Abstract
Meningococcal tetravalent polysaccharide vaccines were observed to be immunogenic in Saudi children 5 to 9 years of age, with >90% having serum bactericidal antibody titers of ≥8 for serogroups A, Y, and W135; for serogroup C, 77% were putatively protected after vaccination.
The Kingdom of Saudi Arabia has previously experienced epidemics of meningococcal disease which coincide with the Hajj and Umra seasons. These epidemics were mainly due to serogroup A disease. In the period from 2000 to 2002, there was a shift in the epidemiologic pattern of the epidemics observed that was due to serogroup W135 (1, 7).
The Saudi Arabian authorities had previously required that pilgrims attending either Hajj or Umra be vaccinated with the bivalent A/C polysaccharide vaccine (4), and a monovalent serogroup A vaccine was required for children less than 2 years of age. Due to the change observed in the period from 2000 to 2002, the Saudi Ministry of Health recommended the use of the tetravalent ACYW135 polysaccharide vaccine to provide coverage against serogroup W135 for pilgrims and Saudi school children. Further analysis revealed that 58% of reported meningococcal disease was in children less than 5 years of age and 39% was in those below 2 years of age (3). Hence, the Ministry of Health initiated an immunization program in 2003 to vaccinate children from 6 months to 5 years of age.
However, the immunogenicity of the tetravalent ACYW135 polysaccharide vaccine in these age groups was unknown. A follow-up study was performed to assess the serologic responses of children <5 years old to meningococcal tetravalent ACYW135 polysaccharide vaccine (2). Children less than 18 months old received two doses 2 to 3 months apart, and those ≥24 months old received one dose. Poor serum bactericidal antibody (SBA) responses were observed to the serogroup C, W135, and Y components of the vaccine in children ≤18 months old, whereas 42% of children had SBA titers of ≥8 for serogroup A. In children ≥2 years old, ≥80% had SBA titers of ≥8 for serogroup A, with a similar number observed for serogroup Y for children 4 years of age. However, poor responses to serogroup C and W135 were still observed for children 4 years of age. As a result of this study, the use of the tetravalent polysaccharide vaccine in children ≤2 years old has been discontinued in Saudi Arabia.
The study also demonstrated that responses to meningococcal polysaccharides are both age and polysaccharide dependent, with serogroup A polysaccharide being more immunogenic in younger children, confirming previous reports (5, 8, 12). It is well known that the immune systems of young children cannot process polysaccharide antigens in a manner appropriate for stimulating an effective response, but it is unclear at what age the immune system is mature enough to provide the correct environment for this process. Hence, a study was performed with Saudi children 5 to 9 years of age to ensure that the responses to the tetravalent ACYW135 polysaccharide vaccine merited the continuation of its use in this age group.
Two hundred forty-one children were recruited to the study from Qassim Province and were split into the following age groups: 5 years old (n = 47), 6 years old (n = 45), 7 years old (n = 49), 8 years old (n = 50), and 9 years old (n = 50). Each group received one dose of Mencevax ACWY (GSK, Rixensart, Belgium), and blood samples were obtained from each child prior to and 1 month after vaccination following agreement to participate by the child's guardian. Serogroup-specific antibody responses were determined by SBA assays as previously described (11) by using the same meningococcal strains as those in the previous study of Saudi children <5 years old (2). Serogroup-specific immunoglobulin G (IgG) levels were determined using a tetraplex bead assay as previously described (9).
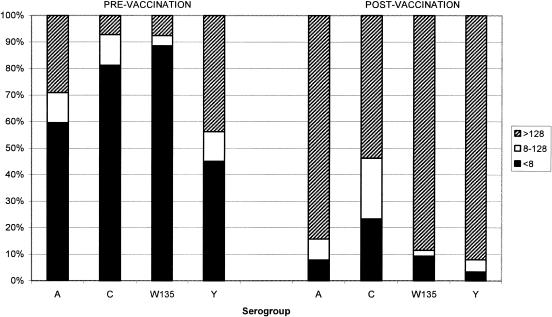
Unlike the previous report of responses in Saudi children <5 years old (2), there was no age-dependent trend observed for either the SBA responses or the IgG concentrations, and hence all age groups have been combined and results presented for each individual serogroup. The numbers of individuals with SBA titers of ≥8 for before and after vaccination and ≥4-fold increases in SBA titers from before vaccination to after vaccination are given in Table 1. Forty percent of children had SBA titers of ≥8 for serogroup A, and 55% had similar titers for serogroup Y before vaccination. Significant increases in the numbers of individuals with SBA titers of ≥8 from before vaccination to after vaccination for all serogroups were observed (McNemar's test; P < 0.001). The proportions of individuals with SBA titers of ≥8 ranged from 77% for serogroup C to 97% for serogroup Y. The number of children who demonstrated ≥4-fold increases from before to after vaccination was high, ranging from 65% for serogroup C to 88% for serogroup W135. Analysis of the SBA titers with respect to a more conservative threshold of 128 (Fig. 1) illustrates the magnitude of the response to each of the individual serogroups. For serogroups A, W135, and Y, >84% of children had titers of >128, whereas only 54% achieved this titer level after vaccination for serogroup C.
TABLE 1.
Proportions of Saudi children 5 to 9 years of age for serogroups A, C, Y, and W135 with SBA titers of ≥8 before and 1 month after vaccination with tetravalent polysaccharide vaccine and with ≥4-fold increases from before to after vaccination
| Serogroup | No. of individuals/no. of individuals tested (%)
|
||
|---|---|---|---|
| SBA titer of ≥8 before vaccination | SBA titer of ≥8 after vaccination | ≥4-fold increase from before to after vaccination | |
| A | 90/227 (40) | 207/225 (92) | 178/212 (84) |
| C | 44/234 (19) | 184/240 (77) | 165/233 (69) |
| W135 | 31/240 (13) | 214/236 (91) | 207/235 (88) |
| Y | 130/236 (55) | 230/238 (97) | 184/233 (79) |
FIG. 1.
Percentages of subjects before and 1 month after vaccination at serum bactericidal antibody titer cutoffs of <8, 8 to 128, and >128 for serogroups A, C, Y, and W135.
The geometric mean concentrations (GMCs) and 95% confidence intervals (CIs) for serogroup-specific IgG levels before and after vaccination are given in Table 2. Low GMCs were observed prior to vaccination for serogroups C, W135, and Y; however, serogroup A had a GMC significantly higher than those of the other three serogroups (paired t test; P < 0.001). All serogroups showed significant increases in IgG GMCs after vaccination (paired t test; P < 0.001), with that of serogroup A again significantly higher than those of the other serogroups (paired t test; P < 0.001). It is interesting that for serogroup A high numbers of individuals had prevaccination SBA titers of ≥8, corresponding to high anticapsular IgG GMCs, whereas for serogroup Y the high numbers of individuals with prevaccination SBA titers of ≥8 corresponded with low GMCs of anticapsular IgG. Inhibition studies indicated that the SBA prevaccination titers observed for serogroup Y were directed mainly towards the capsule, because 100% of samples tested (n = 16) with a range of positive titers (32 to 512) had SBA titers of 2 upon the addition of serogroup Y polysaccharide (data not shown). It is unclear why such high prevaccination SBA titers against serogroup Y were evident, because there are few cases of serogroup Y disease in Saudi Arabia. Furthermore, the carriage of serogroup Y meningococci is unlikely to contribute greatly in inducing high levels of SBA, because the carriage rate of serogroup Y is low, approximately 1%, as illustrated in the United Kingdom (10). One possible explanation may be exposure to cross-reactive epitopes, as has been described for other meningococcal polysaccharides (6, 13, 14).
TABLE 2.
Serogroup-specific IgG GMCs and 95% CIs for Saudi children 5 to 9 years of age for serogroups A, C, Y, and W135 before and 1 month after vaccination with tetravalent polysaccharide vaccine
| Serogroup | Before vaccination
|
After vaccination
|
||
|---|---|---|---|---|
| n | GMC (95% CI) | n | GMC (95% CI) | |
| A | 240 | 4.84 (4.3-5.5) | 241 | 25.00 (20.9-29.9) |
| C | 240 | 0.73 (0.6-0.8) | 241 | 6.11 (4.9-7.6) |
| W135 | 240 | 0.34 (0.3-0.4) | 241 | 3.10 (2.5-3.8) |
| Y | 240 | 0.43 (0.4-0.5) | 241 | 4.31 (3.5-5.3) |
In conclusion, tetravalent polysaccharide vaccines are immunogenic and elicit functional antibodies in greater than 90% of 5- to 9-year-old children for serogroups A, W135, and Y; however, for the serogroup C portion, only 77% of children reached putative protective levels after vaccination.
Acknowledgments
We thank the children and parents who participated in this study and the staffs at the primary health care centers who were involved.
REFERENCES
- 1.Al-Mazrou, Y., M. H. Al-Jeffri, M. N. Abadía, S. A. Elgizouli, and A. A. Mishskas. 2004. Changes in epidemiological pattern of meningococcal disease in Saudi Arabia. Does it constitute a new challenge for prevention and control? Saudi Med. J. 10:1410-1413. [PubMed] [Google Scholar]
- 2.Al-Mazrou, Y., M. Khalil, R. Borrow, P. Balmer, J. Bramwell, G. Lal, N. Andrews, and M. Al-Jeffri. 2005. Serologic responses to ACYW135 polysaccharide meningococcal vaccine in Saudi children under 5 years of age. Infect. Immun. 73:2932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mazrou, Y. Y., E. K. Musa, M. N. Abadía, M. H. Al-Jeffri, S. H. Al-Hajjar, and O. M. Mohammed. 2003. Disease burden and case management of bacterial meningitis among children under 5 years of age in Saudi Arabia. Saudi Med. J. 24:1300-1307. [PubMed] [Google Scholar]
- 4.Anonymous. 1992. Pilgrimage to Mecca (Hajj) 1992. Commun. Dis. Rep. CDR Wkly. 2(10):43. [PubMed] [Google Scholar]
- 5.Gold, R., M. L. Lepow, I. Goldschneider, T. L. Draper, and E. C. Gotschlich. 1975. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 56:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guirguis, N., R. Schneerson, A. Bax, W. Egan, J. B. Robbins, J. Shiloach, I. Orskov, F. Orskov, and A. el Kholy. 1985. Escherichia coli K51 and K93 capsular polysaccharides are crossreative with the group A capsular polysaccharide of Neisseria meningitidis. Immunochemical, biological, and epidemiological studies. J. Exp. Med. 162:1837-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajjeh, R. A., and J. Lingappa. 2000. Meningococcal disease in the Kingdom of Saudi Arabia: an evaluation of disease surveillance and control after an outbreak of serogroup W-135 meningococcal disease. CDC Rep. 23:2000. [Google Scholar]
- 8.Kayhty, H., V. Karanko, H. Peltola, S. Sarna, and P. H. Makela. 1980. Serum antibodies to capsular polysaccharide vaccine of group A Neissera [sic] meningitidis followed for three years in infants and children. J. Infect. Dis. 142:861-868. [DOI] [PubMed] [Google Scholar]
- 9.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a multiplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W135. Clin. Diagn. Lab. Immunol. 11:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiden, M. C., J. M. Stuart, and the UK Meningococcal Carriage Group. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 349:1829-1831. [DOI] [PubMed] [Google Scholar]
- 11.Maslanka, S. E., L. L. Gheesling, D. E. LiButti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltola, H., H. Makela, H. Kayhty, H. Jousimies, E. Herva, K. Hallstrom, A. Sivonen, O. V. Renkonen, O. Pettay, V. Karanko, P. Ahvonen, and S. Sarna. 1977. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N. Engl. J. Med. 297:686-691. [DOI] [PubMed] [Google Scholar]
- 13.Robbins, J. B., L. Myerowitz, J. K. Whisnant, M. Argaman, R. Schneerson, Z. T. Handzel, and E. C. Gotschlich. 1972. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and III. Infect. Immun. 6:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vann, W. F., T. Y. Liu, and J. B. Robbins. 1976. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect. Immun. 13:1654-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]