticles (AgNPs), chitosan, and sodium fluoride concentrations in mg/mL.33 Other studies specify Ag⁺ ion concentrations, while others refer to AgNPs, often using the terms interchangeably. The analytical methods used to determine these concentrations are not always described, making it difficult to assess the accuracy of the reported values.26,28,32, 33 This is particularly relevant given the predominance of AgNPs in NSF compared to SDF, where silver is present primarily as Ag⁺ ions.
The reduction of Ag⁺ to metallic silver (Ag⁰) in the presence of sugars or proteins is known to cause staining, suggesting that lower Ag⁺ concentrations may reduce discoloration.37 Variability in AgNP size and shape may influence the equilibrium between AgNPs and Ag⁺ ions. s. Accurate quantification of Ag⁺ prior to testing is essential for valid comparisons with SDF or other materials, especially if AgNPs are the primary silver species in NSF. It also remains unclear whether the observed antimicrobial effects are primarily due to AgNPs, Ag⁺ release, or the presence of chitosan, which itself has known antimicrobial properties.38, 39, 40 Information on the purity of NSF formulations post-synthesis is limited. Residual byproducts such as sodium or borate salts and acetic acid may be present and could influence toxicity.41 Similarly, data on nanoparticle surface charge, an important factor in stability, cellular uptake, and biodistribution, are often lacking, as are comprehensive analyses of particle size, shape, and composition.
While the safety of silver nanoparticles in medical applications remains an active area of investigation,42,43, 44, 45, 46 current dental literature sometimes equates NSF with 38% SDF, despite notable differences in composition and silver speciation. In SDF, silver is present as free Ag⁺ ions that interact directly with carious dentin, whereas in NSF, Ag⁺ ions must be released from AgNPs through surface oxidation. Both forms of silver can cross biological barriers, including the blood-brain barrier, which underscores the importance of rigorous safety assessments.46 For clinical use, AgNPs should exhibit stability in biological fluids, uniformity in size and shape, and well-characterised, non-toxic surface chemistry. Heterogeneous formulations may behave unpredictably, complicating dosage control and safety evaluations. Consistency in interactions between AgNPs, NaF, and stable suspension media is crucial. Without these controls, safety assumptions at low doses may be premature. This study aims to assess the safety of the newly marketed product ClearDefense (Young Specialties, Lot 271581, U.S. Patent 11,690,790), purchased and tested before its expiration date (2026-01-17).
Methods and results
The sample contains 3% NaF, equivalent to 1.36% F−, and 0.2% Ag. The concentrations of acetic acid and plant-derived chitosan are not provided. The size, shape, purity, and concentration of AgNPs or silver ions (Ag+) are not specified.
Size and shape distribution
To assess size and shape distribution, a viscous brown Ag-Chitosan-NaF solution was deposited on a 300 Å copper grid and imaged via TEM. ImageJ analysis showed agglomerated AgNPs with diverse morphologies, including spheres, triangles, hexagons, and rods. TEM analysis showed 86% of AgNPs were irregular spheres (32 ± 26 nm), 6% triangles (101 ± 100 nm), and 8% rods (42 ± 28 nm long, 16 ± 10 nm wide) (Figure 1A-B). This heterogeneity and agglomeration are aligned with UV-Vis spectra, which displayed a broadened LSPR band (O.D. 2.73, λₘₐₓ 423 nm) extending into the NIR region. Dynamic light scattering (DLS) confirms the agglomeration of Ag-Chitosan-NaF observed by TEM. The hydrodynamic radius of AgNPs in suspension was 213 ± 64 nm, larger than the core sizes of the nanoparticles, attributed to the bulky chitosan coating and agglomeration from NaF-induced interactions. Larger sizes and non-spherical shapes should have been considered in the reported hydrodynamic radii. The Ag-Chitosan-NaF product label does not clarify whether silver is present as nanoparticles (AgNPs) or as ionic silver (Ag⁺). Although the associated patent permits either form, provided the antimicrobial agent is under 100 nm, the ClearDefense packaging does not include information on particle size, shape, purity, or homogeneity. The variability observed in particle morphology differs from other NSF-registered formulations, which have reported spherical AgNPs with sizes ranging from 3.2 ± 1.2 nm to 5.9 ± 3.8 nm in studies involving patients with dental caries.26,32 Consequently, variability in nanoparticle size and shape may affect dose uniformity.
Fig. 1.
(A) Representative TEM image of ClearDefense (Ag-Chitosan-NaF) with (B) distribution histogram, (C) UV-Vis spectra and (D) DLS measurement.
Stability
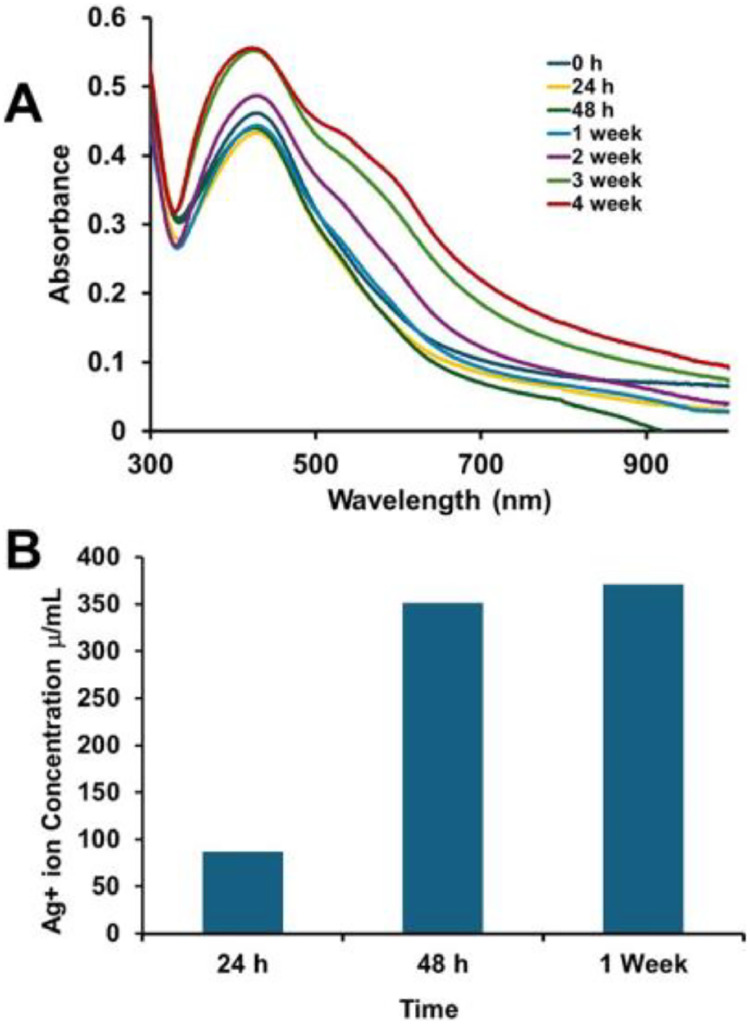
The stability, functionality, safety, shelf life, and reproducibility of biomedical applications are influenced by size, shape, metal core composition, and surface properties.47 Instability may cause aggregation, altered reactivity, or reduced bioavailability.48 To evaluate the stability of Ag-Chitosan-NaF after bottle opening, a diluted stock solution (O.D. 1.1, LSPR λₘₐₓ at 426 nm) was monitored using UV-Vis spectroscopy for four weeks. A 9% increase in optical density was observed, along with the emergence of 2 additional LSPR shoulders at λₘₐₓ 537 nm and 600 nm (Figure 2, A). These spectral changes suggest a shift in refractive index, likely due to nanoparticle agglomeration or material decomposition.49, 50, 51
Fig. 2.
(A) Representative UV-vis spectra of ClearDefense (Ag-Chitosan-NaF) over weeks and (B) ICP-MS analysis of filtrates to monitor Ag+ ion leaching over time.
Product composition, formulation, and purity
The composition and purity of AgNP formulations are crucial; an imbalance of Ag⁺ ions can lead to degradation and unwanted toxicity, while residual synthesis byproducts may exacerbate toxicity.41 The ratio of AgNP to Ag⁺ indicates stability, which affects shelf life, biological performance, toxicity levels, dosing consistency, efficacy, and potential for staining.52 Studies show that AgNPs with uniform sizes, shapes, and protective surface coatings against oxidation are key to ensuring their safety.35, 36,53, 54, 55, 56, 57 A variety of analytical techniques are employed, including UV–Vis spectroscopy, DLS, zeta potential (which measures charge), X-ray photoelectron spectroscopy (XPS), NMR for surface coatings, and inductively coupled plasma mass spectrometry (ICP-MS) to analyse composition, purity, and formulation,58, 59, 60 while techniques such as ultracentrifugation and dialysis will remove smaller nanoparticles, free ligands, or salts.61, 62 To detect free Ag⁺ ions in solution, 1 mL samples of 0.4 O.D. Ag-Chitosan-NaF was filtered using ultracentrifugation with a 3 kDa polyethersulfone membrane. ICP-MS analysis of the filtrates revealed Ag⁺ concentrations of 87 μg/mL after one day and 371 μg/mL after one week, indicating progressive oxidation and limited stability of the formulation. Although the product is stored in a dark bottle to minimise light exposure, the absence of an inert atmosphere likely contributes to oxidation upon opening, resulting in a mixture of free Ag⁺ ions and unoxidised AgNPs. This may account for the light staining observed in studies using similar NSF formulations, which rely on Ag⁺ ion release from oxidised AgNP surfaces.32 This preliminary study did not confirm the concentrations of chitosan and NaF in ClearDefense, and the product label does not disclose these values, so we cannot directly assess their impact on toxicity. However, chitosan is present in the sample as confirmed by FTIR spectra (Figure S1). Though, it is unclear whether the chitosan is bound to or free from the nanoparticles in the sample. It is also essential to note that the fluoride content in ClearDefense and other Brazilian NSF formulations is lower than that of the standard 38% silver diamine fluoride (SDF), raising questions about their effectiveness in remineralising softened dentin.63
Preliminary safety assessment of Ag-Chitosan-NaF
A preliminary safety assessment of AgNPs is essential due to the growing use of AgNPs in consumer and medical products, such as the Ag-Chitosan-NaF formulation. Zebrafish have been used for decades in nanomaterial toxicity testing (ISO/TS 22082:2020). Their embryos are ideal for studies due to their rapid development, transparency, and similarities to human biology. They progress from undifferentiated cells to fully functional larvae in just 5 days, a timeline conserved among vertebrates. Embryos were exposed to various concentrations of the final formulation (n = 24/concentration), either directly or after simulating application and drying. All experiments were performed in compliance with national care and use guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at Oregon State University. Our toxicity testing led to mortality in embryos exposed to 0.125 mg Ag/L, equivalent to 25 mg Ag/kg body mass (Figure 3). The margin of exposure (MOE) approach showed a wide safety margin for adults but raised concerns for children under 6, with MOE values of 8 to 20 mg/kg, depending on the number of teeth treated. MOEs above 100 are generally considered to be protective. Toxicity decreased when formulations were dried before embryo exposure, but clinicians with small children often can't control moisture during treatment. Currently, the WHO has not set tolerable daily intake levels or paediatric guidelines for silver exposure. The FDA does not recognise colloidal silver (AgNPs) as safe and advises against its use in children. In the absence of human data, the Margin of Exposure (MOE) approach provides a conservative risk assessment based on animal studies extrapolated to paediatric populations. These findings highlight the need for rigorous safety testing and evaluation of nanoparticle formulations before widespread use.
Fig. 3.
Concentration-response of embryonic zebrafish mortality after 5 days of exposure to varying concentrations of ClearDefense formulation. Data represent 2 experimental replicates (n = 12 for each) for a total of n = 24 for each exposure condition.
Discussion and conclusions
This study assesses the safety and characteristics of the ClearDefense for potential off-label use in children to prevent dental caries. The formulation includes heterogeneous AgNPs in a chitosan matrix with NaF and acetic acid. While the product has received FDA clearance for treating tooth sensitivity in adults aged 21 and older, its use in paediatric dentistry has not yet been supported by sufficient evidence regarding safety and efficacy. Current findings indicate that the formulation may not meet established standards for purity, stability, or paediatric use. ClearDefense differs from 38% SDF in effectiveness, with concerns about consistency and regulatory clarity. Manufacturer reassessment is recommended, including clear labelling for paediatric off-label use. Clinicians should avoid such use in children, and discolored products should be discarded as a precaution. Manufacturers should adhere to NIST quality standards, including stability testing and clear shelf-life labelling. AgNP-based formulations should be clearly labelled as metal nanomaterials due to their distinct properties from ionic silver. All components must be verified for consistency and safety. Clinicians should also be informed of the lack of paediatric safety data and potential toxicity risks associated with off-label use. By prioritising evidence-based practices and compliance, we can ensure innovations like ClearDefense are safe and effective for our most vulnerable patients.
Conflict of interest
This research was supported by an unrestricted gift to the Departments of Chemistry and Environmental and Molecular Toxicology at Oregon State University from Elevate Oral Care, LLC and Advantage Silver Dental Arrest, LLC. Note that Elevate Oral Care, LLC, and Advantage Silver Dental Arrest, LLC did not have a role in the research or the content of the paper.
Acknowledgments
Author contributions
Stacey L. Haper: Conceptualisation, Methodology, Formal analysis, Writing—original draft, Writing—review and editing. Marilyn Mackiewicz: Investigation, Resources, and Writing—original draft.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.identj.2025.103889.
Appendix. Supplementary materials
References
- 1.Egwu C.O., Aloke C., Onwe K.T., Umoke C.I., Nwafor J., Eyo J., et al. Nanomaterials in drug delivery: strengths and opportunities in medicine. Molecules. 2024;29(11):2584. doi: 10.3390/molecules29112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.R., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han X., Xu K., Taratula O., Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale. 2019;11(3):799–819. doi: 10.1039/c8nr07769j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C.H., Grodzinski P. Nanotechnology for cancer imaging: advances, challenges, and clinical opportunities. Radiol Imaging Cancer. 2021;3(3) doi: 10.1148/rycan.2021200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darwish M.A., Abd-Elaziem W., Elsheikh A., Zayed AA. Advancements in nanomaterials for nanosensors: a comprehensive review. Nanoscale Adv. 2024;6(16):4015–4046. doi: 10.1039/d4na00214h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansuriya B.D., Altintas Z. In: Fundamentals of sensor technology. 3d ed. Barhoum A., Altintas Z., editors. Woodhead Publishing; Cambridge MA: 2013. Carbon nanomaterials for sensing applications; pp. 367–400. [Google Scholar]
- 8.Arora P P., Sindhu A., Dilbaghi N., Chaudhury A., Rajakumar G., Rahuman A.A. Nano-regenerative medicine towards clinical outcome of stem cell and tissue engineering in humans. J Cell Mol Med. 2012;16(9):1991–2000. doi: 10.1111/j.1582-4934.2012.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury K., Kumar V., Kandasamy J., RoyChoudhury S. Regenerative nanomedicine: current perspectives and future directions. Int J Nanomed. 2014;9:4153–4167. doi: 10.2147/IJN.S45332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreenivasalu P.K.P., Dora C.P., Swami R., Jasthi V.C., Shiroorkar P.N., Nagaraja S., et al. Nanomaterials in dentistry: current applications and future scope. Nanomaterials (Basel) 2022;12(10):1676. doi: 10.3390/nano12101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priyadarsini S., Mukherjee S., Mishra M. Nanoparticles used in dentistry: a review. J Oral Biol Craniofac Res. 2018;8(1):58–67. doi: 10.1016/j.jobcr.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik S., Waheed Y. Emerging applications of nanotechnology in dentistry. Dent J. 2023;11(11):266. doi: 10.3390/dj11110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umapathy V.R., Natarajan P.M., SumathiJones C., Swamikannu B., Johnson W.M.S., Alagarsamy V., et al. Current trends and future perspectives on dental nanomaterials – an overview of nanotechnology strategies in dentistry. J King Saud Univ Sci. 2022;34(7) [Google Scholar]
- 14.Sen D., Patil V., Smriti K., Varchas P., Ratnakar R., Naik N., et al. Nanotechnology and nanomaterials in dentistry: present and future perspectives in clinical applications. Eng Sci. 2022;20:14–24. [Google Scholar]
- 15.Fakeeha G., AlHarbi S., Auda S., Balto H. The impact of silver nanoparticles’ size on biofilm eradication. Int Dent J. 2025;75(2):1213–1222. doi: 10.1016/j.identj.2024.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin I.X., Xu V.W., Xu G.Y., Yu O.Y., Niu J.Y., Chu CH. Synthesis and application of silver nanoparticles for caries management: a review. Pharmaceuticals (Basel) 2024;17(10):1264. doi: 10.3390/ph17101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijle M.N., Naseer T.K., Yiu C. Physical properties and enamel remineralization potential of arginine-fluoride varnishes. J Dent. 2024;148 doi: 10.1016/j.jdent.2024.104965. [DOI] [PubMed] [Google Scholar]
- 18.Yin I.X., Zhang J., Zhao I.S., Mei M.L., Li Q., Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. 2020;15:2555–2562. doi: 10.2147/IJN.S246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin I.X., Yu O.Y., Zhao I.S., Mei M.L., Li Q.L., Tang J., Chu CH. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019 Jun;102:106–112. doi: 10.1016/j.archoralbio.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Yin I.X., Yu O.Y., Zhao I.S., Mei M.L., Li Q.L., Tang J., Lo E.C.M., Chu CH. Inhibition of dentine caries using fluoride solution with silver nanoparticles: an in vitro study. J Dent. 2020;103 doi: 10.1016/j.jdent.2020.103512. [DOI] [PubMed] [Google Scholar]
- 21.Ammar N., El-Tekeya M.M., Essa S., Essawy M.M., Talaat DM. Antibacterial effect and impact on caries activity of nanosilver fluoride and silver diamine fluoride in dentin caries of primary teeth: a randomized controlled clinical trial. BMC Oral Health. 2022;22(1):657. doi: 10.1186/s12903-022-02697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhri E., SadrHaghighi A., Sarvari R., Torabi M., Azizi Y., Eskandarinezhad M., et al. Colloidal chitosan-silver nanoparticles-fluoride nanocomposite as an antibacterial mouthwash against salivary Streptococcus mutans in orthodontic patients (a randomized clinical trial) Clin Oral Investig. 2024;28(8):435. doi: 10.1007/s00784-024-05802-3. [DOI] [PubMed] [Google Scholar]
- 23.Raghav P., Khera A.K., Bisht S. Comparative evaluation of antimicrobial properties of silver nanoparticles and chlorhexidine mouthwashes on the colonization of microflora and oral health during orthodontic treatment: a double-blind randomized controlled trial. Dental Press J Orthod. 2025;30(01) doi: 10.1590/2177-6709.30.1.e2524112.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quritum M., Abdella A., Amer H., El Desouky L.M., El Tantawi M. Effectiveness of nanosilver fluoride and silver diamine fluoride in arresting early childhood caries: a randomized controlled clinical trial. BMC Oral Health. 2024;24(1):701. doi: 10.1186/s12903-024-04406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atteya S.M., Amer H.A., Saleh S.M., Safwat Y. Self-assembling peptide and nano-silver fluoride in remineralizing early enamel carious lesions: randomized controlled clinical trial. BMC Oral Health. 2023;23(1):577. doi: 10.1186/s12903-023-03269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos V.E., Jr, Vasconcelos Filho A., Targino A.G., Flores M.A., Galembeck A., Caldas A.F., Jr, et al. A new "silver-bullet" to treat caries in children–nano silver fluoride: a randomised clinical trial. J Dent. 2014;42(8):945–951. doi: 10.1016/j.jdent.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Turton B., Horn R., Durward C. Caries arrest and lesion appearance using two different silver fluoride therapies on primary teeth with and without potassium iodide: 12-month results. Clin Exp Dent Res. 2021;7(4):609–619. doi: 10.1002/cre2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freire P.L.L., AAlbuquerque A.J.R., Sampaio F.C., Galembeck A., Flores M.A.P., Stamford T.C., et al. AgNPs: the new allies against S. Mutans Biofilm - a pilot clinical trial and microbiological assay. Braz Dent J. 2017;28(4):417–422. doi: 10.1590/0103-6440201600994. [DOI] [PubMed] [Google Scholar]
- 29.Taurozzi J., Hackley V., Wiesner M. Reporting Guidelines for the Preparation of Nanoparticle Dispersions from Dry Materials. CEINT/NIST Protocol. 2010 https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=905966 (Accessed June 10, 2025) [Google Scholar]
- 30.Lin P.C., Lin S., Wang P.C., Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32(4):711–726. doi: 10.1016/j.biotechadv.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Targino A.G.R., Flores M.A.P., dos Santos Junior V.E., de Godoy Bené Bezerra F., de Luna Freire H., Galembeck A., et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25(8):2041–2047. doi: 10.1007/s10856-014-5221-5. [DOI] [PubMed] [Google Scholar]
- 32.Espíndola-Castro L.F., Rosenblatt A., Galembeck A., Monteiro G. Dentin staining caused by nano-silver fluoride: a comparative study. Oper Dent. 2020;45(4):435–441. doi: 10.2341/19-109-L. [DOI] [PubMed] [Google Scholar]
- 33.dos Santos Junior V.E., Targino A.G.R., Flores M.A.P., Rodríguez-Díaz J.M., Teixeira J.A., Heimer M.V., et al. Antimicrobial activity of silver nanoparticle colloids of different sizes and shapes against Streptococcus mutans. Res Chem Intermed. 2017;43(10):5889–5899. [Google Scholar]
- 34.Bélteky P., Rónavári A., Igaz N., Szerencsés B., Tóth I.Y., Pfeiffer I., et al. Silver nanoparticles: aggregation behavior in biorelevant conditions and its impact on biological activity. Int J Nanomedicine. 2019;14:667–687. doi: 10.2147/IJN.S185965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engstrom A.M., Wu H., Mackiewicz M.R., Harper S. Controlling silver ion release of silver nanoparticles with hybrid lipid membranes with long-chain hydrophobic thiol anchors decreases in vivo toxicity. Int J Eng Res. 2020;10(9):12–28. [Google Scholar]
- 36.Cunningham B., Engstrom A.M., Harper B.J., Harper S.L., Mackiewicz MR. Silver nanoparticles stable to oxidation and silver ion release show size-dependent toxicity in vivo. Nanomaterials (Basel) 2021;11(6):1516. doi: 10.3390/nano11061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins I.D. Tollens's test, fulminating silver, and silver fulminate. J Chem Educ. 1987;64(2):164. [Google Scholar]
- 38.Atay HY. Antibacterial activity of chitosan-based systems. Functional Chitosan. 2020:457–489. [Google Scholar]
- 39.Guarnieri A., Triunfo M., Scieuzo C., Ianniciello D., Tafi E., Hahn T., et al. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci Rep. 2022;12(1):8084. doi: 10.1038/s41598-022-12150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Fawares O., Alshweiat A., Al-Khresieh R.O., Alzarieni K.Z., Rashaid A.H.B. A significant antibiofilm and antimicrobial activity of chitosan-polyacrylic acid nanoparticles against pathogenic bacteria. Saudi Pharm J. 2024;32(1) doi: 10.1016/j.jsps.2023.101918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harper B., Sinche F., Wu R.H., Gowrishankar M., Marquart G., Mackiewicz M., Harper SL. The impact of synthesis method and purity on the toxicity of glutathione-coated gold nanoparticles. Nanomedicine. 2014;4(2):355–371. doi: 10.3390/nano4020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domb A.J., Sharifzadeh G., Nahum V., Hosseinkhani H. Safety evaluation of nanotechnology products. Pharmaceutics. 2021;13(10):1615. doi: 10.3390/pharmaceutics13101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferdous Z., Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21(7):2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie P., Zhao Y., Xu H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: a review. Ecotoxicol Environ Saf. 2023;253 doi: 10.1016/j.ecoenv.2023.114636. [DOI] [PubMed] [Google Scholar]
- 45.Karunakaran H., Krithikadatta J., Doble M. Local and systemic adverse effects of nanoparticles incorporated in dental materials- a critical review. Saudi Dent J. 2024;36(1):158–167. doi: 10.1016/j.sdentj.2023.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K., Wang S., Yin J., Yang Q., Yu Y., Chen L. Long-term application of silver nanoparticles in dental restoration materials: potential toxic injury to the CNS. J Mat Sci Mat Med. 2023;34(11):52. doi: 10.1007/s10856-023-06753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan H.T., Haes AJ. What does nanoparticle stability mean? J Phys Chem C Nanomater Interfaces. 2019;123(27):16495–16507. doi: 10.1021/acs.jpcc.9b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha S., Wang B., Dutta P. Nanoparticle processing: Understanding and controlling aggregation. Adv Colloid Interface Sci. 2020;279 doi: 10.1016/j.cis.2020.102162. [DOI] [PubMed] [Google Scholar]
- 49.Havelikar U., Ghorpade K.B., Kumar A., Patel A., Singh M., Banjare N., et al. Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines. Discov Nano. 2024;19(1):165. doi: 10.1186/s11671-024-04118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruinink A., Wang J., Wick P. Effect of particle agglomeration in nanotoxicology. Arch Toxicol. 2015;89(5):659–675. doi: 10.1007/s00204-015-1460-6. [DOI] [PubMed] [Google Scholar]
- 51.Ha M.K., Shim Y.J., Yoon TH. Effects of agglomeration on in vitro dosimetry and cellular association of silver nanoparticles. Environ Sci Nano. 2018;5(2):446–455. [Google Scholar]
- 52.Nabgui A., Brik A., Agayr K., Gouhier G., Vidović E., El Haskouri J., et al. Long-term impact of surfactants on colloidal stability and antibacterial properties of biogenic silver nanoparticle. J Bionanosci1. 2023;3(4):2006–2021. [Google Scholar]
- 53.Nieves Lira C., Carpenter A.P., Baio J.E., Harper B.J., Harper S.L., Mackiewicz M.R. Size- and shape-dependent interactions of lipid-coated silver nanoparticles: an improved mechanistic understanding through model cell membranes and in vivo toxicity. Chem Res Toxicol. 2024;37(6):968–980. doi: 10.1021/acs.chemrestox.4c00053. [DOI] [PubMed] [Google Scholar]
- 54.Miesen T.J., Engstrom A.M., Frost D.C., Ajjarapu R., Ajjarapu R., Lira C.N., et al. A hybrid lipid membrane coating “shape-locks” silver nanoparticles to prevent surface oxidation and silver ion dissolution. RSC Adv. 2020;10(27):15677–15693. doi: 10.1039/d0ra01727b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marquart G.W., Stoddard J., Kinnison K., Zhou F., Hugo R., Ryals R., et al. Increasing the efficacy of gold nanorod uptake in stem cell-derived therapeutic cells: implications for stem cell labeling and optical coherence tomography imaging. ACS App Nano Mat. 2022;5(5):6995–7008. doi: 10.1021/acsanm.2c00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harper B.J., Engstrom A.M., Harper S.L., Mackiewicz MR. Impacts of differentially shaped silver nanoparticles with increasingly complex hydrophobic thiol surface coatings in small-scale laboratory microcosms. Nanomaterials (Basel) 2024;14(8):654. doi: 10.3390/nano14080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engstrom A.M., Faase R.A., Marquart G.W., Baio J.E., Mackiewicz M.R., Harper S.L. Size-dependent interactions of lipid-coated gold nanoparticles: developing a better mechanistic understanding through model cell membranes and in vivo toxicity. Int J Nanomedicine. 2020;15:4091–4104. doi: 10.2147/IJN.S249622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu R.H., Nguyen T.P., Marquart G.W., Miesen T.J., Mau T., Mackiewicz MR. A facile route to tailoring peptide-stabilized gold nanoparticles using glutathione as a synthon. Molecules. 2014;19(5):6754–6775. doi: 10.3390/molecules19056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu S., Zhou X., Liu J. In: Silver nanoparticles in the environment. Liu J., Jiang G., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2015. Separation and determination of silver nanoparticles; pp. 9–42. [Google Scholar]
- 60.Dong F., Valsami-Jones E., Kreft J-U. New, rapid method to measure dissolved silver concentration in silver nanoparticle suspensions by aggregation combined with centrifugation. J Nanopart Res. 2016;18(9):259. doi: 10.1007/s11051-016-3565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson J.D., Rizzello L., Avila-Olias M., Gaitzsch J., Contini C., Magoń M.S., et al. Purification of nanoparticles by size and shape. Sci Rep. 2016;6(1) doi: 10.1038/srep27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins C.S.M., Sousa H.B.A., Prior JAV. From impure to purified silver nanoparticles: advances and timeline in separation methods. Nanomaterials (Basel) 2021;11(12):3407. doi: 10.3390/nano11123407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao I.S., Yin I., Mei M.L., Lo E.C.M., Tang J., Li Q., et al. Remineralising dentine caries using sodium fluoride with silver nanoparticles: an in vitro study. Int J Nanomedicine. 2020;15:2829–2839. doi: 10.2147/IJN.S247550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.