Abstract
Objective
To investigate the effects of Modified Hupo San on reproductive hormone levels and microcirculation in patients with menstrual disorders of cold congelation and blood stasis pattern.
Methods
A total of 102 patients from the gynecological outpatient department of our hospital were randomly assigned into a control group (n=51) and a study group (n=51). Randomization was performed using a computer-generated random number table The study group was treated with Modified Hupo San for 3 cycles, while the control group received routine therapy. Blood samples were collected on the 2nd day of menstruation before treatment and again on the 2nd day of the 4th cycle. Serum levels of TXB2, 6-keto-PGF1α, t-PA, PAI-1, estradiol (E2), and progesterone (P) were measured. Safety monitoring was performed throughout the study. Statistical analysis was conducted using SPSS 22.0, with t-tests and chi-square tests applied, and P<0.05 considered statistically significant.
Results
The total effective rate of Modified Hupo San in treating menstrual disorders of cold congelation and blood stasis pattern was 96.30%. Compared with baseline, serum TXB2 and PAI-1 levels significantly decreased, while 6-keto-PGF1α, t-PA, and t-PA/PAI-1 ratio significantly increased after treatment in the study group. E2 levels significantly increased after treatment, and were higher than those in the control group (P<0.05). Progesterone showed no significant difference.
Conclusion
Modified Hupo San demonstrated significant therapeutic effects in improving reproductive hormones and regulating microcirculation in patients with menstrual disorders of cold congelation and blood stasis pattern, with good safety.
Keywords: cold congelation and blood stasis, menstrual disorder, modified hupo san, reproductive hormones, microcirculation, clinical study
Introduction
With the acceleration of social modernization, the pace of life is increasing, interpersonal relationships are becoming more complex, and long-term high-pressure situations pose many challenges to women’s health. Epidemiological data show that the incidence of dysmenorrhea and menstrual disorders among women of childbearing age in China is rising annually.1 Among them, cold-congelation and blood stasis–type menstrual disorders are common in clinical practice, seriously impairing women’s quality of life and reproductive health.2
From the perspective of traditional Chinese medicine (TCM), this disorder is classically explained as the invasion of cold pathogens into the uterus, impairing qi and blood circulation, and causing stasis in the Chong and Ren meridians.3 For non-specialist readers, this theory can be summarized as a traditional conceptual model in which “cold” represents a pathological factor that slows circulation, leading to blood stasis and symptoms such as irregular menstruation, abdominal pain, and the passage of dark clots.
From the perspective of modern medicine, such patients often exhibit microcirculatory disturbances, including vasoconstriction, reduced blood flow velocity, and increased blood viscosity.4 In particular, Thromboxane B2 (TXB2) and 6-keto-prostaglandin F1α (6-keto-PGF1α) are considered important biomarkers of platelet activation and vascular endothelial function. TXB2 promotes platelet aggregation and vasoconstriction, whereas 6-keto-PGF1α exerts vasodilatory and anti-aggregatory effects. An elevated TXB2/6-keto-PGF1α ratio is associated with impaired microcirculation and serves as a potential biological correlate of this syndrome.5 Furthermore, imbalance of the fibrinolytic system—manifested as a reduced tissue-type plasminogen activator (t-PA)/plasminogen activator inhibitor-1 (PAI-1) ratio—may further weaken fibrinolytic activity and increase the risk of thrombosis.6
Reproductive hormone abnormalities are also frequently observed. Estradiol (E2) is crucial for endometrial proliferation, whereas progesterone (P) is essential for pregnancy maintenance and menstrual cycle regulation.7 Reduced E2 impairs endometrial growth, resulting in hypomenorrhea and delayed cycles, while P abnormalities are linked to luteal insufficiency and menstrual dysfunction.8
Hupo San, derived from classical medical texts, is a well-known prescription for gynecological disorders involving blood stasis.9 The modified formula, refined through clinical practice, has demonstrated therapeutic potential in treating cold-congelation and blood stasis–type menstrual disorders.10 However, its mechanisms remain insufficiently elucidated. Previous studies have not systematically quantified its effects on both reproductive hormones and microcirculation markers such as TXB2 and 6-keto-PGF1α, leaving an important research gap.
Therefore, this study, as the first randomized controlled trial (RCT) to evaluate the dual effects of Modified Hupo San, hypothesizes that the intervention can simultaneously improve reproductive hormone balance and microcirculation parameters. By measuring changes in TXB2, 6-keto-PGF1α, t-PA, PAI-1, E2, and P before and after treatment, we aim to clarify the potential mechanisms underlying its clinical efficacy, thereby providing an objective scientific basis for its rational application and contributing to the integration of TCM and modern medical approaches in gynecology.
Materials and Methods
General Information
All participants were recruited from the gynecological outpatient department, with a total of 102 cases randomly divided into two groups (study group and control group, 51 cases each). In the control group, there were 20 married patients, aged 14–35 years, with an average age of (25.24 ± 5.68) years; in the study group, there were 21 married patients, aged 15–36 years, with an average age of (25.69 ± 4.25) years. Baseline characteristics showed no statistically significant differences between groups (P > 0.05), indicating comparability (Table 1).
Table 1.
General Data Comparison (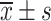 )
)
| Group | n | Age (Years) | Course of Illness (Years) | Number of Married Cases |
|---|---|---|---|---|
| Control group | 51 | 25.24±5.68 | 11.02±3.86 | 20 |
| Study Group | 51 | 25.69±4.25 | 10.01±3.75 | 21 |
| t/χ2 | 0.33 | 1.41 | 0.04 | |
| P | >0.05 | >0.05 | >0.05 |
A priori sample size estimation (power analysis) was performed based on a pilot study. Assuming α = 0.05, power (1-β) = 0.80, and an expected difference of 20% in treatment efficacy, at least 45 participants were required per group. Considering a 10% dropout rate, the final sample size was set at 51 per group.
This study was approved by the ethics committee of Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine (Ethics No: ZYLC-11). All enrolled patients agreed to the use of their clinical data for scientific analysis, and their privacy was strictly protected. The research process followed the relevant ethical principles of the Declaration of Helsinki.
Diagnostic Criteria
Diagnostic criteria were established with reference to “Obstetrics and Gynecology” and the “Guiding Principles for Clinical Research of New Traditional Chinese Medicine”.11,12 (1) Dysmenorrhea: No organic lesion in reproductive organs, with recurrent lower abdominal pain, lumbar soreness, abdominal distension, or other discomfort during or prior to menstruation. (2) Hypomenorrhea: Normal menstrual cycle but significantly reduced volume compared with before, lasting <2 days, or only scant spotting. (3) Delayed menstruation: Menstrual cycle delayed by >7 days, normal volume, occurring in ≥2 consecutive cycles.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) Meet the above diagnostic standards; (2) aged 14–40 years, disease duration >1 year; (3) voluntary participation with signed informed consent.Exclusion criteria: (1) Organic lesions of reproductive organs such as fibroids or adenomyosis; (2) severe systemic or psychiatric disorders; (3) use of drugs affecting menstruation within 3 months; (4) physiological menstrual delay or amenorrhea; (5) drug allergy or multiple allergies; (6) poor compliance.
Research Methods
Study Group (Modified Hupo San)
The formula contained: Amber 3 g, Sparganii Rhizoma 10 g, Curcumae Rhizoma 10 g, Cortex Moutan 12 g, Cinnamomi Cortex 10 g, Corydalis Rhizoma 12 g, Linderae Radix 10 g, Herba Lysimachiae 12 g, Angelica Sinensis 12 g, Paeoniae Radix Rubra 18 g, Rehmanniae Radix 12 g. Herbal decoction preparation followed the Chinese Pharmacopoeia (2020 edition) standards. Herbs were decocted twice with 500 mL water each time, combined, and concentrated to 200 mL daily dose, taken orally in two divided doses.
Control Group
Patients received Yuanhu analgesic tablets (standardized proprietary TCM, widely used for dysmenorrhea) plus ginger syrup, 3 tablets per time, 2 times daily, orally. The choice of this regimen was based on its recognized efficacy and ethical considerations, as withholding treatment (placebo) in symptomatic patients would be inappropriate.
Treatment Course and Blinding
Both groups were treated for 3 consecutive menstrual cycles (≈12 weeks). Outcome assessors and laboratory technicians were blinded to group allocation. Patients were randomized by computer-generated random number sequence, and allocation concealment was maintained using sealed opaque envelopes.
Sample Collection
Blood samples were collected from both groups twice, before treatment and after the 3-cycle course (4th cycle, day 2 of menstruation). Four mL of fasting venous blood was drawn, placed in anticoagulant tubes, centrifuged (3000 r/min, 10 min), and the serum stored at –20 °C until testing.
Outcome Measures
Serum TXB2, 6-keto-PGF1α, E2, and P were measured using radioimmunoassay (same batch kits, following manufacturer’s instructions).
Serum t-PA and PAI-1 were measured using double-antibody sandwich ELISA (same batch kits).
Clinical efficacy was assessed according to “Diagnosis and Efficacy Criteria for Traditional Chinese Medicine Diseases”:13 Cured: Pain completely relieved, no recurrence for 3 consecutive cycles. Improved: Pain relieved but not fully sustained. Not cured: No improvement.
Pain assessment (VAS score):14 The severity of dysmenorrhea was assessed using the Visual Analogue Scale (VAS). Patients were asked to indicate their pain intensity on a 10-cm horizontal line, with 0 representing “no pain” and 10 representing “unbearable pain”. The VAS score was recorded before treatment and after the completion of treatment. Changes in VAS scores were used to evaluate the degree of pain relief.
Statistical Analysis
Data were analyzed using SPSS 22.0. Continuous variables were expressed as mean ± SD. Between-group comparisons used independent-sample t test, within-group comparisons used paired-sample t test, and categorical data used χ²-test. Missing data were handled with last observation carried forward (LOCF). Outliers (values ±3 SD) were verified against original case records; if data entry error was excluded, values were retained to avoid bias. P < 0.05 was considered statistically significant.
Results
Changes in Serum TXB2 and 6-Keto-PGF1α
After treatment, the serum TXB2 levels in both groups decreased significantly (P < 0.05), and the reduction in the study group was more pronounced than that in the control group (P < 0.05). The level of 6-keto-PGF1α increased in both groups, with the study group showing significantly higher levels than the control group (P < 0.05) (Figure 1).
Figure 1.
Changes in serum TXB2 and 6-keto-PGF1α levels before and after treatment in two groups of patients (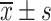 ).
).
Changes in Serum t-PA, PAI-1, and t-PA/PAI-1
After treatment, the serum t-PA levels in both groups increased significantly (P < 0.05), with higher levels in the study group than in the control group (P < 0.05). Meanwhile, PAI-1 levels decreased significantly in the study group compared with the control group (P < 0.05). Consequently, the t-PA/PAI-1 ratio increased significantly after treatment, with a greater improvement observed in the study group (P < 0.05) (Table 2).
Table 2.
Changes in Serum t-PA, PAI-1, and t-PA/PAI-1 in Two Groups (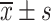 )
)
| Group | n | Time | t-PA/(ng·mL) | PAI-1/(ng·mL) | t-PA/PAI-1 |
|---|---|---|---|---|---|
| Control group | 51 | Before treatment | 3.60±0.38 | 18.00±3.00 | 0.20±0.03 |
| After treatment | 4.20±0.60# | 15.00±2.00# | 0.28±0.05# | ||
| Study Group | 51 | Before treatment | 3.56±0.36 | 18.23±3.25 | 0.19±0.02 |
| After treatment | 5.06±0.85#Δ | 12.15±1.35#Δ | 0.41±0.07#Δ |
Notes: Compared with the same group before treatment, #P < 0.05; compared with the Control group after treatment ΔP < 0.05.
Comparison of Reproductive Hormone Indicators Before and After Treatment
As shown in Table 3, after treatment, the serum E2 levels in both groups were significantly higher than before treatment (P < 0.05), with the study group showing significantly higher E2 levels than the control group (P < 0.05). P levels showed a slight upward trend in both groups, but the difference was not statistically significant (P > 0.05).
Table 3.
Comparison of Changes in Serum E2 and P Before and After Treatment in Two Groups (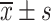 )
)
| Group | n | Time | E2 (pg/mL) | P (ng/mL) |
|---|---|---|---|---|
| Control group | 51 | Before treatment | 25.89±13.02 | 0.61±0.30 |
| After treatment | 28.56±12.11# | 0.75±0.28# | ||
| Study Group | 51 | Before treatment | 26.42±12.38 | 0.58±0.32 |
| After treatment | 33.79±11.54#Δ | 1.23±0.45#Δ |
Notes: Compared with the same group before treatment, #P < 0.05; compared with the Control group after treatment, ΔP < 0.05.
Comparison of Effective Treatment Outcomes in Patients with Cold Coagulation and Blood Stasis Dysmenorrhea
The total effective rate of the study group was significantly higher than that of the control group (P < 0.05). No drug-related adverse reactions were observed in either group during the treatment period (Figure 2).
Figure 2.
Comparison of effective treatment of cold coagulation blood stasis type dysmenorrhea [n(%)].
Patient-Reported Pain Experience (VAS Score)
As shown in Table 4, to better reflect the subjective pain experience, patients were assessed with the VAS before and after treatment. The mean VAS score decreased significantly in both groups after treatment (P < 0.05). The reduction was greater in the study group compared with the control group (P < 0.05), indicating superior pain relief with Modified Hupo San.
Table 4.
Comparison of VAS Scores Between the Two Groups Before and After Treatment (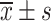 , Points)
, Points)
| Group | n | Time | VAS |
|---|---|---|---|
| Control group | 51 | Before treatment | 7.73±1.21 |
| After treatment | 4.05±1.08# | ||
| Study Group | 51 | Before treatment | 7.85±1.12 |
| After treatment | 2.43±0.96#Δ |
Notes: Compared with the same group before treatment, #P < 0.05; compared with the Control group after treatment ΔP < 0.05.
Discussion
Menstrual disorders of the cold coagulation and blood stasis pattern are common gynecological conditions. Their pathogenesis integrates the TCM theory of “cold causes contraction and blood stasis” with modern biomedical concepts of “microcirculatory disturbance and endocrine imbalance”.15 This study applied Modified Hupo San to evaluate its clinical efficacy and its potential impact on microcirculation and reproductive hormones, aiming to provide preliminary evidence for its mechanisms of action.
The formula is characterized by the combined use of Sparganii Rhizoma and Curcumae Rhizoma, which are traditionally regarded as potent agents for promoting blood circulation and alleviating stasis. In TCM, this action is described as targeting the liver meridian and addressing the core pathogenesis of cold coagulation and blood stasis, thereby relieving uterine obstruction.16 Additional herbs such as Angelica Sinensis and Paeonia contribute to nourishing and activating blood, while Moutan Cortex and Cinnamomi Cortex warm the meridians and disperse cold. Corydalis and Lindera regulate qi and alleviate pain, leading to a synergistic effect of warming, activating, and regulating circulation.16,17 In the present study, the total effective rate reached 96.30%, indicating significant symptom improvement, which suggests—but does not definitively prove—the clinical benefits of this prescription.
Microcirculatory disturbance is a key pathological basis of this syndrome. Our findings showed that, prior to treatment, patients exhibited increased serum TXB2 and PAI-1 and decreased 6-keto-PGF1α and t-PA, consistent with platelet activation, vasoconstriction, and impaired fibrinolysis.18,19 After treatment, TXB2 decreased, 6-keto-PGF1α increased, and the t-PA/PAI-1 balance improved. These results suggest that Modified Hupo San may improve uterine blood supply and restore microcirculatory homeostasis.20 While this is in line with TCM theory, it should be emphasized that our results only imply potential mechanisms, as no molecular or cellular experiments were conducted to directly confirm them.
Regarding reproductive hormones, E2 levels were low at baseline, while P showed no significant abnormality. After treatment, E2 increased significantly, whereas P remained unchanged. This pattern suggests that the prescription may primarily influence estrogen synthesis and follicular development, with weaker or slower effects on luteal function.21 From a TCM perspective, cold coagulation and blood stasis impair the Chong and Ren meridians, hindering ovarian function.22 The observed E2 improvement may reflect better ovarian blood flow and modulation of the hypothalamic–pituitary–ovarian axis.23 The lack of significant change in P could be attributed to factors such as the short treatment duration (three cycles), small sample size, or the complex regulation of progesterone secretion.24 Future studies with longer follow-up and larger cohorts are warranted.
This study has several limitations. First, although randomization and blinded assessment of outcomes were applied, the absence of a double-blind, placebo-controlled design may have introduced bias and potentially overestimated treatment efficacy. Moreover, we did not monitor adherence in detail, and a placebo effect cannot be ruled out. Second, the follow-up duration was limited to three menstrual cycles, restricting conclusions regarding long-term efficacy. Third, mechanistic interpretations remain speculative, as no direct molecular or cellular assays were performed. Fourth, comparison with previous literature was limited; conflicting evidence from prior studies warrants a more in-depth discussion. Finally, the sample size was relatively small, limiting generalizability.
Future research should aim to conduct larger, multicenter randomized controlled trials with rigorous blinding, adherence monitoring, and active comparators such as NSAIDs. At the molecular level, investigations into whether Modified Hupo San modulates prostaglandin synthesis pathways (eg, COX-2 inhibition) or hormone receptor signaling would help establish a stronger causal framework. Such work will be crucial to extend the relevance of these findings beyond the TCM field and to integrate them into broader biomedical discourse.
Conclusion
Modified Hupo San can significantly improve microcirculation indicators (TXB2 ↓, t-PA ↑) and E2 levels in patients with cold coagulation and blood stasis pattern, suggesting potential benefits for menstrual disorders. However, due to the study’s limitations—including the absence of a placebo control, short follow-up, and limited sample size—these findings should be interpreted with caution. Further randomized controlled trials with active comparators, longer follow-up, and mechanistic investigations are warranted to confirm causal relationships, clarify optimal dosing, and identify patient populations most likely to benefit.
Funding Statement
Hebei Province Traditional Chinese Medicine Scientific Research Project Plan. Project Name: Effects of Modified Amber Powder on Microcirculation of Reproductive Organs and Reproductive Hormones in Patients with Menstrual Disorders and Cold Coagulation and Blood Stasis Syndrome (Project Number: 2021339).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lai S, Jin Q, Wang D, Li T, Wang X. Effects of menstrual disorders and dysmenorrhea on cardiovascular disease: a Mendelian randomization study. Front Endocrinol. 2024;15:1302312. PMID: 38375191; PMCID: PMC10875084. doi: 10.3389/fendo.2024.1302312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li N, Li J, Gai P. Effects of modified Wenjing decoction combined with online publicity and education on the treatment of primary dysmenorrhea of cold coagulation and blood stasis. J Healthc Eng. 2022;2022:1899356. PMID: 35070226; PMCID: PMC8769821. doi: 10.1155/2022/1899356 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Sun Y, Chu JZ, Geng JR, et al. Label-free based quantitative proteomics analysis to explore the molecular mechanism of gynecological cold coagulation and blood stasis syndrome. Anat Rec. 2023;306(12):3033–3049. PMID: 36136292. doi: 10.1002/ar.25035 [DOI] [PubMed] [Google Scholar]
- 4.Li T, Wang SY, Huang ZQ, et al. CO2 laser moxibustion for endometriosis related pelvic pain of cold coagulation and blood stasis: a randomized controlled trial. Zhongguo Zhen Jiu. 2022;42(4):397–401. PMID: 35403398. Chinese. doi: 10.13703/j.0255-2930.20210421-0001 [DOI] [PubMed] [Google Scholar]
- 5.Xing Y, Guo JY, Liu P, et al. Mechanism of Liangfang Wenjing decoction in treatment of hypoxia on endometriosis with cold coagulation and blood stasis by regulating CHCHD4 expression. Zhongguo Zhong Yao Za Zhi. 2024;49(14):3818–3827. PMID: 39099355. Chinese. doi: 10.19540/j.cnki.cjcmm.20240207.401 [DOI] [PubMed] [Google Scholar]
- 6.Asawadethmetakul P, Xie F, Xie C, Ma J, You Y, Yao F. Effect of tuina combined with chinese herbal compress on primary dysmenorrhea with cold coagulation and blood stasis syndrome: a study protocol for a randomized controlled trial. Complement Med Res. 2024;31(1):20–29. PMID: 38011840. English. doi: 10.1159/000534335 [DOI] [PubMed] [Google Scholar]
- 7.Bauer J, Cooper-Mahkorn D. Reproductive dysfunction in women with epilepsy: menstrual cycle abnormalities, fertility, and polycystic ovary syndrome. Int Rev Neurobiol. 2008;83:135–155. PMID: 18929079. doi: 10.1016/S0074-7742(08)00007-X [DOI] [PubMed] [Google Scholar]
- 8.Jahanfar S, Mortazavi J, Lapidow A, et al. Assessing the impact of hormonal contraceptive use on menstrual health among women of reproductive age - a systematic review. Eur J Contracept Reprod Health Care. 2024;29(5):193–223. PMID: 39007750. doi: 10.1080/13625187.2024.2373143 [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Li H, Li S-W. Study on syndrome differentiation of ancient arthralgia syndrome and drug use rules based on latent structure model. Zhongguo Zhong Yao Za Zhi. 2020;45(19):4784–4791. PMID: 33164446. Chinese. doi: 10.19540/j.cnki.cjcmm.20200726.501 [DOI] [PubMed] [Google Scholar]
- 10.Wang YY, Ma K. Analysis of questionnaire and clinical characteristics of 2000 cases of dysmenorrheal. Zhongguo Zhong Yao Za Zhi. 2015;40(20):3920–3924. PMID: 27062802. Chinese. [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists. Dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018;132(6):e249–e258. PMID: 30461694. doi: 10.1097/AOG.0000000000002978 [DOI] [PubMed] [Google Scholar]
- 12.Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. 2017;167(2):112–121. PMID: 28654980. doi: 10.7326/M16-2977 [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. PMID: 15621359. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 14.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale (VAS Pain), numeric rating scale for pain (NRS Pain), mcgill pain questionnaire (MPQ), short-form mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63(S11):S240–S252. PMID: 22588748. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wu DX, Hou N, Liu M, Zhang YL, Qiao YJ. Nature-effect relationship research of cold and warm medicinal properties of traditional Chinese medicine for promoting blood circulation and removing blood stasis based on nature combination. Zhongguo Zhong Yao Za Zhi. 2019;44(2):212–217. PMID: 30989935. Chinese. doi: 10.19540/j.cnki.cjcmm.20180903.001 [DOI] [PubMed] [Google Scholar]
- 16.Hao EW, Deng JG, Du ZC, et al. Experimental study on two-way application of traditional Chinese medicines capable of promoting blood circulation and removing blood stasis with neutral property in cold and hot blood stasis syndrome I. Zhongguo Zhong Yao Za Zhi. 2012;37(21):3302–3306. PMID: 23397734. Chinese. [PubMed] [Google Scholar]
- 17.Wei Y, Chen X, Wang T, Dong X, Zhu Z. Effects of Du Meridian moxibustion combined with mild moxibustion on female pelvic floor myofascial pain syndrome: a retrospective cohort study. Evid Based Complement Alternat Med. 2022;2022:7388864. PMID: 36425258; PMCID: PMC9681563. doi: 10.1155/2022/7388864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nattero G, Allais G, De Lorenzo C, et al. Relevance of prostaglandins in true menstrual migraine. Headache. 1989;29(4):233–238. PMID: 2714974. doi: 10.1111/j.1526-4610.1989.hed22904233.x [DOI] [PubMed] [Google Scholar]
- 19.Pandey AN, Yadav PK, Premkumar KV, Pandey AK, Chaube SK Therapeutic potential of Shatavari (asparagus racemosus) for psychological stress-mediated Women’s reproductive health disorders. Innov Discov. 2025;2(3):11. doi: 10.53964/id.2025011 [DOI] [Google Scholar]
- 20.Koike H, Egawa H, Ohtsuka T, Yamaguchi M, Ikenoue T, Mori N. Correlation between dysmenorrheic severity and prostaglandin production in women with endometriosis. Prostaglandins Leukot Essent Fatty Acids. 1992;46(2):133–137. PMID: 1502250. doi: 10.1016/0952-3278(92)90219-9 [DOI] [PubMed] [Google Scholar]
- 21.Grandi G, Napolitano A, Xholli A, Tirelli A, Di Carlo C, Cagnacci A. Effect of oral contraceptives containing estradiol and nomegestrol acetate or ethinyl-estradiol and chlormadinone acetate on primary dysmenorrhea. Gynecol Endocrinol. 2015;31(10):774–778. PMID: 26291811. doi: 10.3109/09513590.2015.1063118 [DOI] [PubMed] [Google Scholar]
- 22.Dawood MY, Khan-Dawood FS. Differential suppression of menstrual fluid prostaglandin F2a, prostaglandin E2, 6-keto prostaglandin F1a and thromboxane B2 by suprofen in women with primary dysmenorrhea. Prostaglandins Other Lipid Mediat. 2007;83(1–2):146–153. PMID: 17259081. doi: 10.1016/j.prostaglandins.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Grant LK, Gooley JJ, St Hilaire MA, et al. Menstrual phase-dependent differences in neurobehavioral performance: the role of temperature and the progesterone/estradiol ratio. Sleep. 2020;43(2):zsz227. PMID: 31670824; PMCID: PMC7457328. doi: 10.1093/sleep/zsz227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen JT, Parke S, Mellinger U, Machlitt A, Fraser IS. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787. PMID: 21422847. doi: 10.1097/AOG.0b013e3182118ac3 [DOI] [PubMed] [Google Scholar]




