Abstract
Pneumococcal polysaccharide vaccine (PPV) is of limited immunogenicity in infants and immunocompromised patients. Our prospective randomized controlled trial investigated whether priming with pneumococcal conjugate vaccine (PCV) induced specific immunological memory in previously nonresponders to PPV. Of a total of 33 children (2 to 18 years) with polysaccharide-specific immunodeficiency (PSI), group A (n = 16) received two doses of 7-valent PCV in a 4- to 6-week interval, and a booster dose of 23-valent PPV after one year. Group B (n = 17) received two doses of PPV in a 1-year interval exclusively. Specific antibody concentrations for serotypes 4, 5, 6B, 9V, 14, 18C, 19F, and 23F were determined (enzyme-linked immunosorbent assay) before and at 7 and 28 days after administration of the PPV booster and compared to an opsonophagocytosis assay. Of group A, 64 to 100% had antibody concentrations of ≥1 μg/ml on day 28 after the booster versus 25 to 94% of group B. Group A had significantly higher antibody concentrations for all PCV-containing serotypes already on day 7, indicating early memory response. Antibody concentrations were in accordance with functional opsonic activity, although opsonic titers varied among individuals. Pneumococcal vaccination was well tolerated. The incidence of airway infections was reduced after priming with PCV (10/year for group A versus 15/year for group B). Following a PPV booster, even patients primarily not responding to PPV showed a rapid and more pronounced memory response after priming with PCV.
Pneumococcal infections cause at least one million deaths worldwide annually, mostly in young children (52). Immunization against Streptococcus pneumoniae has the potential to face this burden of disease. An ideal vaccine should rapidly elicit protective immunity and generate memory cells, which respond efficiently to subsequent antigen exposure. Generation of memory B cells and long lived plasma cells is associated with isotype switching and hypermutation of the immunoglobulin genes, resulting in selection of B cells with high-affinity B-cell receptors (27, 28). The established 23-valent pneumococcal polysaccharide vaccine (PPV-23) induces in adults primarily immunoglobulin M (IgM), with hardly any class switching, affinity maturation, or immunological memory (29, 47). It is ineffective in infants and of limited efficacy in high-risk patients, since it elicits a solely T-cell-independent immune response. In 2000, a 7-valent pneumococcal conjugate vaccine (PCV-7) was introduced in the United States, resulting in a dramatic decrease of invasive pneumococcal disease in the following years (8, 51).
The efficacy of the conjugate vaccine is due to several mechanisms: increased amounts of circulating antibodies, higher avidity, and an induction of immunological memory (2, 9, 16, 18). In addition, a reduction of nasopharyngeal carriage of pneumococci was described for the PCV-7 (13, 14, 26, 31).
In light of the clinical background, the induction of memory appears mandatory for long-term protection against pneumococcal disease. In addition, antibody concentrations gradually diminish after primary series of PPV, and may fall below a protective threshold, underlining the importance of memory versus circulating antibodies. However, while conjugate pneumococcal vaccine apparently confers protection against pneumococci, the presence of polysaccharide specific immunological memory is difficult to demonstrate in individual subjects. Pneumococcal polysaccharide specific memory in subjects immunized with glycoconjugates is demonstrated by challenge with PPV, and the anamnestic response of polysaccharide-specific IgG is considered a surrogate marker for memory (12).
Here, we investigated the immunogenicity of PCV with a focus on its ability to induce an anamnestic response in patients with recurrent infections and an inability to respond to PPV immunization sufficiently (55). This so-called polysaccharide-specific immunodeficiency (PSI) is defined as an impaired immune response to polysaccharide antigens, while antibody response to protein antigens is intact. In this regard, PSI is an excellent in vivo model to study the immunogenicity of protein-coupled polysaccharide vaccines such as PCV. Typically, these patients suffer from recurrent respiratory infections, which are mostly due to encapsulated bacteria and require frequent antibiotic treatment (3, 38, 49, 56). An effective vaccine strategy for this patient group is still pending. In such risk groups, immunological memory is a crucial secondary parameter to characterize functional antibody activity and long-term protective responses. In order to study the nature and kinetics of the immune response, we detected pneumococcal antibodies on day 7 after vaccination as early postchallenge samples, and on day 28, representing late response. Additionally, since the host's protection against pneumococcal infections is mediated mainly by phagocytosis, we also determined the opsonophagocytic activity (OPA) of the induced anticapsular antibodies. This functional activity of antibodies in killing of pneumococci is thought to correlate better with the efficacy needed to prevent infections than antibody levels measured solely by enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Our study cohort encompassed 33 children fulfilling the following criteria for PSI: recurrent airway infections (e.g., pneumonia or otitis media; more than three per year), a lacking immune response to repeat (at least two; range, two to three) PPV applications, and a normal immune response to protein vaccines. A lacking immune response was defined as pneumococcal serotype-specific IgG antibody concentrations of <1.0 μg/ml in at least five of seven serotypes 4 weeks after the last immunization with PPV. Exclusion criteria were a history of allergic or serious adverse reactions to previous vaccinations. Other exclusion criteria were progressive neurological disease or any current illness. All parents supplied written informed consent prior to the study. Human experimentation guidelines of Good Clinical Practice, the German Drug Act, and the declaration of Helsinki/Hong Kong were followed in the conduct of clinical research. Local ethical committee approval was obtained. During the 1-year period before the PPV-23 booster was administered, clinically defined airway infections were documented for both groups on diary cards.
Group A received two doses of the 7-valent PCV and a booster dose of the 23-valent PPV 1 year after the first PCV-7 dose. Group B received one dose of PPV and another dose of PPV after 1 year without previous PCV priming.
Vaccines.
The 7-valent PCV (Prevenar, Wyeth-Lederle, Germany) contains the polysaccharide capsular serotypes 4, 9V, 14, 18C, 19F, and 23F at 2 μg/ml, and 6B at 4 μg/ml, coupled with a carrier protein, a nontoxic variant of diphtheria toxin (cross-reactive material 197; CRM197). The 23-valent PPV (Pneumovax23, Merieux MSD, Germany) contains serotypes 1 through 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F at 25 μg each. Both vaccines were administered intramuscularly, and local and systemic side effects were recorded on diary cards in the 3 days after each immunization.
Serological analysis.
Serological determinations were performed blinded at our laboratory. The staff were not aware of age, vaccination status, or medical background of the vaccinees at the time of sampling. Serum antibodies against two highly immunogenic (14 and 19F) and two low-immunogenic serotypes (6B and 23F) and against serotypes 4, 9V, 18C, and the exclusively PPV-23-containing type 5 were determined before and 7 and 28 days after the PPV booster was administered. Serotype-specific IgG-antibody concentrations were measured by a modified ELISA technique using Nunc CovaLink microtiter plates (Nunc, Germany) and serum lot 89-SF as the standard serum (FDA, Bethesda, MD). Serum samples were preincubated with 10 μg/ml pneumococcal polysaccharide C (CPS, Statens Seruminstitut, Denmark) for blocking of unspecific anti-CPS antibodies. Reference serum 89-SF (provided by C. Frash, Rockville, MD) was used for assay standardization. In addition, serum samples were adsorbed against serotype 22F to remove cross-reactive antibodies (11). Sera with known high antibody titers were used as reference and quality control sera (Endobulin HP, Immuno, Austria). The minimum antibody detection level was 0.1 μg/ml. IgG subclasses were measured by nephelometry (4a, 57).
A recent consensus conference by the World Health Organization recommends pneumococcal antibody concentrations of ≥0.35 μg/ml as a surrogate for conjugate vaccine efficacy against invasive disease in general (http://www.who.int/biologicals/Guidelines/vaccines.html). This threshold level is only reliable after vaccination with the conjugate vaccine (PCV) where memory cells are induced. Thus, the estimation of seroprotection after vaccination with PPV-23 is even more difficult. In contrast to PCV, PPV-23 does not prime the immune system for a rapid booster response. Therefore, Sanders defined antibody titers of ≥1.0 μg/ml for at least five of seven measured serotypes as predictive for a successful pneumococcal immunization after PPV-23 vaccination (40). Since our patients did not respond to solely polysaccharide vaccination and seemed to be at an increased risk of pneumococcal infections, we also chose antibody titers of ≥1.0 μg/ml as correlate of seroprotection.
Opsonophagocytosis assay.
The complement-dependent opsonic activity of serum antibodies was assessed using an opsonophagocytosis assay. We determined antibody activity against serotype 23F in a modified protocol (25). This method uses granulocytes from a promyeloic leukemia cell line (HL 60) as effector cells, and paraformaldehyde fixed 5.6-carboxyfluorescin succinimidyl ester (FAM-SE)-labeled Streptococcus pneumoniae isolates as bacterial targets. Granulocytes and bacteria are used in 1:4 relation and incubated with serum and complement opsonization, subsequent phagocytosis of the bacteria follows. Serum samples were preincubated at 56°C for 30 min for complement inactivation. Baby rabbit serum (Pel-Freez) was used as a complement source. As quality controls, a complement and a cell control were run with each assay. Sera with known high antibody titers (Sandoglobin; Sandoz) were used as quality control sera. Antipneumococcal opsonophagocytic activity was determined by collecting fluorescent signals on the HL-60 granulocyte population by light scatter (FALS and 90LS). FAM-SE was excited at 488 nm, and fluorescent signals were measured at 525 nm. Results were reported as percentage of gated cells with fluorescence higher than the cell control. Opsonophagocytic titers were reported as the reciprocal of the dilution with at least 50% of the maximum uptake of the fluorescent labeled bacteria by HL-60 granulocytes.
Statistical analysis.
Geometric mean concentrations (GMCs) of antibody with 95% confidence intervals and proportion of patients with antibody concentrations ≥1 μg/ml, respectively are presented for each serotype. Antibody levels before and after vaccinations were compared using the Wilcoxon signed-rank test. The U test (Mann-Whitney) was performed for between-groups comparison. We used the SPSS for Windows version 11.0 software package. Probability values (P) of <0.05 were considered statistical significant. Nonparametric correlations between antibody titers and opsonic activity were calculated with Spearman's correlation coefficient.
RESULTS
Demographics.
Thirty-three patients (age, 2 to 18 years; 19 boys, 14 girls) were enrolled in the study and randomly assigned to group A (16 patients) or B (17 patients) (Table 1). All patients returned completed diary cards. Each pneumococcal vaccination was well tolerated in all patients. Adverse reactions were similar in both groups (Table 2); no severe adverse reaction was observed. The median ages of the two groups differed slightly (the median [range] was 7 [5 to 12] years for group A versus 4 [2 to 14] years for group B; P < 0.05).
TABLE 1.
Demographics, underlying diagnosis, and clinical diagnosed infections of subjects unresponsive to pneumococcal polysaccharide vaccine
| Parameter | Value for indicated group
|
|
|---|---|---|
| A (PCV primed) (n = 16) | B (unprimed) (n = 17) | |
| Age (yr) | ||
| Range | 5-12 | 2-14 |
| Median | 7 | 4 |
| Gender (male/female) | 8/8 | 11/6 |
| Underlying diagnosis | ||
| IgG2 deficiency | 10 | 13 |
| IgA deficiency | 3 | |
| Hypogammaglobulinemia | 1 | 3 |
| Asthma bronchiale | 8 | 9 |
| Chronic bronchitis | 1 | 1 |
| Recurrent pneumonia | 1 | 5 |
| Recurrent otitis | 2 | |
| Recurrent bronchitis | 1 | 3 |
| Infections during 12 mo prior to PPV-23 booster administration | ||
| Bronchitis | 5 | 8 |
| Pneumonia | 5 | |
| Otitis | 2 | 1 |
| Tonsillitis | 3 | 1 |
| Fever of unknown origin | 3 | 4 |
TABLE 2.
Number of infants experiencing systemic reactions in the 3 days following each immunization
| Symptom | No. (%) of infants with symptom in:
|
|
|---|---|---|
| Group A: PCV-7 priming | Group B: solely PPV-23 | |
| Redness | 6 (35) | 6 (35) |
| Induration | 2 (12) | 3 (18) |
| Swelling | 2 (12) | 1 (6) |
| Pain at injection site | 8 (47) | 9 (53) |
| Irritability | 2 (12) | 1 (6) |
| Drowsiness | 2 (12) | 1 (6) |
| Lack of appetite | 0 | 3 (18) |
| Body temperature of >38.0°C | 2 (12) | 1 (6) |
At study entrance, groups were comparable concerning frequency of airway infections (>3/year; see inclusion criteria) and medical history. Remarkable features of the vaccinees included bronchial asthma (group A, 47%; group B, 53%), IgG2 subclass deficiency (group A, 59%; group B, 76%), and 18% of group A suffered from IgA deficiency. After PCV vaccination of group A, there were fewer upper and lower airway infections during the year prior to the administration of the PPV-23 booster (10 cumulative in group A versus 15 in group B and a median of 10, with 5 remarkable episodes of pneumonia in the PCV-7-primed group versus none in the other).
Immunogenicity.
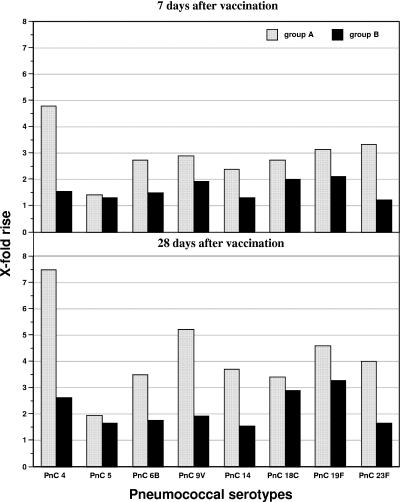
Complete blood sample series were available from all 33 subjects for analysis of the immune responses to the booster dose of PPV. Initial geometric mean antibody concentrations were higher in group A after two PCV-7 applications (0.64 to 3.5 μg/ml) compared to group B after one PPV-23 application (0.17 to 1.13 μg/ml) with significance for the serotypes 4, 6B, 14, 19F, and 23F (Table 3). Following administration of the the PPV booster, antibody concentrations increased even more and were significantly higher in group A: on day 7, group A had 1.76 to 8.39 μg/ml versus 0.21 to 1.67 μg/ml in group B with significance for all serotypes except the non-PCV serotype 5) (Table 2). On day 28, antibodies in group A remained significantly higher than in group B (1.25 to 12.99 μg/ml versus 0.28 to 2.6 μg/ml [significant for all serotypes except 5]). In addition, the mean rise from baseline values of antibody GMCs from day 0 to day 7 was higher in group A (ranging from 1.4- to 4.8-fold) than in group B (1.2- to 2.1-fold) (Fig. 1). Also after 28 days, median antibody GMCs increased higher from baseline values in group A (1.9- to 7.5-fold), compared to 1.6- to 3.3-fold in group B (P < 0.01 for serotype 4) (Fig. 2).
TABLE 3.
Serotype-specific IgG antibody GMCs of patients previously not responding to pneumococcal polysaccharide vaccinationa
| PnC | GMC (μg/ml) (95% CI)
|
Comparison of groups A and B
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A (PCV-7 primed) (n = 16)
|
Group B (PPV-23) (n = 17)
|
||||||||
| Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | |
| 4 | 0.75 (0.45-1.05) | 3.59** (0.00-8.25) | 5.62* (1.80-9.44) | 0.39 (0.17-0.61) | 0.60* (0.21-0.99) | 1.02 NS (0.50-1.54) | ** | *** | *** |
| 5 | 0.64 (0.54-0.75) | 0.91* (0.74-1.08) | 1.25* (0.92-1.58) | 0.73 (0.48-0.99) | 0.95 NS (0.31-1.58) | 1.21 NS (0.33-2.10) | NS | NS | NS |
| 6B | 0.64 (0.00-1.44) | 1.76** (0.00-3.85) | 2.24 NS (0.18-4.29) | 0.18 (0.02-0.33) | 0.27* (0.00-0.62) | 0.32 NS (0.07-0.57) | * | * | *** |
| 9V | 0.82 (0.19-1.45) | 2.37** (0.00-7.22) | 4.28** (0.00-10.4) | 0.43 (0.00-2.18) | 0.83* (0.00-1.68) | 0.83 NS (0.00-2.17) | NS | * | ** |
| 14 | 3.50 (2.50-4.49) | 8.39** (0.00-18.4) | 13.0* (0.00-28.7) | 1.13 (0.54-1.73) | 1.48* (0.86-2.11) | 1.75 NS (1.38-2.12) | *** | *** | *** |
| 18C | 0.86 (0.00-2.09) | 2.35* (0.63-4.08) | 2.94 NS (1.54-4.33) | 0.39 (0.20-0.59) | 0.78** (0.41-1.15) | 1.13 NS (0.29-1.96) | NS | * | * |
| 19F | 2.30 (0.00-5.27) | 7.20** (0.00-15.02) | 10.59* (2.35-18.8) | 0.79 (0.34-1.24) | 1.67** (1.25-2.10) | 2.60** (1.22-3.99) | * | *** | *** |
| 23F | 0.88 (0.14-1.63) | 2.93** (0.00-7.22) | 3.54 NS (0.25-6.83) | 0.17 (0.03-0.31) | 0.21* (0.00-0.62) | 0.28 NS (0.00-0.88) | ** | *** | *** |
PnC, pneumococcal serotype; n, number of patients; NS, not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG. 1.
Increase of specific pneumococcal antibodies after vaccination.
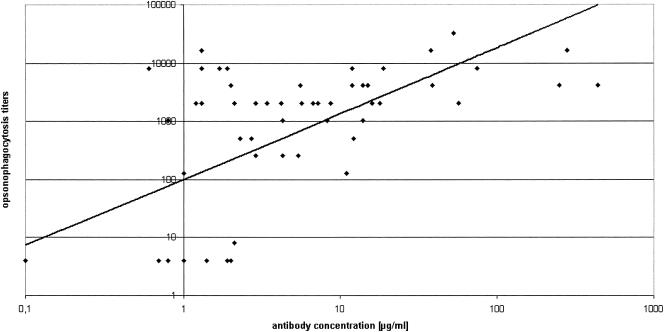
FIG. 2.
Pneumococcal opsonophagocytosis titers versus antibody concentrations (r = 0.72; P < 0.01).
In summary, the antibody response after PCV administration and after booster vaccination was higher and faster in the PCV-primed group A for all PCV-related serotypes as compared to the unprimed group.
For the exclusively PPV-23 serotype 5, there was no significant difference between groups at any time (Table 3). Before the PCV boost, the GMCs were 0.64 μg/ml in group A (0.54 to 0.75) and 0.73 μg/ml (0.48 to 0.99) in group B. On day 7, the group A GMC was 0.91 μg/ml (range, 0.74 to 1.08) and that for group B was 0.95 μg/ml (range, 0.31 to 1.58). On day 28, the GMCs were 1.25 μg/ml (range, 0.92 to 1.58) for group A and 1.21 μg/ml (range, 0.33 to 2.10) for group B. For group A, the rise of antibody levels from baseline values was 1.4-fold on day 7 and 1.95-fold on day 28 (P < 0.05 each). For group B, there was a 1.3-fold rise on day 7 and a 1.66-fold rise on day 28, which reached no statistical significance.
Opsonophagocytic activity.
OPA was determined for the low-immunogenic serotype 23F before and 7 and 28 days after the booster vaccination. Prior to immunization, median opsonic titers were 1:4 (1:4 to 1:2,048) for group A and 1:4 (1:4 to 1:32) for group B. On day 7, group A showed a median activity of 1:4 (1:4 to 1:1,024) and group B of 1:4 (1:4 to 1:1,024). On day 28, median opsonic titers were 1:256 (1:4 to 1:2,048) for group A and 1:4 (1:4 to 1:512) for group B.
There was a direct correlation (Spearman r = 0.72; P < 0.01) between antibody concentrations and functional OPA (Fig. 2). OPA varied highly, especially in group A, reflecting high antibody activity after conjugate vaccination. However, most individuals with a minimum antibody concentration of 1 μg/ml had high OPA.
Seroprotection.
A positive immune response to pneumococcal serotypes was defined as an antibody concentration of ≥1.0 μg/ml and an opsonophagocytic titer of >1:64. Accordingly, after 28 days, the percentage of responders was determined for each group and revealed significantly more responders for low- immunogenic serotypes 6B and 23F in group A, with 80.0% and 73.7% of patients, compared to group B, with 25.0% each (P < 0.01). There was no significant difference between responders in each group for the highly immunogenic serotypes 14 and 19F, even though the antibody concentration was significantly higher in the primed group. Interestingly, even for serotype 5, a higher percentage of responders was found in the primed group A (64.3% versus 43.7% in group B).
Additionally, to further investigate the kinetics of immune response, we determined the number of nonresponders to all pneumococcal serotypes, remaining below the level of 1.0 μg/ml for each pneumococcal serotype (Table 4). As early as on day 7 postvaccination, between 0 (serotypes 14 and 19F) and 33.3% (serotypes 6B, 18C, and 23F) of group A subjects remained below this critical threshold compared to between 5.9% (serotype 19F) and 82.4% (serotype 23F) of group B subjects. For the non-PCV serotype 5, the percentage was similar in both groups (57.1% in group A versus 62.5% in group B).
TABLE 4.
Percentage of subjects with IgG antibody concentrations below 1.0 μg/ml before and 7 and 28 days after pneumococcal polysaccharide booster vaccination
| PnCa | Group A (PCV-7 primed)
|
Group B (unprimed)
|
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | |
| 4 | 68.8 | 6.7 | 6.7 | 87.5 | 70.6 | 56.3 |
| 5 | 85.7 | 57.1 | 35.7 | 75.0 | 62.5 | 56.3 |
| 6B | 43.8 | 33.3 | 20.0 | 100 | 76.5 | 75.0 |
| 9V | 68.8 | 20.0 | 13.3 | 81.3 | 47.1 | 62.5 |
| 14 | 6.3 | 0.00 | 0.00 | 31.3 | 17.6 | 6.3 |
| 18C | 68.8 | 33.3 | 13.3 | 81.3 | 47.1 | 37.5 |
| 19F | 12.5 | 0.00 | 0.00 | 43.8 | 5.9 | 6.3 |
| 23F | 37.5 | 33.3 | 26.7 | 100 | 82.4 | 75.0 |
PnC, pneumococcal serotypes.
DISCUSSION
Pneumococcal conjugate vaccines are immunogenic in infants and induce long-term protection by inducing systemic anamnestic IgG response (10, 37, 43). This has been demonstrated by studying antibody responses to pure polysaccharide antigens in infants who previously received conjugate vaccines (10, 34). Significant responses to plain polysaccharide vaccine challenge are generally not seen in unprimed infants. Thus, in conjugate-primed toddlers a response upon a polysaccharide challenge is considered indicative for immunological memory (1, 44). In addition, we and others have shown that PCV-vaccination can overcome the impaired immune response to the plain pneumococcal polysaccharide in certain risk groups (22, 30, 32, 39, 41, 46, 55). However, antibody concentrations are not the only correlate of a protective immunity. As we know from Haemophilus influenzae, conjugate vaccine efficacy is also determined by differences in the kinetics of immune response (17). Demonstration of B-cell memory has largely been based on a rapid and strong antibody response to a dose of plain polysaccharide vaccine after priming with a conjugate vaccine (32, 33, 54).
There are only limited data on the kinetics of immunological memory in high-risk groups. In our study, the anamnestic immune response after the PPV booster vaccination was demonstrated by the significantly more rapid and higher antibody increase already within 7 days in the PCV-primed group A. Moreover, the majority of patients primed with PCV-7 had protective pneumococcal antibody levels (≥1.0 μg/ml) for all PCV-7 included serotypes already on day 7. This threshold value also implicates long-term protection. Even for the weak immunogenic serotypes 6B, 18C, and 23F, about two-thirds of our subjects reached this threshold compared to 24%, 53%, and 18%, respectively, in the unprimed group (Table 3). Obviously, the kinetics of pneumococcal antibodies are serotype dependent, an observation also suppported by others (15). As there was only a marginal additional rise in antibody concentrations from day 7 to day 28 in these patients, the IgG antibody peak might have been reached even earlier. This observation is of special clinical importance when natural contact with the organisms occurs and rapid protection is required (e.g., in unvaccinated immunocompromized patients as postexposure immunization). Also, group B individuals previously not responding to PPV-23 demonstrated a moderate immune response seven days after the PPV booster. This might be also due to having grown one year older in the meantime. While group A subjects showed a further increase of specific pneumococcal antibodies (significant for serotypes 4, 5, 9V, 14, and 19F) 28 days after PPV-23 administration, in group B, this was observed only for the highly immunogenic serotype 19F and in a much smaller amount.
Overall the development of a polysaccharide-specific memory seems to be influenced by the age at priming with the glycoconjugate, the route of immunization, the time and features of the booster, and other factors, as also demonstrated in an early-life murine model reproducing the main features of infant responses to pneumococcal vaccination (7, 24). Two other studies in healthy senior subjects could not find a benefit from a PPV-23 booster 1 (42) or 6 months (35) after PCV-7 immunization. The subsequent administration provided no additional antibody response. On the other hand, in a Dutch study 383 children (1 to 7 years old) with recurrent otitis media were immunized with either PCV followed 6 to 7 months later by PPV-23, or by hepatitis A or B vaccine (50). In the PPV-23-boosted group, antipneumococcal antibodies against PCV-containing serotypes were much higher. Also a study in 386 healthy United Kingdom infants showed results in favor of the PPV-23 booster. They received three doses of PCV-7 or placebos in the first year of life, followed by a PPV-23 booster at 13 to 16 months of age. The PPV-23 booster resulted in a 3.4- to 51.7-fold rise of pneumococcal anticapsular IgG antibodies depending on the serotype and immunization schedule (10).
PCV repeated priming like in our protocol has the potential to support a booster response, improving immunogenicity and efficacy. On the other hand, there is evidence that even a single dose of a conjugate vaccine may increase antibody avidity (5, 6), and that higher avidity antibodies are more cross-reactive with closely related pneumococci serotypes. Also, prevaccination antibodies are more cross-reactive than postvaccination antibodies (44). In our study, we documented the frequency of airway infections in our subjects in order to estimate clinical efficacy prior to the PPV-23 challenge. Interestingly, there was a marked reduction of previously frequent infections in the PCV-primed patient group (Table 1).
We are aware of a minor selection bias, since the vaccinees in group A were slightly older than in group B. Individuals up to 5 years of age are regarded as relatively immature, which finds its expression also in vaccination strategies (4). In order to create comparable patient groups, we stratified our study subjects as follows: patients up to 5 years of age and older than 5 years. The analysis of these data confirmed our results with the restriction of smaller group sizes (Table 5). This better immunogenicity of PCV compared to PPV beyond infancy has also been suggested elsewhere (21, 39). Induction of immunological memory after priming with a pneumococcal conjugate vaccine had previously also been shown for infants and patients with Hodgkin′s disease after treatment (9, 15). Like in our patients, a marked booster immune response was seen in individuals who initially did not respond to either polysaccharide or conjugate vaccine alone. Even in patients without detectable induction of circulating antibodies following the primary vaccination with PPV, the combined PCV-PPV schedule provided protection against pneumococcal disease. This is presumably due to the PCV-induced ability of these children to produce an anamnestic response to contact with the wild-type bacteria. We observed only moderate increases of GMCs for serotype 5, which is exclusively included in PPV-23, in both groups. This increase reached statistical significance only in the PCV-primed group. This marginal phenomenon in group A subjects might be due to more contact with wild pneumococci, since they were slightly older and immunologically more mature.
TABLE 5.
Serotype-specific IgG antibody GMCs of patients previously not responding to pneumococcal polysaccharide vaccination, stratified for agea
| PnC | GMC (μg/ml) (95% CI)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A (PCV-7 primed)
|
Group B (PPV-23 only)
|
|||||||||||
| 2-5 yr old (n = 2)
|
>5 yr old (n = 14)
|
2-5 yr old (n = 13)
|
>5 yr old (n = 4)
|
|||||||||
| Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | Day 0 | Day 7 | Day 28 | |
| 4 | 0.63b (0.40-1.00) | 3.50b (1.20-9.01) | 5.42* (1.80-9.88) | 0.77b (0.44-1.10) | 4.23* (1.90-9.40) | 7.15* (3.90-13.1) | 0.41b (0.26-0.55) | 0.69b (0.23-1.14) | 1.20* (0.57-1.84) | 0.33b (0.30-1.20) | 0.39* (0.30-0.92) | 0.62* (0.41-1.17) |
| 5 | 0.73b (0.54-1.00) | 0.91b (0.70-1.13) | 1.18b (0.85-1.51) | 0.63b (0.51-0.74) | 0.88b (0.82-0.94) | 1.75b (1.10-2.80) | 0.82b (0.48-1.15) | 1.03b (0.18-1.89) | 1.25b (0.29-2.21) | 0.53b (0.36-0.70) | 0.72b (0.57-0.87) | 1.12b (0.60-2.03) |
| 6B | 0.27b (0.05-1.50) | 2.12b (0.70-4.54) | 2.43b (0.70-4.83) | 0.72b (0.40-1.62) | 0.53b (0.05-5.60) | 1.32* (0.50-3.50) | 0.18b (0.05-0.37) | 0.22b (0.05-1.31) | 0.30b (0.05-0.63) | 0.17b (0.05-0.45) | 0.52b (0.60-0.99) | 0.40* (0.20-0.71) |
| 9V | 1.32b (0.50-3.50) | 2.21b (0.60-7.97) | 3.93* (0.90-11.2) | 0.77b (0.13-1.40) | 3.79b (1.80-8.00) | 7.43* (4.60-12.0) | 0.50b (0.05-2.90) | 0.87b (0.07-1.68) | 0.87* (0.20-2.70) | 0.28b (0.20-2.91) | 0.70b (0.40-1.20) | 0.72* (0.59-1.52) |
| 14 | 2.66b (1.20-5.90) | 10.0b (3.30-21.6) | 15.7b (3.50-33.8) | 3.64* (2.61-4.67) | 2.62* (1.20-5.70) | 3.74** (2.00-7.00) | 0.99b (0.19-1.79) | 1.32b (0.87-1.77) | 1.65b (1.17-2.12) | 1.70* (1.50-2.08) | 2.18* (2.00-2.54) | 2.10** (1.67-2.35) |
| 18C | 1.94* (1.30-2.90) | 2.27* (0.70-4.32) | 2.92b (1.28-4.57) | 0.76b (0.30-2.16) | 3.02b (2.40-3.80) | 3.03b (2.30-4.00) | 0.37* (0.11-0.64) | 0.73* (0.36-1.10) | 1.11b (0.40-2.25) | 0.45b (0.21-0.64) | 0.98b (0.80-1.78) | 1.17b (0.99-1.40) |
| 19F | 1.70b (1.70-1.70) | 7.73* (1.70-16.9) | 10.8b (1.12-20.5) | 2.40b (0.70-5.77) | 4.55b (3.70-5.60) | 9.17* (6.00-14.0) | 0.62b (0.21-1.04) | 1.50* (1.00-2.00) | 2.61b (1.30-4.43) | 1.59b (0.85-2.14) | 2.39b (1.47-3.86) | 2.60* (1.94-3.46) |
| 23F | 0.77b (0.30-2.00) | 3.22b (0.60-8.26) | 3.98b (0.80-7.79) | 0.90b (0.07-1.73) | 1.58* (0.50-5.00) | 1.67* (0.70-3.90) | 0.17b (0.05-0.33) | 0.21b (0.05-0.82) | 0.23b (0.05-1.03) | 0.17b (0.05-0.33) | 0.21* (0.05-0.70) | 0.48* (0.22-1.03) |
PnC, pneumococcal serotype; n, number of patients. *, P < 0.05; **, P < 0.01. In 2- to 5-year-old group A patients, ranges are provided instead of 95% confidence intervals.
Not significant.
All these concerns emphasize the importance of investigating not only quantitative but also qualitative aspects of pneumococcal immunity in patients for whom antipolysaccharide immunity is a problem. We could demonstrate a good correlation of pneumococcal OPA and antibody concentrations (r = 0.72). Our observations confirm data from a study performed in Filipino infants, who received an 11-valent pneumococcal conjugate vaccine. The Finish group performing this trial found comparable correlations between OPA and antibody titers (r = 0.73 for serotype 23F) (36). The majority of our patients with antibody GMCs of ≥1 μg/ml demonstrated high OPA. In some cases, there was high opsonic activity related to IgG antibody concentrations of less than 1 μg/ml, which would be considered to be a nonresponder. But since polysaccharide immunodeficiency is considered as a regulatory dysfunction of the immune system, alternative compensatory mechanisms might be more important in these patients. In our low-IgG patients, we detected a marked serotype-specific IgM immune response. In addition, there were a few other cases where antibody levels of ≥1 μg/ml were associated with low OPA. These cases may reflect individual variations of immune response and underline the importance of qualitative testing in this patient group. Other influences on the relationship between OPA and antibody levels are the IgG subclass distribution (19, 23), non-type-specific antipneumococcal antibodies (53), or other heat-stable serum opsonins (e.g., mannose-binding lectin).
In conclusion, following a PPV booster, even patients primarily not responding to solely PPV, showed a rapid and more pronounced memory response after priming with PCV. There was a good correlation between serum antibody GMCs and OPA as a functional surrogate for protection.
REFERENCES
- 1.Åhman, H., H. Käyhty, H. Lehtonen, O. Leroy, J. Froeschle, and J. Eskola. 1998. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr. Infect. Dis. J. 17:211-216. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, F., M. C. Steinhoff, M. C. Rodriguez-Barradas, R. G. Hamilton, D. M. Musher, and K. E. Nelson. 1996. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J. Infect. Dis. 173:83-90. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosino, D. M., G. R. Siber, B. A. Chilmonczyk, J. B. Jernberg, and R. W. Finberg. 1987. An immunodeficiency characterized by impaired antibody responses to polysaccharides. N. Engl. J. Med. 316:790-793. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. 2003. Pneumococcal infections, p. 492-497. In L. K. Pickering (ed.), Red book: 2003 Report of the Committee on Infectious Diseases, 26th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 4a.Anonymous. 2004. Human IgG subclass liquid reagent kits. The Binding Site Ltd., Birmingham, United Kingdom.
- 5.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. [DOI] [PubMed] [Google Scholar]
- 6.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnason, S. P., H. Jakobson, G. del Giudice, E. Trannoy, C.A. Siegrist, and I. Jonsdottir. 2005. The advantage of mucosal immunization for polysaccharide-specific memory responses in eary life. Eur. J. Immunol. 35:1037-1045. [DOI] [PubMed] [Google Scholar]
- 8.Black, S. B., H. R. Shinefield, J. Hansen, L. Elvin, D. Laufer, and F. Malinoski. 2001. Postlicensure evaluation of the effectiveness of seven valent pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 20:1105-1107. [DOI] [PubMed] [Google Scholar]
- 9.Chan, C. Y., D. C. Molrine, S. George, N. J. Tarbell, P. Mauch, L. Diller, R. C. Shamberger, N. R. Phillips, A. Goorin, and D. M. Ambrosino. 1996. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin′s disease. J. Infect. Dis. 173:256-258. [DOI] [PubMed] [Google Scholar]
- 10.Choo, S., L. Seymour, R. Morris, S. Quataert, S. Lockhart, K. Cartwright, and A. Finn. 2000. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a Haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr. Infect. Dis. J. 19:854-862. [DOI] [PubMed] [Google Scholar]
- 11.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan, R., R. Melamed, O. Zamir, and O. Leroy. 1997. Safety and immunogenicity of tetravalent pneumococcal vaccines conjugate vaccines containing 6B, 14, 19F and 23F polysaccharide conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr. Infect. Dis. J. 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 13.Dagan, R., M. Muallem, R. Melamed, O. Leroy, and P. Yagupsky. 1997. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphteria toxoid. Pediatr. Infect. Dis. J. 16:1060-1064. [DOI] [PubMed] [Google Scholar]
- 14.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greenberg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 15.Ekström, N., H. Åhman, J. Verho, J. Jokinen, M. Väkeväinen, T. Kilpi, H. Käyhty, and the Finnish Otitis Media Study Group. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PnOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskola, J., and M. Antilla. 1999. Pneumococcal conjugate vaccines. Pediatr. Infect. Dis. J. 18:543-551. [DOI] [PubMed] [Google Scholar]
- 17.Granoff, D. M., E. L. Anderson, M. T. Osterholm, S. J. Holmes, J. E. McHugh, R. B. Belshe, F. Medley, and T. V. Murphy. 1992. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J. Pediatr. 121:187-194. [DOI] [PubMed] [Google Scholar]
- 18.Jodar, L., J. Butler, G. Carlone, R. Dagan, C. Frasch, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 19.Kaniuk, A. C., J. E. Lortan, and M. A. Monteil. 1992. Specific IgG subclass antibody levels and phagocytosis of serotype 14 pneumococcus following immunization. Scand. J. Immunol. 36:96-98. [DOI] [PubMed] [Google Scholar]
- 20.Kauppi, M., J. Eskola, and H. Käyhty. 1995. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr. Infect. Dis. J. 14:286-294. [DOI] [PubMed] [Google Scholar]
- 21.King, J. C., Jr., P. E. Vink, J. J. Farley, M. Parks, M. Smilie, D. Madore, R. Lichenstein, and F. Malinoski. 1996. Comparison of the safety and immunogenicity of a pneumococcal conjugate with a licensed polysaccharide vaccine in human immunodeficiency virus and non-human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 15:192-196. [DOI] [PubMed] [Google Scholar]
- 22.King, J. C., Jr., P. E. Vink, J. J. Farley, M. Smilie, M. Parks, and R. Lichenstein. 1997. Safety and immunogenicity of three doses of a five-valent pneumococcal conjugate vaccine in children younger than two years with and without human immunodeficiency virus infection. Pediatrics 99:575-580. [DOI] [PubMed] [Google Scholar]
- 23.Lortan, J. E., A. C. Kaniuk, and M. A. Monteil. 1993. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin. Exp. Immunol. 91:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLennan, J., S. Obaro, J. Deeks, D. Lake, C. Elie, G. Carlone, E. R. Moxon, and B. Greenwood. 2001. Immunologial memory 5 years after meningococcal A/C conjugate vaccination in infancy. J. Infect. Dis. 183:97-104. [DOI] [PubMed] [Google Scholar]
- 25.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytosis assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 27.McHeyzer-Williams, M. G., and R. Ahmed. 1999. B cell memory and the long lived plasma cell. Curr. Opin. Immunol. 11:172-179. [DOI] [PubMed] [Google Scholar]
- 28.McHeyzer-Williams, M. G., D. J. Driver, and M. G. McHeyzer-Williams. 2001. Germinal center reaction. Curr. Opin. Hematol. 8:52-59. [DOI] [PubMed] [Google Scholar]
- 29.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 30.Nachman, S., S. Kim, J. King, E. J. Abrams, D. Margolis, A. Petru, W. Shearer, E. Smith, J. Moye, S. Blanchard, E. Hawkins, P. Bouquin, P. Vink, M. Benson, S. Estep, and F. Malinoski. 2003. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics 112:66-73. [DOI] [PubMed] [Google Scholar]
- 31.Obaro, S. K., R. A. Adegbola, W. A. S. Banya, and B. M. Grennwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 32.Obaro, S. K., Z. Huo, W.A. Banya, D.C. Henderson, M.A. Monteil, A. Leach, and B. M. Greenwood. 1997. A glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr. Infect. Dis. J. 16:1135-1140. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien, K. L., A. J. Swift, and J. A. Winkelstein. 2000. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 among infants with sickle cell disease. Pediatrics 106:965-972. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, K. L., M. C. Steinhoff, K. Edwards, H. Keyserling, M. L. Thoms, and D. Madore. 1996. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr. Infect. Dis. J. 15:425-430. [DOI] [PubMed] [Google Scholar]
- 35.Powers, D. C., E. L. Anderson, K. Lottenbach, and C. M. Mink. 1996. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J. Infect. Dis. 173:1014-1018. [DOI] [PubMed] [Google Scholar]
- 36.Puumalainen, T., N. Ekström, R. Zeta-Capeding, J. Ollgren, K. Jousimies, M. Lucero, H. Nohynek, and H. Käythy. 2003. Functional antibodies elicited by an 11-valent diphtheria-tetanus toxoid-conjugated pneumococcal vaccine. J. Infect. Dis. 187:1704-1708. [DOI] [PubMed] [Google Scholar]
- 37.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1988. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 38.Rijkers, G. T., L. A. Sanders, and B. J. M. Zegers. 1993. Anti-capsular polysaccharide antibody deficiency states. Immunodeficiency 5:1-21. [PubMed] [Google Scholar]
- 39.Rose, M. A., C. Hey, S. Kujumdshiev, V. Gall, R. Schubert, and S. Zielen. 2004. Immunogenicity of pneumococcal vaccination in patients with cochlear implants. J. Infect. Dis. 190:551-557. [DOI] [PubMed] [Google Scholar]
- 40.Sanders, L. A., G. T. Rijkers, W. Kuis., A. J. Tenbergen-Meekes, B. R. de Graeff-Meeder, I. Hiemstra, and B. J. Zegers. 1993. Defective anti-pneumococcal polysaccharide antibody response in children with recurrent respiratory tract infections. J. Allergy Clin. Immunol. 91:110-119. [DOI] [PubMed] [Google Scholar]
- 41.Schubert, R., J. Reichenbach, M. Rose, and S. Zielen. 2004. Immunogenicity of the seven valent pneumococcal conjugate vaccine in patients with ataxia-telangiectasia. Pediatr. Infect. Dis. J. 23:269-270. [DOI] [PubMed] [Google Scholar]
- 42.Shelly, M. A., H. Jacoby, G. J. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 44.Sigurdardottir, S. T., G. Ingolfsdottri, K. Davidsdottir, T. Gudnason, S. Kjartansson, K. G. Kristinsson, F. Bailleux, O. Leroy, and I. Jonsdottir. 2002. Immune response to octavalent diphtheria- and tetanus-conjugated pneumococcal vaccines is serotype- and carier-specific: the choice for a mixed vaccine. Pediatr. Infect. Dis. J. 21:548-554. [DOI] [PubMed] [Google Scholar]
- 45.Soininen, A., G. van den Dobbelsteen, L. Oomen, and H. Kayhty. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen, R. U., L. E. Leiva, P. A. Giangrosso, B. Butler, F. C. Javier III, D. M. Sacerdote, N. Bradford, and C. Moore. 1998. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr. Infect. Dis. J. 17:685-691. [DOI] [PubMed] [Google Scholar]
- 47.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165:49-52. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Umetsu, D. T., D. M. Ambrosino, I. Quinti, G. R. Siber, and R. S. Geha. 1985. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N. Engl. J. Med. 5:1247-1251. [DOI] [PubMed] [Google Scholar]
- 50.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, E. IJzerman, P. Hermans, R. de Groot, B. Zegers, W. Kuis, G. Rijkers, A. Schilder, and E. Sanders. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomized study. Lancet 361:2189-2195. [DOI] [PubMed] [Google Scholar]
- 51.Whitney, C. G., M. M. Farley, and J. Hadler. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. 1999. Pneumococcal vaccines. Wkly. Epidemiol. Rec. 74:177-183.10437429 [Google Scholar]
- 53.Yu, X., C. Frasch, N. Conception, and M. H. Nahm. 1996. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zepp, F., H. J. Schmitt, A. Kaufhold, A. Schuind, M. Knuf. P. Habermehl, C. Meyer, H. Bogaerts, M. Slaoui, and R. Clemens. 1997. Evidence for induction of polysaccharide specific B-cell-memory in the 1st year of life: plain Haemophilus influenzae type b-PRP (Hib) boosters in children primed with a tetanus-conjugate Hib-DTPa-HBV combined vaccine. Eur. J. Pediatr. 156:18-24. [DOI] [PubMed] [Google Scholar]
- 55.Zielen, S., I. Buhring, N. Strnad, J. Reichenbach, and D. Hofmann. 2000. Immunogenicity and tolerance of a 7-valent pneumococcal conjugate vaccine in non-responders to the 23-valent pneumococcal vaccine. Infect. Immun. 68:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zielen, S., I. Bühring, F. Ewald, P. Ahrens, and D. Hofmann. 1997. Immunoglobulin subclasses and polysaccharide specific immuno-deficiency states in patients with recurrent respiratory infections. Paediatr. Pulmonol. 16:146-147. [DOI] [PubMed] [Google Scholar]
- 57.Zielen, S., M. Broker, N. Strnad, L. Schwenen, P. Schoen, G. Gottwald, and D. Hofmann. 1996. Simple determination of polysaccharide specific antibodies by means of chemically modified ELISA-Plates. J. Immunol. Methods 193:1-7. [DOI] [PubMed] [Google Scholar]