Abstract
Diagnostic techniques for invasive pneumococcal disease (IPD) in children are insensitive and underestimate both the burden of disease and the cost-effectiveness of pneumococcal conjugate vaccination (PCV). Consequently, there is little demand for the highly effective PCV outside the United States and Europe. In Kenya, diagnosis of pneumococcal pneumonia in adults was achieved with a sensitivity of 0.70 and a specificity of 0.98 using enzyme-linked immunosorbent assays (ELISAs) of paired plasma samples for immunoglobulin G (IgG) to pneumococcal surface adhesin A (PsaA). We aimed to validate the same technique in children. We assayed paired blood samples from 98 children with IPD, 95 age-matched children with malaria/anemia, and 97 age-matched healthy controls by using an ELISA for anti-PsaA IgG. Sensitivity and specificity were determined in IPD patients and healthy controls. Specificity (0.97; 95% confidence interval [CI], 0.91 to 0.99) and sensitivity (0.42; 95% CI, 0.32 to 0.52) were optimized at a 2.7-fold rise in anti-PsaA antibody concentration. Sensitivity was improved to a maximum of 0.50 by restricting testing to children of <2 years old, by excluding IPD patients who were not sampled on the first day of presentation, and by incorporating high existing antibody concentrations in the analysis. Assay performance was independent of nasopharyngeal carriage of pneumococci at recruitment. This assay improves on existing diagnostic tools for IPD in children but would still leave over half of all cases undetected in epidemiological studies. Effective diagnosis of pneumococcal disease in children is urgently required but poorly served by existing technology.
The incidence of invasive pneumococcal disease (IPD) in young children has decreased by over two-thirds following the programmatic introduction of pneumococcal conjugate vaccination in the United States (26). In the developing world, the prospects for prevention by vaccination are uncertain. In South Africa, vaccination was shown to reduce IPD by 83% among human immunodeficiency virus-negative children (11); in The Gambia, vaccine efficacies were 77% against IPD and 37% against radiologically proven pneumonia (5). The case for introducing pneumococcal conjugate vaccination to the developing world, however, depends critically on local demonstrations of disease burden to justify its considerable cost.
In sub-Saharan Africa, diagnostic facilities are limited (8), and children with pneumonia, meningitis, and septicemia are treated according to syndromic algorithms rather than by individual laboratory investigations (27). The detection of IPD is also needed, therefore, to validate treatment algorithms. The emphases of individual diagnosis and epidemiological validation differ; individual diagnosis is advanced by techniques with high sensitivities and negative predictive values, and epidemiological studies by techniques with high specificities and positive predictive values. Isolation of Streptococcus pneumoniae from cultures of normally sterile sites is insensitive in untreated patients; for example, in The Gambia, despite comprehensive blood culture screening in pneumonia cases, the absolute effectiveness of conjugate vaccine against radiological pneumonia was more than seven times greater than that against pneumococcal disease defined by blood or lung aspirate cultures, suggesting that most cases of pneumococcal pneumonia are culture negative (5). The natural insensitivity of cultures is compromised further by prior use of antibiotics, which are available without prescription in many developing countries. Techniques that are unaffected by antibiotic use, such as PCR, antigen detection, and serology, may therefore be advantageous.
Pneumococcal surface adhesin A (PsaA) is a conserved, common protein of S. pneumoniae expressed at the cell surface of all 90 pneumococcal serotypes and visible to the humoral immune system (4, 15). In mouse models, immunization with PsaA elicits protection against nasopharyngeal carriage and invasive disease, and PsaA is proposed as a potential vaccine antigen (3, 10, 23). An immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) using native or recombinant PsaA has been developed (25), and among Kenyan adults with pneumococcal pneumonia, this test has a sensitivity of 0.70 and a specificity of 0.98 (21). Natural IgG to PsaA is observed in healthy Kenyan infants at levels similar to those found in adults (13), and in Finland, episodes of acute otitis media have been associated with serum and salivary anti-PsaA responses (18, 22). It is possible, therefore, that a rising concentration of anti-PsaA in the presence of a compatible clinical illness may provide a specific diagnosis of IPD. Here we test this possibility in pediatric patients with IPD and in two control populations.
MATERIALS AND METHODS
Setting.
The study took place at Kilifi District Hospital, a rural government hospital on the Indian Ocean coast of Kenya between January 2000 and September 2003. Each child admitted to the hospital was investigated with a blood culture procedure (1). Lumbar puncture was undertaken for children with impaired consciousness, meningism, prostration, or seizures (other than febrile seizures) and in all children ≤60 days old for whom sepsis was suspected. The commonest presentations were with malaria, invasive bacterial disease, anemia, and malnutrition. The prevalences of human immunodeficiency virus among outpatients and admissions to the pediatric service were 2.4% and 7.1%, respectively (1, 2).
Study populations.
IPD patients were defined as children of 1 month to 7 years of age admitted to Kilifi District Hospital whose cultures of blood, cerebrospinal fluid (CSF), or pleural aspirate grew S. pneumoniae and who survived for at least 10 days and provided convalescent-phase blood specimens.
For every patient with IPD, one sick and one healthy control child were recruited and frequency matched by sex and by age in seven strata (0 to 5, 6 to 11, 12 to 17, 18 to 23, 24 to 35, 36 to 59, and 60 to 83 months of age). After identifying a patient with IPD, sick control children were recruited on the pediatric ward as the next qualifying admissions, subject to the constraints of matching. A child was eligible for inclusion as a sick control if he or she had a blood culture negative for S. pneumoniae and had either anemia (hemoglobin level of <6.0 g/dl) or malaria, defined as pyrexia and a blood smear positive for Plasmodium falciparum. Later, a fieldworker traveled to the home of the IPD patient and, spinning a pencil on the ground, walked in the direction indicated looking for a homestead with a suitable healthy control child. Children were eligible for inclusion if they were well on the day of recruitment and had no histories of cough or clinic attendance in the last 14 days.
Sampling procedures.
Blood for culture was inoculated into BACTEC PedsPlus media, incubated for 5 days in a BACTEC 9050 instrument (Becton Dickinson, New Jersey), and subcultured to 7% horse blood agar as signaled. From each child, samples of plasma, one at recruitment and another 10 or more days later, were taken and frozen at −70°C. Among controls, a Dacron-tipped flexible wire swab was passed to the nasopharynx, twisted, and withdrawn; the swab was inoculated promptly onto gentamicin blood agar and incubated overnight at 37°C in 5% CO2. All healthy control children were sampled at the hospital clinic close to the laboratory. Pneumococci from blood, CSF, and nasopharyngeal swab cultures were identified by colony morphology, optochin sensitivity, and capsular serotyping by the Quellung technique with polyclonal rabbit sera (Statens Seruminstitut, Denmark).
ELISA for anti-PsaA IgG.
The method of Tharpe and colleagues was used (24, 25) and is described in detail elsewhere (21). An in-house standard serum, BW2000, was used to calculate concentrations; this in-house serum was given a value of 578 ng/ml anti-PsaA when assayed in the laboratory of J. S. Sampson against reference serum IS1644 (CDC, Atlanta, Georgia). A commercial pooled serum (Sandoglobulin) was used for quality control. Paired plasma samples from each patient were always assayed on the same plate, including those for any repeat assays.
Blood samples for ELISA were taken from IPD patients when blood or CSF cultures became positive at 1 to 6 days after admission. Plasma residual volumes from hemoglobin estimations done upon admission were available for some children. To take advantage of these earlier acute-phase samples and maintain consistency across acute-convalescent-phase pairings, all blood samples in the study were assayed as plasma. The use of plasma was first validated in 25 serum-plasma pairs taken from the same blood draw.
Analytical methods.
Analysis was performed using STATA v 8.2 (StataCorp, College Station, TX). For consistency, the terms acute- and convalescent-phase plasma are used to refer to the first and second samples, respectively, drawn from study subjects in all groups. Receiver operating characteristic (ROC) curves for acute concentrations and for the ratio rises in concentration between the two samples, comparing IPD patients to healthy controls, were generated. Optimal thresholds were selected from these curves to estimate sensitivities and specificities. Differences in categorical data were tested by chi-square test, and differences in concentrations and ratio rises between groups were tested by t test after logarithmic transformation. For proportions, exact binomial 95% confidence limits were calculated.
The study was approved by the National Ethical Review Committee of Kenya and by the Institutional Review Board of the Centers for Disease Control and Prevention.
RESULTS
Ninety-eight patients with IPD, 97 healthy controls, and 95 sick controls were recruited to the study. From the patients with IPD, pneumococci were cultured from cerebrospinal fluid samples for 19, from pleural fluid samples for 4, and from blood samples for the remaining 75. Among sick controls, 34 (36%) had anemia and 86 (91%) had malaria. The three groups were well matched for age and sex (Table 1).
TABLE 1.
Characteristics of the three study groups
| Characteristic | IPD patients
|
Healthy controls
|
Sick controls
|
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 58 | 59.2 | 58 | 59.8 | 55 | 57.9 |
| Female | 40 | 40.8 | 39 | 40.2 | 40 | 42.1 |
| Age (mo) | ||||||
| 0-5 | 10 | 10.2 | 8 | 8.2 | 7 | 7.4 |
| 6-11 | 13 | 13.3 | 14 | 14.4 | 17 | 17.9 |
| 12-17 | 15 | 15.3 | 15 | 15.5 | 13 | 13.7 |
| 18-23 | 8 | 8.2 | 8 | 8.2 | 9 | 9.5 |
| 24-35 | 17 | 17.3 | 17 | 17.5 | 17 | 17.9 |
| 36-59 | 23 | 23.5 | 23 | 23.7 | 21 | 22.1 |
| 60-83 | 12 | 12.2 | 12 | 12.4 | 11 | 11.6 |
| Admission-acute-phase interval (days)a | ||||||
| 0 | 48 | 49.0 | 67 | 70.5 | ||
| 1 | 3 | 3.1 | 19 | 20.0 | ||
| 2 | 13 | 13.3 | 5 | 5.3 | ||
| 3 | 19 | 19.4 | 4 | 4.2 | ||
| 4 | 10 | 10.2 | 0 | 0.0 | ||
| 5 | 4 | 4.1 | 0 | 0.0 | ||
| 6 | 1 | 1.0 | 0 | 0.0 | ||
| Acute-convalescent-phase interval (days) | ||||||
| 10-13 | 18 | 18.4 | 6 | 6.2 | 3 | 3.2 |
| 14-20 | 48 | 49.0 | 81 | 83.5 | 77 | 81.1 |
| 21-27 | 17 | 17.3 | 8 | 8.2 | 15 | 15.8 |
| 28-34 | 11 | 11.2 | 1 | 1.0 | 0 | 0.0 |
| 35-46 | 4 | 4.1 | 1 | 1.0 | 0 | 0.0 |
| Prevalence of pneumococci in NP swabsb | ||||||
| Yes | 40 | 41.2 | 31 | 32.6 | ||
| No | 57 | 58.8 | 64 | 67.4 | ||
Admission-acute interval is the time from admission to the first (acute) blood sample.
NP, nasopharyngeal.
For 25 paired samples of serum and plasma, the mean difference in concentrations of anti-PsaA was not significant (paired t test P = 0.97), and the Pearson correlation coefficient, r, was 0.999. In the ELISA of all plasma samples, the interplate coefficient of variation was 14%.
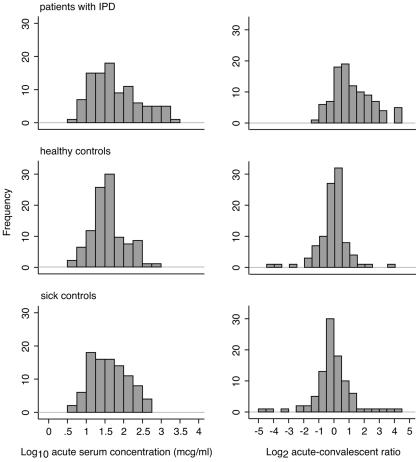
The distributions of anti-PsaA concentrations in acute-phase samples and of the ratios of convalescent/acute-phase concentrations of anti-PsaA are shown in Fig. 1. The distribution of ratios shows a significant rightward spread (indicating rising concentrations) among IPD patients compared to the control groups. A smaller rightward shift is seen for the concentrations of anti-PsaA in acute-phase plasma samples from IPD patients, suggesting that some patients already had elevated concentrations of anti-PsaA at the point when they were admitted to the study. The median (interquartile range) concentrations of acute-phase plasma in μg/ml for IPD cases and healthy and sick controls were 51 (18 to 163), 36 (21 to 64), and 36 (16 to 97), respectively.
FIG. 1.
Distributions of the concentrations of anti-PsaA in acute-phase samples and of the ratios of convalescent/acute-phase concentrations in IPD patients and in two control groups. Bars show the frequencies of children in each group against concentrations of anti-PsaA in acute-phase plasma samples (left side) and ratio rise/fall in anti-PsaA concentrations between acute- and convalescent-phase samples (right side). The numbers in each group are as follows: patients with IPD, 98; healthy controls, 97; and sick controls, 95.
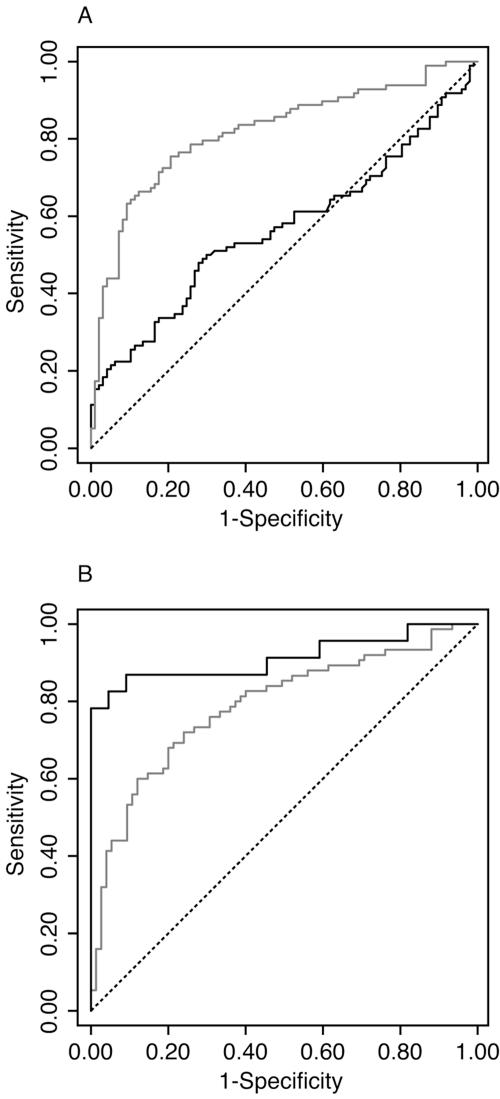
In the ROC curve analyses (Fig. 2A), the area under the curve for convalescent/acute-phase ratios is 0.82, significantly better than that for acute-phase concentrations alone (0.57). Thresholds were selected from these curves to emphasize specificity over sensitivity (Table 2). Using paired samples and a 2.7-fold rise as the threshold, sensitivity was 0.42 (95% CI, 0.32 to 0.52) and specificity was 0.97 (95% CI, 0.91 to 0.99). However, at a threshold with high specificity (≥0.96), a single measurement of anti-PsaA in the acute-phase specimen had a sensitivity of less than 0.20.
FIG. 2.
Receiver operating characteristic curves for the anti-PsaA ELISA in IPD patients and healthy controls. (A) The gray curve describes thresholds defined by the convalescent/acute-phase concentration ratio; the black curve describes thresholds defined by the acute-phase plasma concentration alone. The areas under the two ROC curves are 0.82 and 0.57, respectively (chi-square value, 19.6; P < 0.0005). (B) Both curves describe thresholds defined by the convalescent/acute-phase concentration ratios. Data for children of <2 years old are in black; The data for children of 2 to 6 years old are in gray. The areas under the two ROC curves are 0.91 and 0.79, respectively (chi-square value, 4.2; P = 0.04).
TABLE 2.
Sensitivities and specificities of different diagnostic criteria estimated among patients with invasive pneumococcal disease and healthy controls, respectively
| Characteristic of sample tested | Study subsample | Criterion | Patients with IPD
|
Healthy controls
|
||||
|---|---|---|---|---|---|---|---|---|
| No. of subjects | Sensitivity | 95% CI | No. of subjects | Specificity | 95% CI | |||
| Ratio rise in convalescent/acute-phase concentration | All children | >2.0-fold rise | 98 | 0.48 | 0.38-0.58 | 97 | 0.93 | 0.86-0.97 |
| All children | >2.7-fold rise | 98 | 0.42 | 0.32-0.52 | 97 | 0.97 | 0.91-0.99 | |
| Age <2 yr | >2.7-fold rise | 46 | 0.46 | 0.31-0.61 | 45 | 1.00 | 0.92-1.00 | |
| Age ≥2 yr | >2.7-fold rise | 52 | 0.38 | 0.25-0.53 | 52 | 0.94 | 0.84-0.99 | |
| Acute-phase serum taken on admission | >2.7-fold rise | 48 | 0.48 | 0.33-0.63 | 97 | 0.97 | 0.91-0.99 | |
| Acute-phase serum taken after admission | >2.7-fold rise | 50 | 0.36 | 0.23-0.51 | 97 | 0.97 | 0.91-0.99 | |
| Age <2 yr and acute-phase serum taken on admission | >2.7-fold rise | 24 | 0.50 | 0.29-0.71 | 45 | 1.00 | 0.92-1.00 | |
| Acute-phase concentration alone | >250 ng/ml | 98 | 0.19 | 0.12-0.29 | 97 | 0.96 | 0.90-0.99 | |
| >290 ng/ml | 98 | 0.18 | 0.11-0.27 | 97 | 0.97 | 0.91-0.99 | ||
ROC curve analyses also indicate the superior performance of the assay among infants compared with that among older children (Fig. 2B). This is due to a narrower distribution of ratio rises in the infant controls; the optimum threshold in this group is at a ratio rise of 1.5, where sensitivity is 0.78 (95% CI, 0.56 to 0.93) and specificity is 1.00 (95% CI, 0.85 to 1.00). Although it is valid to set different thresholds for different age groups, it is not practical for numerous age strata. Most epidemiological studies of IPD in childhood focus on those children of <2 years of age rather than on infants. Table 2 presents the results of the standard threshold (ratio of >2.7) for children above and below 2 years of age.
Acute-phase samples were available from the day of admission in half of the IPD patients (Table 1). Restriction of the analysis to these children improved the sensitivity of the assay to 0.48 (Table 2).
Nasopharyngeal swabs from 40 (41%) healthy controls and 31 (33%) sick controls were positive on cultures for S. pneumoniae. Colonization prevalences were 43%, 35%, and 22% among children of <2 years, 3 to 4 years, and 5 to 6 years of age, respectively. We hypothesized that healthy control children with positive swabs would be more likely than those with negative swabs to express rises in anti-PsaA and also that the presence of S. pneumoniae in the nasopharynges of sick control children may be a marker for undetected pneumococcal disease. However, the geometric mean ratio change in anti-PsaA between acute- and convalescent-phase plasma was not significantly different among colonized (1.01) and uncolonized (0.84) healthy controls. Similarly the mean ratio change in anti-PsaA was not significantly different among colonized (0.94) and uncolonized (0.96) sick controls.
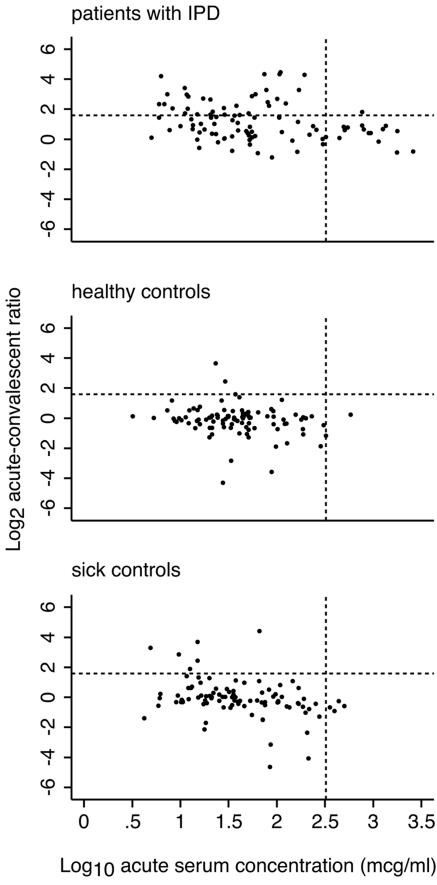
Given the small excess of very high concentration values in the acute-phase samples from IPD cases (Fig. 1), we explored the value of including these in the analysis. From the scatter graphs of the acute-phase concentrations and ratio rises (Fig. 3), new threshold criteria were selected to exclude the main cluster of results in the healthy control group, allowing only outliers to be considered as positive. These thresholds represent an acute concentration of >323 μg/ml and a ratio rise of >3-fold. In this analysis, a positive test was achieved by meeting either threshold; sensitivity was 0.48 (95% CI, 0.38 to 0.58), and specificity was 0.97 (95% CI, 0.91 to 0.99)
FIG. 3.
Scatter plots of convalescent/acute-phase concentration ratios against acute-phase concentrations of anti-PsaA for three study groups. Dots represent individual patients within the three study groups: (A) patients with IPD, n = 98; (B) healthy controls, n = 97; (C) sick controls, n = 95. Convalescent/acute-phase ratios have been transformed to log2 and acute-phase concentrations to log10. Dotted lines indicate the thresholds selected from the scatter plots to optimize sensitivity in IPD patients and specificity in healthy controls.
Among 95 sick controls with anemia and/or malaria, 6 (6.3%) had acute-phase anti-PsaA concentrations of ≥250 μg/ml, and 6 (6.3%) had rises in anti-PsaA concentrations between acute- and convalescent-phase plasma samples of ≥2.7. The proportions meeting these criteria among sick control children of <2 years of age were 7% (3/46) and 2% (1/46), respectively.
DISCUSSION
Serological techniques for diagnosis of IPD have attracted little support, despite several decades of innovation and inquiry (14). Where they appear clinically useful they have often not been validated against control populations (12, 17), and where they have been validated they often lack specificity (16, 20). The assay described here was first validated among adult pneumonia patients in Kenya; sensitivity, estimated in 109 patients with blood or lung aspirate cultures positive for S. pneumoniae, was 0.70 (95% CI, 0.60 to 0.78); specificity, estimated in 49 healthy controls, was 1.00 and, in 56 sick outpatients with diagnoses not associated with pneumococcal infection, was 0.98 (95% CI, 0.90 to 1.00).
Our objective was to validate the performance of the anti-PsaA ELISA for the diagnosis of pediatric IPD with adequate numbers and appropriate control groups. Pneumococcal disease is more common among children than among adults admitted to hospitals, and given the insensitivity of blood and CSF cultures, we anticipated that the sick control group would contain moderate numbers of occult pneumococcal patients. To illustrate, at Kilifi Hospital 21% of childhood deaths associated with P. falciparum parasitemia have concurrent bacteremia, for which S. pneumoniae is the commonest pathogen (1). For this reason, we estimated specificity here among healthy controls. The sick control group was included to test the hypothesis that nonspecific factors associated with illness, particularly with fever, may frequently elicit immunological changes that mimic a measurable anti-PsaA ELISA response. In Kilifi, it is quite plausible that 6% of patients admitted with malaria or anemia have occult pneumococcal disease, and therefore the results do not support the hypothesis of nonspecific immunological changes.
The performance of the anti-PsaA ELISA was not as impressive in children as it had been in adults (21). Optimized at a 2.7-fold rise in concentration, sensitivity was 0.42 and specificity was 0.97. Two factors suggest that the study performance would be slightly better in the context of an epidemiological study. First, in a surveillance study or vaccine efficacy trial, blood specimens would normally be collected from all study participants on admission. Second, research and surveillance studies focus almost invariably on children in the first 2 years of life, because that is the age of maximum disease risk. In our subset analyses, both factors yielded higher sensitivity estimates, albeit marginally, and in the group of children satisfying both factors, the assay correctly identified half of all cases of IPD and correctly classified all of the healthy controls.
An assay of IgM rather than IgG antibodies might have discriminated better between healthy children and patients with IPD. However, Kenyan children attain adult levels of anti-PsaA IgG in the first year of life (13), and as IPD affects only a small percentage of the population, the most likely stimulus is early nasopharyngeal colonization with S. pneumoniae. Later exposures leading to disease are likely therefore to elicit secondary memory immune responses.
The fact that colonization prevalence is much higher in children than in adults might explain the inferior performance of the serological assay in this age group. This problem was first described for a PCR assay of the pneumolysin gene, for which the proportion of false-positive results by age among healthy controls was shown to parallel the prevalence of nasopharyngeal carriage (6). We hypothesized that a rising concentration of anti-PsaA, captured during a 2-week observation period, might be stimulated by recent nasopharyngeal acquisition in healthy controls but found no evidence to support this; it may be that acquisition during, but not before, the sampling period was driving false-positive results, and in hindsight the study may have benefited from nasopharyngeal swab cultures taken with the convalescent-phase samples.
Deriving a standard curve from 8 dilutions and assaying test samples at 7 dilutions allows a precise definition of the IgG concentration. On a continuous concentration scale, the ROC analysis permits fine optimization of the test performance. Applying fitted thresholds for both the ratio rise and the acute-phase concentration together extended the sensitivity of the assay to 0.48 by including those who exhibited high concentrations on admission, presumably because they had been exposed to the pneumococcus several days prior to admission. The thresholds were derived closely from the data, and before generalizing this approach to another setting, it would be prudent to validate it locally. It does, however, illustrate the practical problems of serological diagnosis and a potential solution.
Advances in the diagnosis of invasive pneumococcal disease in children are rare and have been confined recently to the detection of C polysaccharide antigen in urine and CSF (19). The specificity of this antigen assay in urine is too low for consideration in epidemiological studies (7, 9). The anti-PsaA IgG ELISA has a sensitivity of 0.42 at an acceptable specificity of 0.97. It is possible that assay refinements (e.g., employing different blocking agents or wash routines or prior absorption with potentially cross-reactive antigens) could improve performance, though in this evaluation we adhered to the published methodology (21). Sampling children at first presentation, examining only those under 2 years of age, and using a two-threshold analysis all tended to improve sensitivity without compromising specificity. In any of these situations, the anti-PsaA ELISA can detect approximately half of culture-positive cases; if some of this benefit extends to culture-negative cases, especially those with prior antibiotic use, it would augment our currently meager diagnostic toolbox. However, the excellent performance of the assay in adults has clearly not been reproduced in children, and what gains can be claimed for the anti-PsaA ELISA are more indicative of the extremely poor state of diagnostics, both epidemiological and clinical, for invasive pneumococcal disease in children.
Acknowledgments
Recombinant PsaA was kindly provided by Elaine Wang and Mary Ewasyshyn of Aventis, Canada. This paper is published with the permission of the director of KEMRI.
The work was supported by the Wellcome Trust of Great Britain, and J.A.G.S. is supported by a fellowship from the Wellcome Trust (no. 061089).
REFERENCES
- 1.Berkley, J. A., B. S. Lowe, I. Mwangi, T. Williams, E. Bauni, S. Mwarumba, C. Ngetsa, A. Hart, K. Maitland, M. English, K. Marsh, and J. A. Scott. 2004. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39-47. [DOI] [PubMed] [Google Scholar]
- 2.Brent, A., I. Ahmed, M. Ndiritu, P. Lewa, C. Ngetsa, B. S. Lowe, E. Bauni, M. English, J. A. Berkley, and J. A. G. Scott. 2005. Incidence of clinically significant bacteremia among children presenting to hospital in Kenya. Submitted for publication. [DOI] [PubMed]
- 3.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook, J., J. A. Tharpe, S. E. Johnson, D. B. Williams, A. R. Stinson, R. R. Facklam, E. W. Ades, G. M. Carlone, and J. S. Sampson. 1998. Immunoreactivity of five monoclonal antibodies against the 37-kilodalton common cell wall protein (PsaA) of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 5:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutts, F. T., S. M. A. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139-1146. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., O. Shriker, I. Hazan, E. Leibovitz, D. Greenberg, F. Schlaeffer, and R. Levy. 1998. Prospective study to determine clinical relevance of detection of pneumococcal DNA in sera of children by PCR. J. Clin. Microbiol. 36:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell, S. F., R. L. Garman, G. Liu, O. S. Levine, and Y. H. Yang. 2001. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin. Infect. Dis. 32:824-825. [DOI] [PubMed] [Google Scholar]
- 8.English, M., F. Esamai, A. Wasunna, F. Were, B. Ogutu, A. Wamae, R. W. Snow, and N. Peshu. 2004. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet 363:1948-1953. [DOI] [PubMed] [Google Scholar]
- 9.Hamer, D. H., J. Egas, B. Estrella, W. B. MacLeod, J. K. Griffiths, and F. Sempertegui. 2002. Assessment of the Binax NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage. Clin. Infect. Dis. 34:1025-1028. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, S. E., J. K. Dykes, D. L. Jue, J. S. Sampson, G. M. Carlone, and E. W. Ades. 2002. Inhibition of pneumococcal carriage in mice by subcutaneous immunization with peptides from the common surface protein pneumococcal surface adhesin a. J. Infect. Dis. 185:489-496. [DOI] [PubMed] [Google Scholar]
- 11.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 12.Korppi, M., M. Koskela, E. Jalonen, and M. Leinonen. 1992. Serologically indicated pneumococcal respiratory infection in children. Scand. J. Infect. Dis. 24:437-443. [DOI] [PubMed] [Google Scholar]
- 13.Laine, C., T. Mwangi, C. M. Thompson, J. Obiero, M. Lipsitch, and J. A. G. Scott. 2004. Age-specific immunoglobulin g (IgG) and IgA to pneumococcal protein antigens in a population in coastal Kenya. Infect. Immun. 72:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leinonen, M. 1994. Serological diagnosis of pneumococcal pneumonia—will it ever become a clinical reality. Semin. Respir. Infect. 9:189-191. [PubMed] [Google Scholar]
- 15.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musher, D. M., R. Mediwala, H. M. Phan, G. Chen, and R. E. Baughn. 2001. Nonspecificity of assaying for IgG antibody to pneumolysin in circulating immune complexes as a means to diagnose pneumococcal pneumonia. Clin. Infect. Dis. 32:534-538. [DOI] [PubMed] [Google Scholar]
- 17.Ortqvist, A., J. Hedlund, L. A. Burman, E. Elbel, M. Hofer, M. Leinonen, I. Lindblad, B. Sundelof, M. Kalin, et al. 1998. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 351:399-403. [DOI] [PubMed] [Google Scholar]
- 18.Rapola, S., T. Kilpi, M. Lahdenkari, P. H. Makela, and H. Kayhty. 2001. Antibody response to the pneumococcal proteins pneumococcal surface adhesin A and pneumolysin in children with acute otitis media. Pediatr. Infect. Dis. J. 20:482-487. [DOI] [PubMed] [Google Scholar]
- 19.Samra, Z., H. Shmuely, E. Nahum, D. Paghis, and J. Ben-Ari. 2003. Use of the NOW Streptococcus pneumoniae urinary antigen test in cerebrospinal fluid for rapid diagnosis of pneumococcal meningitis. Diagn. Microbiol. Infect. Dis. 45:237-240. [DOI] [PubMed] [Google Scholar]
- 20.Scott, J. A. G., A. J. Hall, and M. Leinonen. 2000. Validation of immune-complex enzyme immunoassays for diagnosis of pneumococcal pneumonia among adults in Kenya. Clin. Diagn. Lab. Immunol. 7:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott, J. A. G., J. Obiero, A. J. Hall, and K. Marsh. 2002. Validation of immunoglobulin G enzyme-linked immunosorbent assay for antibodies to pneumococcal surface adhesin A in the diagnosis of pneumococcal pneumonia among adults in Kenya. J. Infect. Dis. 186:220-226. [DOI] [PubMed] [Google Scholar]
- 22.Simell, B., M. Korkeila, H. Pursiainen, T. M. Kilpi, and H. Kayhty. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin a, pneumolysin, and pneumococcal surface protein a in children. J. Infect. Dis. 183:887-896. [DOI] [PubMed] [Google Scholar]
- 23.Talkington, D. F., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 24.Tharpe, J. A., and H. Russell. 1996. Purification and seroreactivity of pneumococcal surface adhesin A (PsaA). Clin. Diagn. Lab. Immunol. 3:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tharpe, J. A., H. Russell, M. Leinonen, B. D. Plikaytis, R. F. Breiman, G. M. Carlone, E. W. Ades, and J. S. Sampson. 1998. Comparison of a pneumococcal common protein (PsaA) antibody ELISA and a PsaA immune complex ELISA for detection of pneumococcal serum antibody. Pathobiology 66:77-83. [DOI] [PubMed] [Google Scholar]
- 26.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2000. Management of the child with a serious infection or severe malnutrition. Guidelines for care at first-referral level in developing countries. World Health Organization, Geneva, Switzerland.