Abstract
Murine norovirus 1 (MNV-1) is a newly recognized pathogen of mice that causes lethal infection in mice deficient in components of the innate immune response but not in wild-type 129 mice. In this study, in vitro-propagated MNV-1 was used as antigen to develop a multiplexed fluorescent immunoassay (MFI) to detect antibodies to MNV-1 in infected mice. The MNV-1 MFI was 100% specific and 100% sensitive in detecting anti-MNV-1 antibody in sera from experimentally infected mice. Testing of a large number of mouse serum samples (n = 12,639) submitted from contemporary laboratory mouse colonies in the United States and Canada revealed that 22.1% of these sera contained antibodies to MNV-1, indicating infection with MNV-1 is widespread in research mice. In addition, a reverse transcriptase PCR primer pair with a sensitivity of 25 virus copies was developed and used to demonstrate that MNV-1 RNA could be detected in the spleen, mesenteric lymph node, and jejunum from some experimentally infected mice 5 weeks postinoculation. These diagnostic assays provide the necessary tools to define the MNV-1 infection status of research mice and to aid in the establishment of laboratory mouse colonies free of MNV-1 infection.
Members of the genus Norovirus are nonenveloped viruses with a linear, positive-sense, single-stranded RNA genome (6). Noroviruses are in the family Caliciviridae, which also includes the genera Sapovirus, Lagovirus, and Vesivirus (6, 22). Formerly known as “Norwalk-like viruses” or “small round structured viruses,” noroviruses cause acute gastroenteritis in humans, typically lasting 24 to 48 h, and infect people of all ages (6). Outbreaks occur all over the world and have been reported to occur in schools, nursing homes, hospitals, restaurants, and cruise ships (1, 3, 10, 12, 18). The virus is shed in the feces and vomit and is transmitted by exposure to infected individuals or contact with contaminated foods, water, or surfaces. In addition, noroviruses have a very low infective dose of less than 100 viral particles, are persistent in the environment, and require at least 10 mg/liter of chlorine to be inactivated (4, 15, 24, 26). Noroviruses are considered to be one of the most important causes of acute nonbacterial gastroenteritis in the world and have been detected in as many as 94% of outbreaks (2, 3). In addition, noroviruses account for an estimated 33 to 67% of all food-borne cases of gastroenteritis (1, 9, 23, 28).
Recently, the first murine norovirus, murine norovirus 1 (MNV-1), was isolated from mice lacking recombination-activating gene 2 and signal transducer and activator of transcription 1 (RAG2/STAT1−/− mice) (14). Mice lacking the alpha/beta and gamma interferon receptors or STAT1 succumbed to MNV-1 infection after oral inoculation, while wild-type 129 mice were asymptomatic. STAT1−/− mice showed histopathologic signs of pneumonia, liver fibrosis, and loss of splenic architecture after 3 or 7 days postinfection. In addition, virus was detected in multiple organs, including the intestines, by quantitative real-time reverse transcriptase PCR (RT-PCR) and was shed in feces. Subsequent studies identified a tropism of MNV-1 for murine macrophages and dendritic cells, including the murine macrophage cell line RAW 264.7, which is used for propagation of MNV-1, providing the first report of in vitro cultivation of a norovirus (29).
In this report, we describe the development of a high-throughput, microsphere-based, flow cytometric serologic assay to screen mouse sera for anti-MNV-1 antibodies. Use of this assay to screen sera from mice in research colonies in North America demonstrated that infection with MNV-1 is widespread. In addition, an RT-PCR assay specific for the detection of MNV-1 in fecal and tissue samples was developed as a method for identifying MNV-1 infection in mice.
MATERIALS AND METHODS
Propagation of MNV-1.
Murine norovirus 1 (MNV-1.CW1, passage 3) was a kind gift from Herbert W. Virgin, Washington University School of Medicine (St. Louis, Mo.). MNV-1 was grown as previously described with some modifications (14). RAW 264.7 cells (TIB-71; American Type Culture Collection, Manassas, Virginia) were maintained in Dulbecco's modified Eagle's medium (DMEM) (SH30243.02; HyClone, Logan, Utah) supplemented with 10% low-endotoxin fetal bovine serum (14-501F; Cambrex, East Rutherford, N.J.), 10 mM HEPES, and 10 μg/ml of ciprofloxacin. For large virus preparations, RAW cells were cultivated in 1-liter MagnaFlex suspension spinner flasks (Wheaton Science Products, Millville, N.J.) and infected with MNV-1 when the cell count was approximately 106 cells/ml at a multiplicity of infection of 0.1. Cell viability was monitored by trypan blue exclusion assay; after 36 to 48 h, when cells displayed 90 to 100% cytopathic effect, cellular material was pelleted at 2,000 × g for 10 min at 4°C, and the clarified supernatants containing virus were frozen at −80°C or immediately concentrated and purified.
MNV-1 concentration and purification.
Viral particles were precipitated and concentrated by adding 1 M NaCl and 8% (wt/vol) polyethylene glycol 8000 (Fisher Scientific, Fair Lawn, N.J.) to clarified supernatants, followed by slow stirring overnight at 4°C. The precipitated material was pelleted by centrifugation at 10,000 × g for 15 min at 4°C and resuspended in phosphate-buffered saline (PBS) and 1% Nonidet P-40 substitute ([Octylphenoxy]polyethoxyethanol; USB Corp., Cleveland, Ohio) to 1/20 the original volume. The virus was purified using a 10% to 40% cesium chloride gradient and centrifugation at 26,500 rpm in an SW28 rotor (Beckman Coulter, Inc., Fullerton, Calif.) for 4 h at 4°C in an ultracentrifuge (Beckman Coulter, Inc.). Following centrifugation, a blue band was visualized at a density of 1.30 g/cm3 and harvested. The recovered virus was dialyzed through a 10,000-Da-cutoff membrane (Pierce Biotechnology, Inc., Rockford, Ill.) overnight at 4°C in 4 liters of PBS with stirring and multiple changes of PBS. Purified virus in PBS was aliquoted and stored at −80°C until use. Protein concentrations were determined by a bicinchoninic acid procedure (11).
Plaque assay.
The plaque assay was adapted from one previously described (Bac-N-Blue transfection kit; Invitrogen, Carlsbad, Calif.). Briefly, RAW 264.7 cells were seeded onto 60-mm-diameter tissue culture plates at a density of 5 × 106 cells and allowed to adhere for 5 h at 37°C. Tenfold serial dilutions of MNV-1 were made on ice using supplemented DMEM and inoculated in duplicate onto 60-mm-diameter plates containing a confluent layer of RAW cells. After incubation for 1 h at 37°C, the inoculum was aspirated and replaced with a mixture of one part 2.5% SeaPlaque agarose and three parts supplemented DMEM, allowed to solidify, and incubated at 37°C for 24 to 48 h until plaques were visible. For better visualization of the plaques, neutral red (0.01% final concentration) was added to 3 ml of the mixture containing 2.5% SeaPlaque agarose and supplemented DMEM, allowed to solidify, and incubated at 37°C for 6 to 8 h.
Multiplexed fluorescent immunoassay (MFI).
Cesium chloride-purified MNV-1 was covalently coupled to 5 × 106 carboxylated polystyrene microspheres (Luminex Corp., Austin, Texas) at the optimal coupling concentration of 4 μg of protein per million microspheres (previously determined, data not shown) according to manufacturer's recommended protocols, except that protein coupling to the microsphere was performed in a final volume of 1 ml of 50 mM MES coupling buffer [2-(N-morpholino)ethanesulfonic acid; Sigma-Aldrich, St. Louis, Missouri]. Microspheres were stored at 4°C in the dark until use.
Testing of mouse serum samples for anti-MNV-1 antibody was done using the LiquiChip Workstation (QIAGEN Inc., Valencia, Calif.). First, microspheres coupled to MNV-1 proteins were resuspended by vortexing and sonication. Multiscreen 96-well filter-bottom plates (MABV N12 50; Millipore Corp., Bedford, Mass.) were prewetted by adding 100 μl of PBS-BSA (PBS, 1% bovine serum albumin, 0.05% sodium azide; Sigma-Aldrich) followed by removal of the liquid using a plate vacuum manifold. Approximately 2,500 microspheres in PBS-BSA and test serum at a final dilution of 1:500 in a total volume of 100 μl were added to each well. Plates were covered and incubated for 90 min on an orbital shaker at 400 rpm at room temperature in the dark. The fluid in the wells was removed using the plate vacuum manifold. Each well was washed twice by adding 100 μl of PBS-BSA, shaking at 900 rpm for 1 min, and then removing the fluid with the vacuum manifold. The microspheres were resuspended in 100 μl of PBS-BSA, and 10 μl of a 1:20 diluted F(ab′)2 fragment goat anti-mouse immunoglobulin G (IgG) (heavy plus light chains [H+L]) phycoerythrin-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was added to each well. The plate was covered and incubated for 90 min on an orbital shaker at 400 rpm at room temperature in the dark. Plates were washed twice as described above, and microspheres were resuspended in 100 μl of fresh PBS-BSA and analyzed on the LiquiChip Workstation that reported the median fluorescent intensity of 100 MNV-1-coated microspheres.
ROC curve.
Test results of serum samples from 189 uninfected or naturally MNV-1-infected mice were used to plot a receiver operating characteristic (ROC) curve (7, 8). The ROC curve plots 1 − specificity on the x axis and sensitivity on the y axis, which were calculated, as the MNV-1 MFI threshold values varied, by using the MNV-1 IFA test result as the reference standard to detect the presence of anti-MNV-1 antibody. The area under the ROC curve represents the accuracy of the diagnostic test, with an area of 1.0 corresponding to a perfect test (100% sensitivity and 100% specificity) and an area of 0.5 representing a test with no diagnostic predictability. Based on the sensitivity and specificity calculations made to plot the ROC curve from the 189 mouse serum samples, the MFI fluorescence value corresponding to a 95% test sensitivity and the MFI fluorescence value corresponding to a 95% test specificity were used as the threshold values to discriminate negative, intermediate, or positive test results in all subsequent serum samples tested by the MNV-1 MFI.
Indirect fluorescent antibody (IFA) assay.
RAW 264.7 cells in suspension culture were infected with MNV-1 as described above and incubated at 37°C for 24 h, at which time there was approximately 20% cytopathic effect as indicated by the trypan blue exclusion assay. Infected cells were mixed with equal numbers of uninfected RAW cells, and 10 μl of this mixture was spotted onto each spot of 12-well Teflon printed slides (Erie Scientific Co., Portsmouth, New Hampshire). Slides were acetone fixed at 4°C for 10 min and stored at −80°C until use. For evaluation of mouse serum samples, 20 μl of test serum diluted 1:5 in PBS was added to each well and incubated at room temperature for 15 min. Slides were then washed and incubated for 15 min in the dark with 20 μl of goat anti-mouse IgG (H+L) fluorescein isothiocyanate-labeled secondary antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Maryland) diluted 1:25 in PBS containing 1% Evan's blue dye. Evidence of specific fluorescence was evaluated using a 480-nm wavelength UV light microscope. Samples were read by three individuals experienced in evaluating IFA assay slides and classified as positive, negative, or having nonspecific fluorescence. If a discrepancy arose, slides were reevaluated by the same individuals, and a consensus determination was made.
Western blot analysis.
Separation and transfer of cesium chloride-purified MNV-1 proteins were done using the XCell SureLock Mini-Cell system (Invitrogen, Carlsbad, Calif.) according to manufacturer's recommended protocol. Proteins were separated using NuPAGE Novex 4 to 12% Bis-Tris gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore Corp., Bedford, Mass.). Membranes were blocked with 5% nonfat dry milk in PBS and probed with mouse serum samples diluted 1:50 in 5% nonfat dry milk in PBS for 1 h at room temperature. Membranes were then incubated with a 1:1,000 dilution of biotinylated donkey anti-mouse IgG (H+L) secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) in 5% nonfat dry milk in PBS for 1 h at room temperature and then with avidin DH and biotinylated horseradish peroxidase (Vector Laboratories, Inc., Burlingame, Calif.) for 30 min before development with TMB (3,3′,5,5′-tetramethylbenzidine; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Maryland).
RNA extraction and RT-PCR.
RNA was extracted from cesium chloride-purified MNV-1 using a QIAamp viral RNA Mini kit (QIAGEN Inc., Valencia, Calif.) according to manufacturer's recommended protocol. RNA was extracted from mouse fecal and tissue samples using the MagAttract RNA tissue Mini M48 kit (QIAGEN Inc.) according to manufacturer's recommended protocol. Briefly, one fecal pellet or approximately 20 mg of tissue was disrupted and homogenized in buffer RLT using 5-mm stainless steel balls and a TissueLyser (QIAGEN Inc.). Fecal samples were disrupted and homogenized at 30 Hz for 10 s and tissue samples at 20 Hz for 2 min twice. Fecal and tissue lysates were centrifuged for 5 min at 300 × g and for 3 min at 13,000 × g, respectively, and the supernatants were used for magnetic silica-based RNA purification on the BioRobot M48 Workstation (QIAGEN Inc.). RNA concentration and purity were determined by measuring the A260 and A280 with a spectrophotometer.
MNV-1-specific RT-PCR primers (MNV Polymerase 5 Forward, 5′-TCTTCGCAAGACACGCCAATTTCAG-3′; MNV Polymerase 5 Reverse, 5′-GCATCACAATGTCAGGGTCAACTC-3′) targeting the polymerase gene of MNV-1 (GenBank accession no. AY228235) were designed using the Discover Studio Gene software package (Accelrys, San Diego, Calif.). This primer pair produces a 318-bp product from nucleotide positions 4228 to 4545 after reverse transcription and PCR amplification of MNV-1 RNA. RT-PCR was performed in duplicate according to manufacturer's recommended protocols using the OneStep RT-PCR kit with Q-Solution (QIAGEN Inc.) and a 0.6 μM concentration of each primer. Reverse transcription was done at 50°C for 30 min followed by 1 cycle of 95°C for 15 min to activate the DNA polymerase, 40 cycles of denaturation (94°C, 1 min), annealing (63°C, 1 min), and extension (72°C, 1 min), and a final extension at 72°C for 10 min. PCR products were evaluated on precast 3% agarose gels containing ethidium bromide (Bio-Rad Laboratories, Hercules, Calif.) and visualized with UV light.
Animals and sample collection.
To evaluate anti-MNV-1 antibody production following experimental exposure to MNV-1, 4-week-old female Hsd:ICR(CD-1) mice (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) that were documented to be free of ectoparasites, endoparasites, Mycoplasma pulmonis, Helicobacter sp., known enteric and respiratory bacterial pathogens, antibodies to mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reovirus 3, Theiler's murine encephalomyelitis virus, ectromelia virus, polyoma virus, lymphocytic choriomeningitis virus, mouse adenovirus, mice minute virus, mouse parvovirus, mouse rotavirus, mouse cytomegalovirus, mouse thymic virus, Encephalitozoon cuniculi, and Clostridium piliforme were obtained.
Baseline serum samples collected from the saphenous veins of all mice before experimental inoculation were negative for anti-MNV-1 antibody. Control mice were sham inoculated with uninfected RAW 264.7 cell lysates by oral gavage (n = 5), and experimental mice were inoculated with 107 PFU of MNV-1-infected RAW cell lysates by oral gavage (n = 10). Mice were group housed by MNV-1 infection status in microisolation cages with filter tops and provided autoclaved acidified water and standard irradiated rodent chow ad libitum. Fresh fecal samples were collected daily on days 0 to 7 postinoculation (p.i.), and blood samples were collected from all mice weekly. All manipulations, including basic husbandry, were done in a class II biological safety cabinet. At 5 weeks p.i., mice were euthanized with an overdose of carbon dioxide. Blood was collected by cardiocentesis, and the serum was separated, diluted 1:5 with PBS, and stored at −20°C. The mesenteric lymph node, sections of the spleen and jejunum, and a fecal pellet from the descending colon were collected aseptically, immediately placed in liquid nitrogen, and stored at −80°C pending RNA extraction. The animal studies were approved by the University of Missouri's Animal Care and Use Committee.
To evaluate the anti-MNV-1 antibody status in mice naturally exposed to MNV-1, serum samples from various strains of immunocompetent mice from a variety of sources submitted to the University of Missouri Research Animal Diagnostic Laboratory (Columbia, Mo.) were tested by the MNV-1 MFI. To determine the threshold fluorescence values to designate a negative, intermediate, or positive test result when evaluated by the MFI, sera from 121 mice positive for anti-MNV-1 antibodies by MNV-1 IFA assay and sera from 68 mice negative for anti-MNV-1 antibodies by MNV-1 IFA assay were used as reference standards. Blood was obtained by cardiocentesis from euthanized mice, and the serum was collected, diluted 1:5 with PBS, and stored at −20°C.
Electron microscopy.
A 200-μl aliquot of cesium chloride-purified MNV-1 was diluted in PBS and centrifuged at 40,000 rpm for 1 h in an SW41Ti rotor (Beckman Coulter, Inc.) at 5°C to pellet the virus. The pellet was resuspended in 100 μl of chilled 1% ammonium acetate overnight at 4°C. A 10-μl drop was placed onto 300-mesh carbon-coated copper grids and allowed to settle for 10 min. Excess solution was wicked off, and a drop of 1% uranyl acetate (aqueous) was added and stained for 10 min. The grids were viewed at 100 kV accelerating voltage in a JEOL 1200EX transmission electron microscope.
Statistical analysis.
MFI values from mice that seroconverted in the experimentally inoculated group were compared to those from the sham-inoculated control group using the Mann-Whitney rank sum test (SigmaStat 3.1; Systat Software, Inc., Point Richmond, Calif.). A P value of less than 0.05 was considered significant. To evaluate the MNV-1 MFI, a receiver operating characteristic curve was generated using Analyze-It statistical software version 1.71 (Analyze-It Software, Ltd., England) with Microsoft Excel 2003 (Microsoft Corp., Redmond, Wash.).
RESULTS
MNV-1 production in suspension culture flasks.
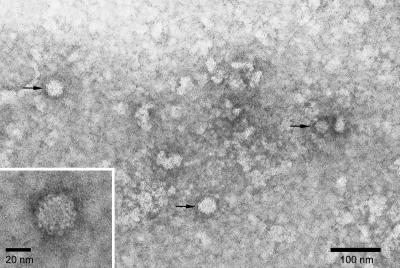
Cesium chloride purification of MNV-1 from 1-liter suspension cultures of RAW 264.7 cells infected with MNV-1 yielded 3.2 mg of purified MNV-1 protein. Electron micrographs of this purified preparation indicated the sample contained MNV-1 virions with minimal contaminating material (Fig. 1). Viral particles were approximately 30 nm in diameter, similar to that previously reported for MNV-1 (14).
FIG. 1.
Transmission electron micrograph of negatively stained murine norovirus 1 particles (arrows) from cesium chloride-purified supernatants of infected RAW 264.7 cells grown in suspension culture.
Evaluation of MFI to detect anti-MNV-1 antibody.
Using the MNV-1 IFA assay as the reference standard, a receiver operating characteristic curve was generated by plotting the false positive rate (1 − specificity) on the x axis and the true positive rate (sensitivity) on the y axis as the threshold values of the MNV-1 MFI varied to obtain an area under the curve of 0.905 (95% confidence interval, 0.858 to 0.953), indicating that test performance is highly accurate (7). Based on the sensitivity and specificity calculated at various threshold values used to generate the curve, an MFI value of 175 corresponded to a 95% test sensitivity (69% specificity), while an MFI value of 600 corresponded to a 95% test specificity (39% sensitivity). To achieve maximum overall test performance, the following testing paradigm was established: sera with MFI values of <175 were classified as negative for anti-MNV-1 antibodies, sera with MFI values from 175 to 600 were classified as intermediate and possibly containing anti-MNV-1 antibodies, and sera with MFI values of >600 were classified as positive for anti-MNV-1 antibodies. To determine the anti-MNV-1 antibody status of sera in the intermediate range (MFI values from 175 to 600), these serum samples were tested by the MNV-1 IFA assay, and the outcome of this test was used to classify sera as positive or negative.
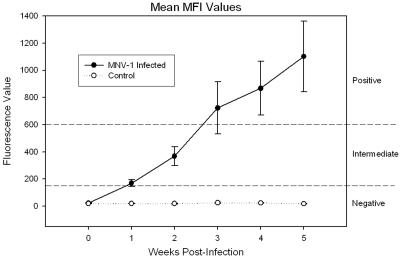
To evaluate the performance of the MNV-1 MFI and the kinetics of seroconversion of mice following oral exposure to MNV-1, sera from experimentally inoculated mice and sham-inoculated control mice were evaluated by the MNV-1 MFI and IFA assay. At 1 week p.i., 2 of 10 (20%) MNV-1-inoculated mice were in the intermediate fluorescence range by the MFI. IFA assays of these two samples were positive for anti-MNV-1 antibody. The remaining eight serum samples tested negative by the MFI, but two of these samples were positive by the IFA assay. However, these two serum samples had MFI fluorescence values of 165.5 and 158.5, just under the threshold value of 175 for an intermediate result. At 2 weeks p.i. and for the remainder of the study, 7 of 10 (70%) MNV-1-inoculated mice were in the intermediate or positive range by the MFI and were positive by the IFA assay. There was a steady rise in the MNV-1 MFI mean fluorescence values over time in these seven mice (Fig. 2) until the end of the experiment, when the mean fluorescence value ± standard deviation at 5 weeks p.i. was 1,101.9 ± 684.6. By 5 weeks p.i., five of these seven samples had reached MFI values in the positive range, while two were in the intermediate range. The remaining 3 of 10 (30%) mice orally inoculated with MNV-1 tested negative for anti-MNV-1 antibody by the MFI and IFA assay during the entire experiment and had MFI fluorescence values of 12.0, 15.0, and 43.0 at week 5 p.i. These three serum samples were also negative for anti-MNV-1 antibodies by Western blot assays at 5 weeks p.i. Collectively, these data suggest that these three mice failed to be infected with MNV-1 rather than that the serologic assays failed to detect anti-MNV-1 antibody. In comparison to mice that seroconverted, serum samples from control mice inoculated with uninfected RAW cell lysates were negative by the MNV-1 IFA assay and MNV-1 MFI at all time points and had a mean MFI fluorescence value ± standard deviation of 17.7 ± 4.1 at 5 weeks p.i., which was statistically significant when compared to the values from the seven mice that seroconverted (P < 0.05).
FIG. 2.
The mean MNV-1 MFI fluorescence values of sera collected weekly from mice experimentally inoculated with MNV-1 by oral gavage that seroconverted (n = 7) and sham-inoculated control mice (n = 5). Mice inoculated with MNV-1 showed a steady increase in MFI fluorescence values over time. An MFI fluorescence value of 175 corresponds to a 95% test sensitivity, while an MFI fluorescence value of 600 corresponds to a 95% test specificity (dashed lines). These fluorescence values were used as thresholds so that MFI fluorescence values <175 were considered negative for anti-MNV-1 antibody, values from 175 to 600 were considered intermediate and possibly containing anti-MNV-1 antibody, and values >600 were considered positive for anti-MNV-1 antibody. Error bars represent the standard errors of the mean.
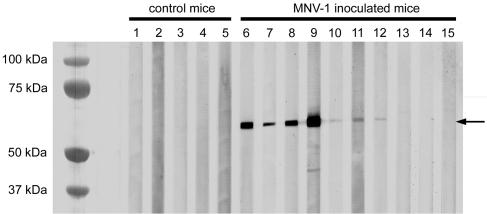
Western blot analysis of the seven serum samples that contained anti-MNV-1 antibody at week 5 p.i. determined by the MFI and IFA assay exhibited a 59-kDa capsid protein as previously described for MNV-1 in all seven of these serum samples (Fig. 3) (29). Western blot analyses of serum samples from control mice at 5 weeks p.i. were negative.
FIG. 3.
Western blot analysis of serum samples from mice 5 weeks after oral inoculation with MNV-1-infected lysates (lanes 6 to 15) or control mice sham inoculated with uninfected RAW 264.7 lysates (lanes 1 to 5). Samples containing anti-MNV-1 antibody (lanes 6 to 12) displayed a band approximately 59 kDa in size (arrow) representing the capsid protein. Control mice and the three mice that did not seroconvert after oral inoculation with MNV-1 (lanes 13 to 15) did not display any specific bands by Western blot analysis.
Prevalence of MNV-1 infection in research mice.
To obtain an estimate of the seroprevalence of MNV-1 in research mice, mouse serum samples submitted to the Research Animal Diagnostic Laboratory at the University of Missouri (Columbia, Mo.) from research facilities in the United States and Canada were evaluated for anti-MNV-1 antibody by the MNV-1 MFI. Serum samples that had an MFI value of >600, corresponding to a test specificity of 95%, were classified as positive for previous infection with MNV-1. Of the 12,639 serum samples tested by the MNV-1 MFI, 2,791 (22.1%) were positive for anti-MNV-1 antibody, indicating widespread exposure to MNV-1 in research mouse colonies.
Sensitivity and specificity of MNV-1 RT-PCR assay.
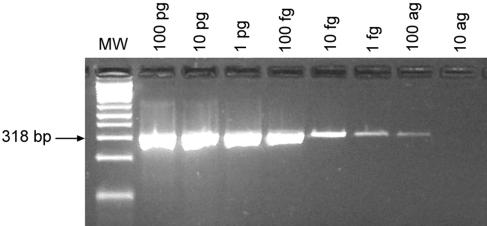
To test the sensitivity of the MNV-1 RT-PCR assay, RNA was extracted from cesium chloride-purified MNV-1-infected cell cultures, and RNA concentrations were determined by a spectrophotometer. Log-fold serial dilutions from 100 pg to 10 ag of MNV-1 RNA were made and tested using the MNV-1 RT-PCR assay. A specific PCR product was detectable in the sample containing 100 ag of starting RNA (Fig. 4), equivalent to a copy number of 25 virus particles assuming the purified RNA contained 100% MNV-1 RNA. The specificity of the MNV-1 RT-PCR assay was tested by using RNA extracted from uninfected RAW 264.7 cells and fecal and tissue samples from uninfected sham-inoculated control mice. No specific or nonspecific PCR products were detected from uninfected samples (data not shown). In addition, a nucleotide BLAST search of each primer did not reveal any significant nucleotide homology to non-MNV-1 sequences.
FIG. 4.
Ethidium bromide-stained agarose gel of MNV-1 RT-PCR products of log-fold serial dilutions of RNA extracted from cesium chloride-purified MNV-1-infected cell cultures. A 318-bp PCR product was generated with as little as 100 ag of RNA, representative of approximately 25 virus copies based on the estimated viral genome molecular mass of 2,366,090 Da per virion.
Detection of MNV-1 RNA in experimental samples.
Fecal samples were collected daily for 7 days p.i. from mice that were given MNV-1 by oral gavage and at necropsy 5 weeks p.i. MNV-1 RNA could be detected in the feces of some mice at all time points from day 0 to day 7 p.i. (Table 1), while fecal samples collected at 5 weeks p.i. were uniformly negative for MNV-1 RNA (Table 2). The three mice that were orally inoculated with MNV-1 but that did not seroconvert had detectable levels of MNV-1 RNA in their feces for only the first few days p.i., most likely due to the passing through of the inoculum dose. MNV-1 RNA was detected in the mesenteric lymph node, the spleen, and the jejunum of some experimentally inoculated mice by RT-PCR at 5 weeks p.i. (Table 2). All uninfected control mouse samples remained negative for MNV-1 by RT-PCR throughout the duration of the study.
TABLE 1.
MNV-1 detection by RT-PCR in fecal pellets collected from day 0 to day 7 postinoculation from mice inoculated with MNV-1 by oral gavage and sham-inoculated mice
| MNV-1 status | No. positive detected by PCR
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Uninfected (n = 5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MNV-1 inoculated (n = 10) | 0 | 10 | 9 | 7 | 2 | 4 | 3 | 2 |
TABLE 2.
MNV-1 detection by RT-PCR in tissue and fecal samples collected at 5 weeks postinoculation from mice inoculated with MNV-1 by oral gavage and sham-inoculated mice
| MNV-1 status | No. positive detected by PCR
|
|||
|---|---|---|---|---|
| MLNa | Spleen | Jejunum | Feces | |
| Uninfected (n = 5) | 0 | 0 | 0 | 0 |
| MNV-1 inoculated (n = 10) | 3 | 1 | 1 | 0 |
MLN, mesenteric lymph node.
DISCUSSION
Murine norovirus 1 is a newly recognized pathogen of mice and is the first norovirus to be adapted to grow in cell culture (14, 29). To date, disease in mice has been described only for mice lacking components of the innate immune system, namely, STAT1 and interferon receptors. In contrast, MNV-1-infected wild-type 129 mice do not show any clinical symptoms of disease or any tissue pathology after oral inoculation (14). High-throughput, sensitive, and specific diagnostic assays are needed to identify infected mice. These assays will aid in estimating the prevalence of MNV-1 in research mouse colonies and will serve as tools for MNV-1 research studies.
The multiplexed fluorescent immunoassay was developed as a high-throughput, automated screening test over the traditional enzyme-linked immunosorbent assay (ELISA) because this assay has been shown to be as sensitive if not more sensitive than ELISA (17, 27). An additional advantage of the MFI over the traditional ELISA is that up to 100 different analytes can be multiplexed in one reaction well (17), compared to an ELISA, where usually only 1 analyte per well is screened. This multiplexing is the basis for the high-throughput capabilities of this diagnostic assay. Simultaneous serodetection of 10 mouse pathogens has recently been reported using this immunoassay format (16), and our laboratory currently uses this methodology to test for antibodies to 22 different mouse pathogens, including MNV-1. The MFI format also requires much less serum than an ELISA, since multiple agents can be screened using one aliquot of serum. For example, only 0.2 μl of undiluted mouse serum is needed in the MFI format to test for up to 100 different agents, while a single-agent ELISA would require approximately 2 μl of undiluted serum per agent tested. This is a significant advantage, since only a small amount of serum can be obtained from a mouse, and allows for the capability to perform comprehensive serologic profiles from a survival blood collection from a mouse.
Based on this diagnostic approach, we were able to develop a high-throughput screening test to detect anti-MNV-1 antibodies in mice. In order to determine the MNV-1 MFI threshold fluorescence values to classify sera as positive or negative for containing anti-MNV-1 antibodies, a receiver operating characteristic curve was generated comparing the MNV-1 MFI fluorescence values to MNV-1 seroreactivity determined by the MNV-1 IFA test (7, 8). This curve is a plot of the false positive rate (1 − specificity) on the x axis and the true positive rate (sensitivity) on the y axis, which are calculated as the threshold values vary. The area under the curve represents the accuracy of the diagnostic test, with an area of 1.0 corresponding to a perfect test (100% sensitivity and 100% specificity) and an area of 0.5 representing a test with no diagnostic predictability. The area under the curve for the MNV-1 MFI was 0.905, indicating an excellent or very accurate test. Calculating the sensitivity and specificity of the MNV-1 MFI at various fluorescence threshold values indicated that the assay was 95% sensitive at a threshold value of 175 and was 95% specific at a threshold value of 600. To achieve maximum test performance, a testing paradigm similar to one proposed previously (8) was developed. MNV-1 MFI fluorescence values less than 175 were classified as negative for anti-MNV-1 antibody, while MFI values greater than 600 were classified as positive for anti-MNV-1 antibody. Sera with MFI values from 175 to 600 were classified as intermediate and possibly containing anti-MNV-1 antibodies. To discern the MNV-1 reactivity of these intermediate sera, they were further tested by the MNV-1 IFA assay, and the result of this test was used for final classification as positive or negative. This testing paradigm has the advantage of providing high-throughput results with a 95% sensitivity or a 95% specificity for fluorescence values below 175 or above 600, respectively, using just the MNV-1 MFI, while using the more labor-intensive IFA assay to characterize the intermediate MFI fluorescence values. Sera from naturally infected research mice were used to develop the threshold values of this testing paradigm, since this population is more representative of the target test population than mice with experimental infections (8). As with any diagnostic assay, there is a possibility of false positive reactions, such as from cross-reactivity of the MNV-1 MFI with antibodies to other mouse pathogens. However, as part of the validation of the MNV-1 MFI, serum samples from mice infected with other known murine pathogens were tested and were consistently negative (data not shown).
In mice experimentally infected with MNV-1, the sensitivity and specificity for detecting anti-MNV-1 antibody based on this testing paradigm were both 100% in mice at 5 weeks p.i. Samples from seven of ten (70%) mice orally inoculated with MNV-1 were correctly identified as positive samples containing anti-MNV-1 antibody by either a positive MFI fluorescence value (which was also confirmed by IFA) or an intermediate MFI fluorescence value and a positive IFA test. In contrast, the remaining three samples from experimentally inoculated mice plus the five control mice which did not contain anti-MNV-1 antibody were correctly classified as negative by both the MFI and IFA assay. These mice were negative by all testing formats, including the MFI, IFA assay, Western blot assay, and PCR of feces and tissues, providing additional support that they did not seroconvert or have any virus present beyond the inoculum dose. We speculate that the three mice failed to seroconvert after oral inoculation with MNV-1 because the MNV-1 used for inoculation was a tissue culture-adapted strain and the infectivity was possibly reduced. In support of this explanation, the original virus was a third-passage plaque-purified strain of MNV-1 that was previously reported to have an attenuated virulence in vivo after serial passage in cell culture (29). Alternatively, the infection simply was not successful after oral inoculation, with the dose, dose frequency, route of inoculation, or immune status of the mouse all potentially playing a role in preventing development of an antibody response. Challenge studies with human volunteers likewise display an incomplete seroconversion rate of all subjects after experimental norovirus infection (13, 19, 20).
A large number of mouse serum samples submitted to the Research Animal Diagnostic Laboratory at the University of Missouri (Columbia, Mo.) from research institutions throughout the United States and Canada were screened by MFI for anti-MNV-1 antibody to estimate the prevalence of MNV-1 infection in research mice. Data collected revealed that 2,791 (22.1%) of 12,639 serum samples were positive for anti-MNV-1 antibody, indicating that MNV-1 infection is widespread in research mouse populations. A previous report of infectious agents in mice by our diagnostic laboratory identified Helicobacter as the most prevalent pathogen in mice, with 32% of all mouse fecal samples testing positive by PCR, while mouse parvovirus (MPV) was the most prevalent viral pathogen in mice, with approximately 2.5% of serum samples containing anti-MPV antibodies (21). Therefore, MNV-1 is among the most prevalent pathogens, and likely the most prevalent known viral pathogen, in mice used for research. It is not surprising that MNV-1 infection rates are so high, since this viral pathogen is newly recognized, clinical signs cannot be used to determine infection status, and practices for exclusion or elimination of this pathogen from research mouse colonies by testing and control measures are only beginning.
In experimental mice orally inoculated with MNV-1, fecal shedding and persistence of the virus in tissues were variable. MNV-1 RNA was detected in the feces by RT-PCR out to day 7 p.i. in 2 of 10 mice given MNV-1 by oral gavage. However, as feces were not evaluated again for virus until 5 weeks p.i., at which point all fecal samples were negative for MNV-1, the endpoint of fecal shedding of MNV-1 was not determined. This duration of viral shedding in the feces of mice is not inconsistent with fecal shedding data in human norovirus infections, as people may shed virus for up to 2 weeks p.i. (5, 25). MNV-1 RNA was detected in the mesenteric lymph node, spleen, and jejunum in some mice even after 5 weeks p.i. These data are in contrast to results obtained previously in which wild-type 129 mice infected orally with MNV-1 did not have any detectable viral RNA by day 3 p.i. in the feces, spleen, or intestines (14). The following two reasons could account for these differences: (i) the immunologic responses to MNV-1 may be different in outbred ICR mice and inbred 129 mice or (ii) we used a high viral inoculation dose of 1 × 107 PFU per mouse while the previous study inoculated mice with 3 × 104 PFU. Although the mechanisms of MNV-1 persistence in tissues are unknown, further studies are planned to evaluate the different immunologic responses from various stocks and strains of mice to MNV-1, the duration of fecal shedding from these various mice, and the presence of virus in different tissues at multiple time points postinoculation.
The MNV-1 seroreactivity data reported here suggest that murine norovirus 1, a recently discovered pathogen in mice, is present in a high percentage of contemporary research mice. Currently, only limited information is known about the effects that MNV-1 has on the mouse immune system and what impact these effects, or any other yet to be discovered effects, have on research. To assist further research on MNV-1, we developed an MNV-1 microsphere-based multiplexed fluorescent immunoassay to use as a high-throughput, sensitive, and specific serologic assay. In addition, we validated an MNV-1 IFA and Western blot assay to be used as confirmatory tests for the MFI screening assay. Finally, we developed a very sensitive and specific RT-PCR primer set to detect MNV-1 in tissues and feces. These assays will provide the diagnostic tools necessary to aid in the detection of MNV-1 infection in mice, enabling further research studies with MNV-1 and facilitating establishment of laboratory or research mouse colonies free of MNV-1.
Acknowledgments
We thank Herbert W. Virgin from Washington University for providing murine norovirus 1, Greg Purdy for cell culture and virus purification, Randy Tindall and the Electron Microscopy Core Facility at the University of Missouri—Columbia for electron microscopy, and John Critser and Steve Mullen for ROC curve analysis.
This work was supported by funds from the National Institutes of Health Postdoctoral Training in Comparative Medicine grant T32-RR07004, the Mutant Mouse Resource and Research Center grant 2 U42 RR014821, the National Institute of Allergy and Infectious Diseases Midwest Regional Center for Excellence U54 AI057160, and the Research Animal Diagnostic Laboratory (RADIL).
REFERENCES
- 1.Deneen, V. C., J. M. Hunt, C. R. Paule, R. I. James, R. G. Johnson, M. J. Raymond, and C. W. Hedberg. 2000. The impact of foodborne calicivirus disease: the Minnesota experience. J. Infect. Dis. 181(Suppl. 2):S281-S283. [DOI] [PubMed] [Google Scholar]
- 2.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 3.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 4.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 5.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34-43. [DOI] [PubMed] [Google Scholar]
- 6.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 7.Greiner, M., D. Pfeiffer, and R. D. Smith. 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 45:23-41. [DOI] [PubMed] [Google Scholar]
- 8.Greiner, M., D. Sohr, and P. Gobel. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185:123-132. [DOI] [PubMed] [Google Scholar]
- 9.Hall, J. A., J. S. Goulding, N. H. Bean, R. V. Tauxe, and C. W. Hedberg. 2001. Epidemiologic profiling: evaluating foodborne outbreaks for which no pathogen was isolated by routine laboratory testing: United States, 1982-9. Epidemiol. Infect. 127:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedlund, K. O., E. Rubilar-Abreu, and L. Svensson. 2000. Epidemiology of calicivirus infections in Sweden, 1994-1998. J. Infect. Dis. 181(Suppl. 2):S275-S280. [DOI] [PubMed] [Google Scholar]
- 11.Hinson, D. L., and R. J. Webber. 1988. Miniaturization of the BCA protein assay. BioTechniques 6:14, 16, 19. [PubMed] [Google Scholar]
- 12.Inouye, S., K. Yamashita, S. Yamadera, M. Yoshikawa, N. Kato, and N. Okabe. 2000. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J. Infect. Dis. 181(Suppl. 2):S270-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, P. C., J. J. Mathewson, H. L. DuPont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 161:18-21. [DOI] [PubMed] [Google Scholar]
- 14.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 15.Keswick, B. H., T. K. Satterwhite, P. C. Johnson, H. L. DuPont, S. L. Secor, J. A. Bitsura, G. W. Gary, and J. C. Hoff. 1985. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 50:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan, I. H., L. V. Kendall, M. Ziman, S. Wong, S. Mendoza, J. Fahey, S. M. Griffey, S. W. Barthold, and P. A. Luciw. 2005. Simultaneous serodetection of 10 highly prevalent mouse infectious pathogens in a single reaction by multiplex analysis. Clin. Diagn. Lab. Immunol. 12:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan, S. S., M. S. Smith, D. Reda, A. F. Suffredini, and J. P. McCoy, Jr. 2004. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin. Cytom. 61:35-39. [DOI] [PubMed] [Google Scholar]
- 18.Koopmans, M., J. Vinje, M. de Wit, I. Leenen, W. van der Poel, and Y. van Duynhoven. 2000. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J. Infect. Dis. 181(Suppl. 2):S262-S269. [DOI] [PubMed] [Google Scholar]
- 19.Lindesmith, L., C. Moe, J. Lependu, J. A. Frelinger, J. Treanor, and R. S. Baric. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 79:2900-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 21.Livingston, R. S., and L. K. Riley. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab. Anim. 32:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147:1655-1663. [DOI] [PubMed] [Google Scholar]
- 23.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moe, C., D. Rhodes, S. Pusek, F. Tseng, W. Heizer, C. Kapoor, B. Gilliam, M. Harb, P. Stewart, S. Miller, M. Sobsey, J. Herrmann, N. Blacklow, and R. Calderon. 1998. Presented at the 98th Annual Meeting of the American Society for Microbiology, Atlanta, Ga., May 1998.
- 25.Okhuysen, P. C., X. Jiang, L. Ye, P. C. Johnson, and M. K. Estes. 1995. Viral shedding and fecal IgA response after Norwalk virus infection. J. Infect. Dis. 171:566-569. [DOI] [PubMed] [Google Scholar]
- 26.Parashar, U., E. S. Quiroz, A. W. Mounts, S. S. Monroe, R. L. Fankhauser, T. Ando, J. S. Noel, S. N. Bulens, S. R. Beard, J. F. Li, J. S. Bresee, and R. I. Glass. 2001. “Norwalk-like viruses”. Public health consequences and outbreak management. Morb. Mortal. Wkly. Rep. Recomm. Rep. 50:1-17. [PubMed] [Google Scholar]
- 27.Rao, R. S., S. R. Visuri, M. T. McBride, J. S. Albala, D. L. Matthews, and M. A. Coleman. 2004. Comparison of multiplexed techniques for detection of bacterial and viral proteins. J. Proteome Res. 3:736-742. [DOI] [PubMed] [Google Scholar]
- 28.Widdowson, M. A., A. Sulka, S. N. Bulens, R. S. Beard, S. S. Chaves, R. Hammond, E. D. Salehi, E. Swanson, J. Totaro, R. Woron, P. S. Mead, J. S. Bresee, S. S. Monroe, and R. I. Glass. 2005. Norovirus and foodborne disease, United States, 1991-2000. Emerg. Infect. Dis. 11:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]