Abstract
mRNA technology might accelerate development of an urgently needed preventive human immunodeficiency virus (HIV) vaccine. We evaluated the safety and immunogenicity of three mRNA-encoded envelope trimers, including two doses of soluble and membrane-anchored forms, in a randomized, open-label, phase 1 clinical trial. Vaccines were generally well-tolerated, although 6.5% (7/108) of participants developed urticaria, a higher proportion than seen with other mRNA vaccines. mRNA-encoded trimers induced strong envelope-specific B and T cell responses. Immunization with membrane-anchored trimers, intended to obscure epitopes at the trimer base targeted by non-neutralizing antibodies, reduced the frequency of base-binding serum antibodies in comparison with soluble trimers. Three immunizations elicited autologous tier 2 serum neutralizing antibodies in 80% of vaccinees receiving the membrane-anchored trimers, in contrast to only 4% receiving the soluble trimer. Thus, with demonstration of more favorable safety, mRNA-encoded membrane-anchored HIV envelope trimers represent a promising platform for HIV vaccine clinical development.
One-Sentence Summary:
Membrane-anchored HIV envelope trimer mRNA vaccines elicited high response rates of serum autologous tier 2 neutralizing antibodies in humans.
Editor’s Summary:
A Better Immunogen for HIV. Vaccines for human immunodeficiency virus (HIV) usually target the envelope (Env) trimer that is present on the surface of virions. However, protein-based vaccines require the Env trimer be soluble; this creates a site at the base of the Env for antibodies to bind that’s unable to confer protection against infection. In two papers, Ramezani-Rad and Cottrell et al. and Parks et al. take advantage of mRNA vaccines to circumvent this problem. mRNAs are translated into proteins within the vaccine recipient’s cells, enabling the authors to produce membrane-bound versions of the Env trimer. Ramezani-Rad and Cottrell et al. demonstrated that this approach elicited antibodies that bound to the intended, non-base regions of the Env in preclinical models, supporting progression of this vaccine to clinical trials. Parks et al. report results of that trial, showing that vaccination of humans with a membrane-bound Env trimer elicits productive antibody responses, consistent with the preclinical studies. This pair of papers highlights the advantages of mRNA vaccines, especially for HIV vaccine design. –Courtney Malo
Introduction
A safe vaccine that induces long term immunity is needed to help end the human immunodeficiency virus (HIV) epidemic (1), but no candidate vaccine regimen evaluated in phase 2b-3 clinical trials has provided protective efficacy sufficient to justify licensure (2, 3). An effective preventive HIV vaccine will likely need to induce potent broadly neutralizing antibodies (bnAbs) that can block acquisition of diverse circulating HIV strains. The field is currently pursuing multiple vaccine approaches to induce bnAbs, including germline-targeting vaccine design (4–10), B cell lineage vaccine design (11), and epitope-focused vaccine design (12, 13). These strategies often involve sequential immunization with different native-like HIV envelope (Env) trimers, which display the various bnAb epitopes. Therefore, identifying the optimal strategies for vaccinating humans with HIV Env trimers to elicit desirable immune responses, including autologous neutralizing antibodies as well as trimer-specific memory B cells and CD4 T cells, will assist the field broadly in its quest for a successful HIV vaccine regimen.
The introduction of the mRNA vaccine platform has the potential to expedite iterative testing and development of new vaccine regimens. The rapid development of safe and highly effective mRNA vaccines for coronavirus disease 2019 (COVID-19) demonstrated the utility of mRNA technology for accelerating vaccine testing by reducing manufacturing timelines while maintaining safety and immunogenicity, generating hope that similar results could be achieved in the field of HIV vaccines. However, sufficient data are not yet available on safety and immune responses to mRNA-encoded HIV Env vaccines in humans.
In addition to expediting vaccine development, mRNA vaccine technology also enables the delivery of viral glycoproteins such as HIV Env in a membrane-anchored form, potentially mimicking protein presentation on the virion more closely than the soluble forms of recombinant HIV Env used in vaccine studies; this difference might result in more effective vaccines. For example, the base of soluble Env trimers is a large, non-glycosylated, highly immunogenic surface on an otherwise heavily glycosylated molecule (14, 15). Thus, base-directed antibodies are relatively easy to generate in response to vaccination with soluble HIV Env; however, such antibodies are typically non-neutralizing because the base is occluded by the viral membrane on the HIV virion. Recent phase 1 studies of conformationally stabilized, soluble HIV Env trimers formulated with alum alone or 3M-052-AF plus alum adjuvant found that serum antibody responses were dominated by base-directed antibodies, and autologous tier 2 neutralizing antibodies were either not elicited (16) or were elicited in only a subset of participants (16, 17). Conceivably, mRNA vaccine technology could overcome such limitations.
Another important question regarding HIV trimer immunization in humans is whether trimer binding to host CD4, and the potential for subsequent trimer conformational changes and CD4-binding-induced intracellular signaling, might negatively impact the magnitude or specificity of antibody responses. For membrane-bound trimers that may interact with CD4+ T cells in a highly multivalent manner through presentation on microvesicles, exosomes, or cell surfaces, the potential for an altered response may be heightened. Testing mutated versions of the Env trimer designed to limit host CD4 binding (CD4KO) may provide insight into the effects of host CD4 binding on immunogenicity and the effectiveness of CD4 binding site mutations in limiting potential interaction effects.
To address these issues, we report recent findings from a phase 1 study (HVTN 302), to evaluate the safety and immunogenicity of mRNA vaccination with membrane-anchored and soluble forms of stabilized HIV Env trimers in humans. We also evaluated whether a modified membrane-bound trimer lacking affinity for human CD4 would elicit improved antibody responses (18). We demonstrate mRNA delivered Env trimers to be generally tolerable, despite low rates of chronic urticaria, and immunogenic, including improved elicitation of tier 2 autologous neutralizing antibodies by membrane display of the HIV Env trimer.
Results
Study design and enrollment
HVTN 302 is a phase 1, open-label, randomized, multicenter trial conducted by the HIV Vaccine Trials Network (HVTN) (Clinical Trials.gov: NCT05217641). Two dose levels (100μg and 250μg) of three trimer vaccines were evaluated: BG505 MD39.3 gp140 (soluble trimer gp140; Groups 1 and 4); BG505 MD39.3 gp151 that includes a transmembrane domain but lacked a C-terminal domain (membrane-bound trimer gp151; Groups 2 and 5); and BG505 MD39.3 gp151 CD4KO that additionally included a point mutation in the CD4 binding site to reduce CD4 binding (membrane-bound gp151 CD4KO; Groups 3 and 6). Each of the trimers include modifications to stabilize the trimer in the prefusion conformation, eliminate the need for furin cleavage, and add two glycosylation sites that are generally conserved across HIV isolates but missing in the BG505 isolate (19). Generally healthy adults aged 18 to 55 years without HIV provided written consent, and were then enrolled and randomized into 6 groups split into Part A (100μg; groups 1 to 3; n=18, 19, 17) and Part B (250μg; groups 4 to 6, n=18 each) (Fig. 1 and fig. S1). The median age was 29, 52% (56/108) were male, and 17% (18/108) were of Hispanic or Latino ethnicity. Part A was enrolled prior to Part B, with a 2-week safety evaluation after the 1st vaccination in a sentinel group (n=12) before enrolling the remaining Part A participants; Part B conducted a similar sentinel group (n=12) safety evaluation. Blood specimens were collected at designated time points in the protocol, and serum and peripheral blood mononuclear cells were cryopreserved for immunological studies.
Figure 1. Trial schema.

(A) Graphic depiction of the HVTN 302 study groups and vaccine doses in Parts A and B, vaccine delivery schedule and enrollment strategy for safety holds. For planned safety hold #1, a safety sentinel cohort of participants in Part A (n=4 each group, 12 total) were enrolled and evaluated for safety for 2 weeks after the first vaccination. If safety criteria were met, the remaining Part A participants were enrolled, as well as a subset of participants from Part B. For planned safety hold #2, a safety sentinel cohort of participants in Part B (n=4 each group, 12 total) were enrolled and evaluated for safety for 2 weeks after the first vaccination. If safety criteria were met, the remaining Part B participants were enrolled. (B) Time points of blood sample collection for immune analyses.
Safety
Solicited adverse events (AEs) reported during the seven days following vaccine administration included increased body temperature, fatigue, generalized myalgia, generalized arthralgia, headache, chills, nausea, and injection site pain/tenderness, induration and erythema. These occurred at rates similar to those observed in the phase 3 Moderna COVID-19 mRNA-lipid nanoparticle (LNP) vaccine trial (20–23) (fig. S2 and tables S1 and S2). During the study, 80 participants reported a total of 190 AEs of any relationship to study product (highest severity reported for each participant: n=29 mild, n=47 moderate, n=4 severe). AEs deemed related to study product included 22 mild events (8 lymphadenopathy, 2 urticaria, 2 axillary pain, 2 rash, 1 diarrhea, 1 hypoaesthesia, 1 injection site pruritis, 1 swelling, 1 musculoskeletal chest pain, 1 decreased neutrophil count, 1 tonsillar hypertrophy, 1 vomiting) and 8 moderate events (5 urticaria, 1 angioedema, 1 syncope, 1 diarrhea). Six participants discontinued vaccinations due to the following unsolicited AEs: urticaria with or without pruritis (n=5) and unrelated severe musculoskeletal chest pain (n=1). One participant discontinued vaccinations due to multiple solicited AEs. Three serious adverse events (SAEs) were reported; two were deemed unrelated to study product, and the third SAE, deemed related to study product, is described below.
An unexpected safety signal was identified during the conduct of the trial: seven of 108 [6.5%, 95% confidence interval (CI) 3.2%–12.8%] participants developed urticarial skin reactions deemed related to study product within 6 to 21 days of the 1st or 2nd study vaccination (fig. S3), as described in detail in Riddler et al. (24) with select case photos and skin biopsy immunocytochemistry analyses. Briefly, five participants had chronic spontaneous urticaria, defined as symptom duration lasting more than 6 weeks; 4 had ongoing chronic urticaria at the final planned study visit at 12 months. Following these events, the protocol was modified to offer participation for these 4 individuals in an extended study follow-up; of these participants, 3 enrolled and had continued symptoms for ≥18 months of follow-up. One participant terminated from extended follow-up at 18 months due to symptom resolution with continued antihistamine use, where antihistamine use was reported at study entry. The other two participants remain in extended follow-up 32 to 35 months from symptom onset. Of note, urticaria was observed with all three study products and both dose levels: 5 of the 7 participants received the 100μg dose, and 2 participants received the 250μg dose. All urticaria AEs were mild to moderate in severity, and improved with single or dual oral antihistamines, with or without courses of oral corticosteroids. One related SAE was classified as a suspected unexpected serious adverse event (SUSAR) due to the need for a brief hospitalization for monitoring and to undergo a diagnostic ultrasound to investigate scrotal swelling with urticaria covering a large body surface area.
Serum binding antibodies were elicited by vaccination
Serum IgG antibodies binding the vaccine-matched BG505 MD39.3 protein were assessed in samples collected two weeks after each vaccination and six months after the last vaccination (Fig. 2A). Antibody responses were detected in all groups but arose earlier in the gp140 vaccinees. Serum IgG responses to the BG505 MD39.3 were detected after one vaccination in the majority of participants receiving the gp140 construct (15/17 in low dose, 11/17 in high dose), but only after the 2nd vaccination in the majority of participants receiving gp151 constructs (Fig. 2A). Despite slower kinetics, the responses in the gp151 groups ultimately reached higher binding antibody AUC magnitudes among positive responders. Response magnitudes were higher for gp151 than gp140 at two weeks post-3rd vaccination for 100μg dose (p = 0.049) and 250μg dose (p < 0.001). Response magnitudes were higher in the gp151 CD4KO group compared to the gp151 group only at 2 weeks post 2nd vaccination for 250 μg dose (p = 0.031). Therefore, the impact of the CD4KO mutation on the serum antibody binding response was transient and did not lead to durable differences in serum antibody binding magnitudes. Median trimer-binding antibody responses declined 2.3- to 9-fold from two weeks post 3rd vaccination to six months post 3rd vaccination. Thus, the mRNA-encoded trimer vaccines induced substantial class-switched serum antibody responses to intact prefusion trimer (Fig. 2A).
Figure 2. Vaccine-specific serum binding antibody responses were elicited by vaccination.

(A and B) IgG binding antibody responses to BG505 MD39.3 (A) and BG505 MD39.3 (B) base epitopes were measured by BAMA and titers reported as area under the curve (AUC) for the different vaccine groups, specified by color-coding defined below (B). Positive response rates and magnitudes among positive responders to BG505 MD39.3 are shown at 2 weeks after the 1st, 2nd, and 3rd vaccinations, and at 6 months after 3rd vaccination. The proportion of positive responders is shown by vaccine group at the top of (A). Boxplots display the distribution of positive responses across participants by vaccine group with medians indicated by mid-lines; the ends of the boxes denote the 25th and 75th percentiles and the whiskers extend to the most extreme data points that are no more than 1.5 times the interquartile range (the height of the box) or if no value meets this criterion, to the data extremes. Circles denote responders; triangles represent non-responders. Significant p-values are shown for comparisons of response rates by Barnard’s test (listed above Resp/total) and magnitudes among positive responders by Wilcoxon test (listed above boxplots) for the following group comparisons: gp140 vs. gp151 at each dose, gp151 vs. gp151 CD4KO at each dose, gp140 100μg vs. gp140 250μg, gp151 100μg vs. gp151 250μg, gp151 CD4KO 100μg vs. gp151 CD4KO 250μg.
Anchoring Env to the membrane in the gp151 constructs aimed to reduce the antibody binding to the trimer base, a region that is not exposed on the native virus. To determine the extent to which membrane display decreased serum IgG responses to the trimer base, we quantified the serum IgG response to base epitopes. All groups elicited base-directed serum IgG. Median base responses at 2 weeks post-3rd vaccination ranged from 27285 to 30037 in the gp140 groups and ranged from 13661 to 15305 in the gp151 and gp151 CD4KO groups (Fig. 2B). Thus, although the gp151 membrane bound platform did not fully eliminate the production of base binding serum IgG, membrane display resulted in 1.78 to 2.20-fold lower medians of base binding serum IgG titers.
We also evaluated serum IgG responses to the BG505 MD39.3 trimer internal epitopes. Internal epitopes could potentially become exposed due to trimer misfolding or incomplete assembly. This is a risk inherent with mRNA-encoded trimer vaccination because the expressed trimer cannot be purified. Internal epitopes could also be exposed due to trimer conformational opening and closing (“breathing”) or due to loss of structural integrity induced by binding of vaccine-elicited trimer-degrading antibodies or due to CD4 binding-induced Env conformational changes (25, 26). We tested serum IgG binding responses to different internal peptides that might be exposed, including peptides from C1, HR1, V3, MPER, and the engineered linker peptide between gp120 and gp41 (table S3). Low or zero response rates were observed for all peptides except V3, which we detected in all groups (fig. S4). Despite modifications to BG505 MD39.3 to reduce V3 loop exposure, serum antibodies to the V3 loop were generated suggesting that at least a minimal degree of trimer breathing around V3 was occurring in vivo. For context, V3 binding antibody responses were also induced by immunization of non-human primates (NHPs) with prefusion-stabilized BG505 trimers delivered as highly purified proteins with adjuvant (27). Overall, this indicates that the in vivo-expressed trimers were properly folded and generally maintained a prefusion or near-prefusion conformation, minimizing exposure of internal, non-neutralizing epitopes.
Neutralizing serum antibody responses were elicited by vaccination
Serum antibody neutralization was measured against the autologous tier 2, BG505/T332N virus (Fig. 3A). We observed neutralization responses in the low and high dose gp151 and CD4KO gp151 groups, with significantly higher response rates in the gp151 versus gp140 groups at 2 weeks after the 3rd vaccination (low dose: 65% vs. 0%, p<0.0001; high dose: 86% vs. 8%, p<0.0001). Overall, 80% (51/64) of recipients in the gp151 or CD4KO gp151 groups produced autologous neutralizing responses two weeks after the 3rd vaccination. Responses in the four gp151 and CD4KO gp151 groups were first observed after the 2nd vaccination at month 2.5 and appeared to increase in magnitude after the 3rd vaccination at month 6.5. Neutralization was evaluated at 6 months after the 3rd vaccination (month 12) on the 27 participants with month 6.5 half-maximal inhibitory dilution (ID50) value ≥ 60 to BG505/T332N. All but one participant in the low dose gp151 group had a positive ID50 at month 12 indicating that antibody neutralization titers, although decreased, were durable up to 6 months post 3rd vaccination. No differences in the magnitude of responses were observed among positive responders across groups. Only one responder was observed in the high dose gp140 group with a low ID50 of 11.5, just above the positivity cutoff titer of 10. Therefore, mRNA-encoded membrane-bound trimers generated robust autologous tier 2 virus neutralizing antibodies.
Figure 3. Neutralizing antibody responses (autologous and glycan mutant panel) and EMPEM responses to vaccination targeted multiple HIV Env epitopes.
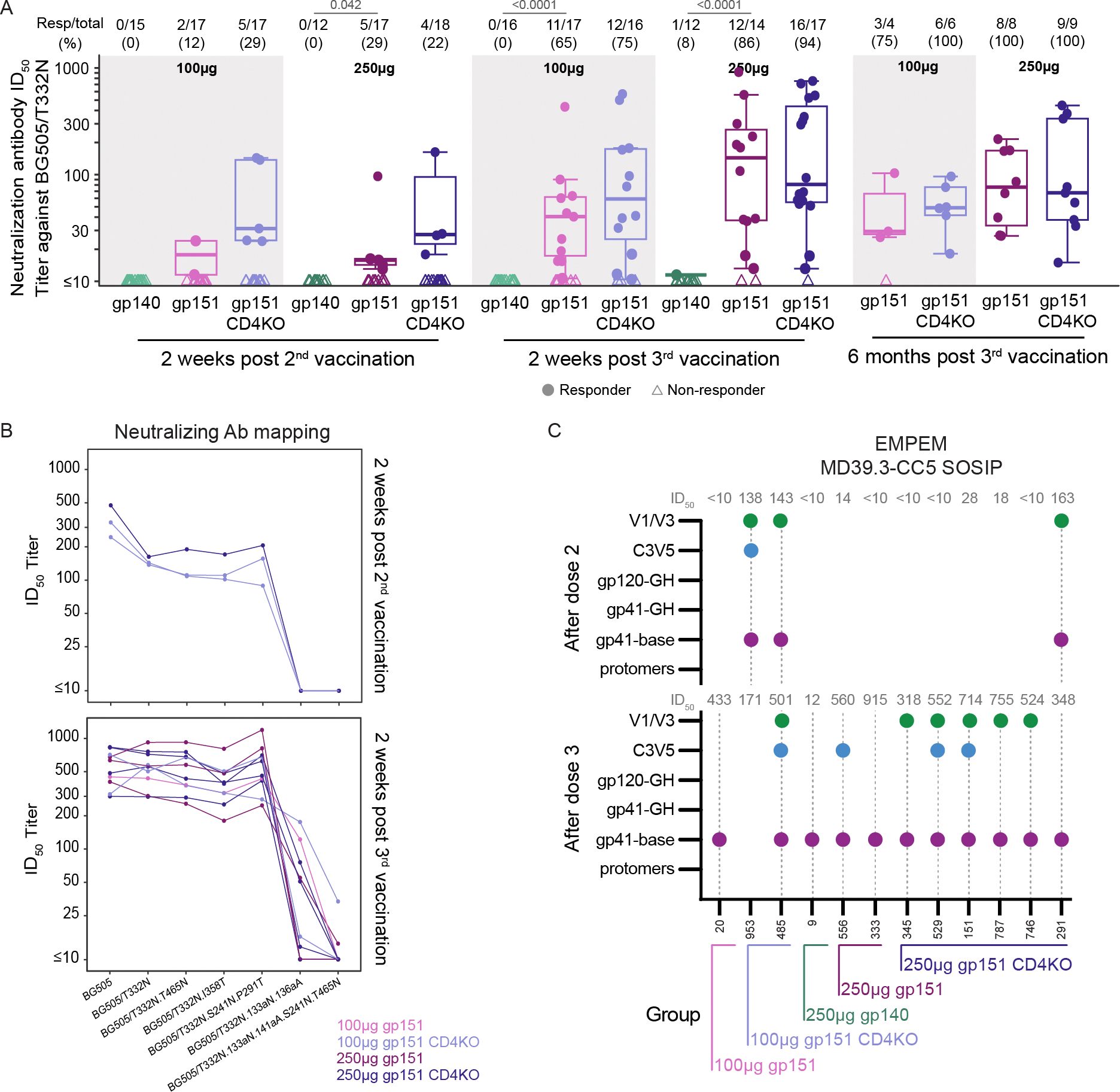
(A) Neutralizing antibody ID50 titers against BG505/T33N were measured as a function of reductions in Tat-regulated luciferase (Luc) reporter gene expression in TZM-bl cells for all participants in each vaccine group. Positive response rates and magnitudes among positive responders against BG505/T33N are shown at 2 weeks after 2nd and 3rd vaccinations, and at 6 months after 3rd vaccination for the 27 participants with month 6.5 ID50 ≥ 60 to BG505/T332N. The proportion of positive responders for each vaccine group is shown at the top of (A) where boxplots show the distribution of positive responses across participants in a given vaccine group with medians indicated by mid-lines; the ends of the boxes denote the 25th and 75th percentiles and the whiskers extend to the most extreme data points that are no more than 1.5 times the interquartile range (the height of the box) or if no value meets this criterion, to the data extremes. Circles denote responders; triangles represent non-responders. Significant p-values are shown for comparisons of response rates by Barnard’s test (listed above Resp/total) with no significant differences in magnitudes among positive responders seen by Wilcoxon test for the following group comparisons: gp140 vs. gp151 at each dose, gp151 vs. gp151 CD4KO at each dose, gp140 100μg vs. gp140 250μg, gp151 100μg vs. gp151 250μg, gp151 CD4KO 100μg vs. gp151 CD4KO 250μg. (B) Any samples resulting in >200 ID50 titer against BG505/T332N at 2 weeks after 2nd (n = 3) or 3rd vaccination (n = 10) were tested against a panel of mutant BG505 viruses. The line color denotes vaccine group. (C) Serum from select participants with neutralizing ID50 values to BG505/T332N were assayed by EMPEM 2 weeks following 2nd vaccination or two weeks following 3rd vaccination or both. Dotted gray lines indicate the sample was assayed. Dots on the graph indicate that reactivity to that epitope was detected. ID50 values are listed above the graph. The group of each participant is indicated on the x-axis.
Serum was also evaluated for antibody neutralization of the tier 1 virus MW965.25 in all groups (fig. S5, table S4). Tier 1 viruses express Env in the open conformation and can be neutralized by antibodies targeting conserved internal epitopes, which are not exposed on tier 2 viruses that mostly express closed-conformation Env (27–29). Tier 1 neutralization epitopes include, but are not limited to, the V3 loop (27). We detected tier 1 neutralizing antibodies to the MW965.25 virus at a high response rate in all groups, but at low titers (group ID50 medians=13.6 to 48.3). These titers are similar to or lower than those reported by others in response to adjuvanted protein immunization with stabilized BG505 trimers in humans (17) or NHPs (27). Thus, the higher titers to BG505/T332N tier 2 virus and lower titers to the MW965.25 virus indirectly indicate that the mRNA-encoded gp151 trimer can maintain its conformational integrity in vivo and preferentially present the epitopes of tier 2 autologous neutralizing antibodies.
Sera from 26 participants with an ID50 antibody neutralization titer > 60 to the BG505/T332N pseudovirus were further evaluated for neutralization of a panel of eight heterologous pseudoviruses derived from participants in the Antibody Mediated Prevention (AMP) studies who had early HIV-1 acquisition (conducted between 2016 and 2020) (30). Low response rates and magnitudes (median ID50 <40) were detected to the heterologous, tier 2 pseudoviruses (table S4).
V1/V3, C3/V5, and base regions are major targets of the serum Ab response
In other pre-clinical and clinical studies, vaccine-induced autologous tier 2 neutralizing antibodies to BG505/T332N typically target strain-specific holes in the glycan shield in the V1 loop or the C3/V5 region, where the location and density of glycosylation sites vary across HIV isolates, or at Env positions 241 and 289, where glycosylation sites conserved across most HIV isolates are absent in BG505 (17, 27, 31–35). The BG505 MD39.3 trimers in this study have modifications to introduce glycosylation sites at positions 241 and 289, but whether the sites were glycosylated on the in vivo-expressed trimers was not known. To determine if strain-specific glycan holes were the target of autologous neutralization in this study, we used a panel of BG505/T332N pseudovirus mutants that eliminated the known neutralization epitopes in V1, C3/V5, and the 241/289 glycan hole. Twelve of 14 samples of participant sera with autologous neutralization ID50 >200 were selected for mapping (n = 3 post 2nd vaccination; n = 10 post 3rd vaccination; only one participant qualified at both timepoints; the two samples not tested were excluded for non-specific neutralization activity) (Fig. 3B, table S4). An ID50 titer less than the observed ID50 to the BG505/T332N virus could indicate the presence of serum neutralization against the mutated epitope. All three of the neutralizing serum samples tested following 2nd vaccination mapped to the V1 epitope based on the absence of neutralization to the BG505/T332N.133aN.136aA pseudovirus. Following the 3rd vaccination, substantial V1-loop directed neutralization was also detected, with complete loss of neutralization to the BG505/T332N.133aN.136aA pseudovirus for 30% (3/10) of the serum samples and low ID50 titers between 13–121.3 in the other seven serum samples tested. Neutralizing responses to C3/V5 were also detected: ID50 titers ranged from 255.1–747.4 to the BG505/T332N.T465N mutant virus and 179.0–800.0 ID50 titers to BG505/T332N.I358T mutant virus. High ID50 titers between 246.3–696.9 for the BG505/T332N.S241N.P291T mutant virus suggest limited contribution to neutralization by sera targeting the 241/289 glycan hole. The pan-epitope mutant (BG505/T332N.133aN.136aA.S241N.T465N) eliminated 100% of detected neutralization in the sera from 8/10 participants. The fact that 2/10 serum samples maintained neutralizing activity to the pan-epitope mutant suggests the existence of another target of serum neutralization not determined here. Based on these data, the majority of BG505/T332N autologous neutralizing activity was directed to V1 with minor contributions from antibodies targeting the C3/V5 and 241/289 glycan hole regions in a subset of participants.
To capture additional epitopes targeted by antigen-specific antibodies not determined by these specific pseudovirus mutants, serum antibody responses from 11 participants with tier 2 neutralization were analyzed by electron microscopy-based polyclonal epitope mapping (EMPEM) (Fig. 3C). Ten participants were from the membrane-bound gp151 groups, and one participant was from the gp140 high dose group. Initial assays using the BG505 MD39.3 recombinant trimer revealed multiple epitopes targeted but substantial amounts of protomer bound by Fab (antigen binding fragment) (fig. S6). This likely was due to the presence of serum antibodies binding the trimers and degrading them into protomers (25, 26, 36). The relatively small mass of gp140 protomers make epitope resolution by EMPEM difficult. Therefore, two disulfide bonds were introduced into BG505 MD39.3 to increase the stability of the trimer (37) and prevent antibody-induced degradation, with the resulting disulfide-stabilized trimer referred to as BG505 MD39.3-CC5 (Fig. 3C). When EMPEM was performed using the BG505 MD39.3-CC5 probe, gp41/base-targeting antibodies were detected in all samples assayed at visits 2 weeks post 2nd vaccination and 2 weeks post 3rd vaccination, indicating the base of the trimer to be a major target of the serum antibody response. Responses to V1/V3 and C3/V5 epitopes were also detected two weeks post 2nd and two weeks post 3rd vaccination. V1/V3 responses were detected in 3/3 and 6/11 vaccine recipients 2 weeks post 2nd vaccination and 2 weeks post 3rd vaccination, respectively. C3/V5 responses were detected in 1/3 and 4/11 vaccine recipients 2 weeks post 2nd vaccination and 2 weeks post 3rd vaccination, respectively. These results are consistent with pseudovirus mutant epitope mapping studies. Thus, whereas other clinical studies evaluating BG505 trimers as adjuvanted proteins detected either antibody base responses only (16) or non-base responses in only a small fraction of participants tested (17), we detected non-base serum antibody responses to different epitopes in the majority of participants tested receiving mRNA-encoded membrane-anchored BG505 trimers.
Memory B cell responses were elicited by vaccination
We next measured memory B cell responses elicited by vaccination. BG505 MD39.3 trimer-specific IgG+ memory B cell responses were detected in all groups after two vaccinations, with response rates of 81.2% to 100% and median frequencies among all IgG+ B cells of 0.63% to 1.96% (Fig. 4A). Median frequencies increased in all groups following the 3rd vaccination, ranging from 1.07% to 3.60%. Trimer-specific IgG+ memory B cell responses persisted to 6 months following the 3rd vaccination, with only modestly lower median frequencies compared to the peak at two weeks post 3rd vaccination. For example, among the group receiving 100μg gp151, the median frequency dropped 3.5-fold, from 2.5% at two weeks post 3rd vaccination to 0.7% at six months post 3rd vaccination. No differences in the magnitude or response rate of the vaccine-induced B cell responses were observed across the three vaccine platforms at either dose. Thus, BG505 MD39.3 gp140 and both gp151 mRNA constructs elicited vaccine-specific memory B cell responses.
Figure 4. BG505 MD39.3-specific memory B cells increased in frequency and somatic hypermutation rate and underwent clonal expansion following vaccination.

(A and B) Frequencies of BG505 MD39.3++ (A) and non-base binding (B) B cells of total IgG+ B cells measured at baseline, 2 weeks post 2nd vaccination, 2 weeks post 3rd vaccination, and 6 months post 3rd vaccination. Each data point for a single participant are shown and colored by vaccine group. Circles denote responders; triangles represent non-responders. The proportion of positive responders by vaccine group is shown at the top of each panel. Significant p-values are shown for comparisons of magnitudes among positive responders by Wilcoxon test (listed above boxplots), with no significant differences in response rates by Barnard’s test for the following group comparisons: gp140 vs. gp151 at each dose, gp151 vs. gp151 CD4KO at each dose, gp140 100μg vs. gp140 250μg, gp151 100μg vs. gp151 250μg, gp151 CD4KO 100μg vs. gp151 CD4KO 250μg. (C) Rates of nucleotide (NT) SHM in V segments of heavy, lambda, and kappa chains for the base- and non-base-specific memory (IgD−) B cell receptors at week 0, week 10 and week 26 after vaccination (vacc.). Each dot represents the median rate of SHM across sequenced B cell receptors from one participant. The size of the dot represents the number of sequences recovered, and dots are colored by group. Statistical comparisons of SHM between base and non-base binders at a given time point, and between time points within base or non-base binders, were performed using a Wilcoxon signed-rank test (two-sided) to account for paired data. In panels (A to C), boxplots show the distribution of responses across participants in a given vaccine group with medians indicated by mid-lines; the ends of the box denote the 25th and 75th percentiles and the whiskers extend to the most extreme data points that are no more than 1.5 times the interquartile range (the height of the box) or if no value meets this criterion, to the data extremes. (D) Number of base- and non-base-specific clonotypes with a given size (number of cells) at week 0, week 10, and week 26 within each vaccine group. Lines are colored by group.
To evaluate the ability of the gp151 platform to direct the immune response to non-base epitopes expressing bnAb binding sites, we quantified the frequency of non-base binding IgG+ memory B cells by flow cytometry using differential binding to a BG505 MD39.3 base knockout probe (BG505 MD39.3 BaseKO). All groups elicited non-base binding IgG+ memory B cell responses. However, the magnitudes of non-base BG505 MD39.3 trimer-specific IgG+ memory B cells among positive responders were significantly higher in the gp151 group compared with the gp140 group at both two weeks post 2nd vaccination (medians 1.0 vs. 0.6, p = 0.047) and two weeks post 3rd vaccination (medians 2.0 vs. 0.8, p=0.013) (Fig. 4B). Furthermore, at 2 weeks post-3rd vaccination, the median percentage of the total BG505 MD39.3-binding IgG+ memory B cells targeted non-base epitopes in each of the gp151 and gp151 CD4KO groups ranged from 55.3 to 64.3 and the median percents for the gp140 groups ranged from 39.6 to 44.5 (fig. S7). Thus, although the total magnitude of the trimer-specific IgG+ B cell response was similar for the gp140 and gp151 platforms, gp151 the mRNA construct appears to improve the fraction of BG505 MD39.3-binding IgG+ memory B cells response targeting non-base epitopes.
We tested for dose effects in the generation of trimer-specific and non-base-targeting IgG+ B cells. The only significant differences in magnitudes among positive responders were those observed for gp151 CD4KO, where we observed approximately 1.5-fold higher frequencies of BG505 MD39.3-specific IgG+ memory B cells for high dose compared with low dose (two weeks post 3rd vaccination medians, 3.6 vs. 2.5, p = 0.008; six months post 3rd vaccination medians 1.0 vs. 0.6, p = 0.027) and 1.7-fold higher frequencies of non-base binding IgG+ memory B cells for high dose compared with low dose (M6.5 medians 2.5 vs. 1.5, p = 0.024; M12 medians 0.5 vs. 0.3, p = 0.033). Thus, the higher dose provided a modest improvement in memory B cell generation after the 3rd vaccination for gp151 CD4KO but not for gp151 or gp140.
To gain deeper insights into the neutralizing responses, we sorted trimer-specific memory B cells (IgD− B cells) and sequenced B cell receptors (BCRs) for a subset of 20 participants who produced autologous tier 2 neutralizing serum responses, all of whom had received membrane-anchored trimer (table S5). Paired heavy and light chain BCR sequences were recovered from a total of 2,684 antigen-specific memory B cells: 1,773 were base-binding and 911 were non-base-binding (table S5), in relation to binding of sorting probes. Somatic hypermutation (SHM) generally increased from post 2nd to post 3rd vaccination timepoints in both heavy and light chain sequences, and by two weeks after the 3rd vaccination, base- and non-base-binding B cells had acquired similar rates of mutation by 2 weeks post 3rd vaccination (Fig. 4C and tables S6 and S7). Both the base- and non-base-binding BCR sequences were predominantly individual clonotypes, but 5.1% of clonotypes contained at least two cells, and clonal families of up to 10 cells were observed (Fig. 4D and table S8). Despite the sampling depth in the non-base-binding population being lower than in the base-binding population (and much lower than in the antigen-negative population), all of the nine largest clonal families observed (containing 6 to 10 B cells each) were non-base-binding (Fig. 4D and table S8). This indicates that a fraction of the non-base-binding memory B cells underwent greater clonal expansion compared with base-binding memory B cells, which likely contributed to the greater frequency of non-base-specific B cells in the periphery (Fig. 1B). Further evidence of differences between the epitope specificities came from distinctions in the patterns of heavy and light chain gene usage. For example, the IGHV3–21 and IGHV3–23 genes appeared to be more frequently used in the non-base-binding population, whereas the IGHV1–69 gene was more frequently used in the base-binding population (fig. S8). In summary, mRNA-encoded trimers induced similar degrees of SHM between base- and non-base-specific B cells, but non-base B cells had greater clonal expansion and expressed BCRs with heavy and light chains preferentially encoded by specific immunoglobulin genes.
Monoclonal antibodies that neutralized the autologous (BG505/T332N) virus targeted V1 and C3/V5 regions
Monoclonal antibodies (mAbs) were produced from BCR sequences isolated from base-specific (n=7) and non-base-specific (n=11) memory (IgD−) B cells from vaccine recipients (table S9). These mAbs were evaluated for neutralization of the BG505/T332N virus. Most (8/11) non-base-specific antibodies neutralized the autologous, tier 2 virus, with IC50 values ranging from 0.01 to 82.98, but none (0/7) of the base-specific antibodies had detectable neutralizing activity. The 8 non-base-specific antibodies with autologous neutralization were screened for neutralization against the heterologous pseudoviruses derived from viral strains isolated from participants in the Antibody Mediated Prevention (AMP) studies. None of these 8 mAbs neutralized any of the heterologous pseudoviruses (table S10).
Negative stain electron microscopy (nsEM), neutralization of BG505/T332N pseudovirus glycan mutants, and surface plasmon resonance (SPR) affinity epitope mapping were utilized to map the epitopes of a subset of mAbs on BG505 MD39.3 Env. nsEM epitopes were determined by imaging complexes of BG505 MD39.3 with monoclonal Fab (fig. S9), and epitopes were determined by neutralization or SPR by a reduction in IC50 to the autologous virus or increase in KD compared with vaccine-matched protein (fig. S10 and tables S11 and S12). Results across the 3 assays were largely congruent and specifically identified the target of non-base antibodies to be mostly V1 with a lesser contribution of C3/V5 (only two antibodies mapped to C3/V5). Only one non-base mAb defined as V1 by pseudovirus mutants and SPR affinity could not be imaged by nsEM. SPR affinity mapping and nsEM were also used to confirm the epitope of one base binding mAb (fig. S9, table S11). The results from the nsEM and SPR affinity epitope mapping studies were consistent with mutant neutralization assays and indicated that V1 was a dominant target of the serum neutralizing response with additional contributions from antibodies targeting C3/V5.
Vaccine-specific T cell responses were elicited by three doses of vaccine
Vaccine-specific T cell responses were measured using intracellular cytokine staining (ICS) (Fig. 5). Peptide pools used to stimulate peripheral blood mononuclear cells (PBMCs) were comprised of 15-mer peptides overlapping by 11 amino acids covering the length of BG505 MD39.3 gp151 with separate pools for the gp120 and gp41 subunits. CD4+ T cells expressing interferon (IFN)-γ or interleukin (IL)-2 provide an overall indicator of a T cell response, and these BG505 MD39.3-specific CD4+ T cells were elicited in all groups two weeks post 3rd vaccination, with response rates from 80 to 100% and median magnitudes among positive responders ranging from 0.23 to 0.51% of CD4+ T cells (Fig. 5A). Env-specific CD4+ T cells expressing Th2 cytokines (IL-4, IL-5 or IL-13 individually or in combination) were also detected at high response rates (40–71%), although magnitudes were about 10-fold lower than for IFN-γ and IL-2 (median magnitudes among positive Th2 responses between 0.03 and 0.06%) (Fig. 5B). CD4+ T follicular helper (Tfh) cells provide help to B cells in the lymph node germinal centers, and these cells can be detected in circulation for a short period after vaccination. Because these cells express high amounts of IL-21(38, 39), cells expressing IL-21 may identify circulating Tfh. These cells were detected in response to Env stimulation in most participants (response rates 58 to 82%) with median magnitudes between 0.06 and 0.13% (Fig. 5C).
Figure 5. CD4+ and CD8+ T cell responses to total Env increased after vaccination.
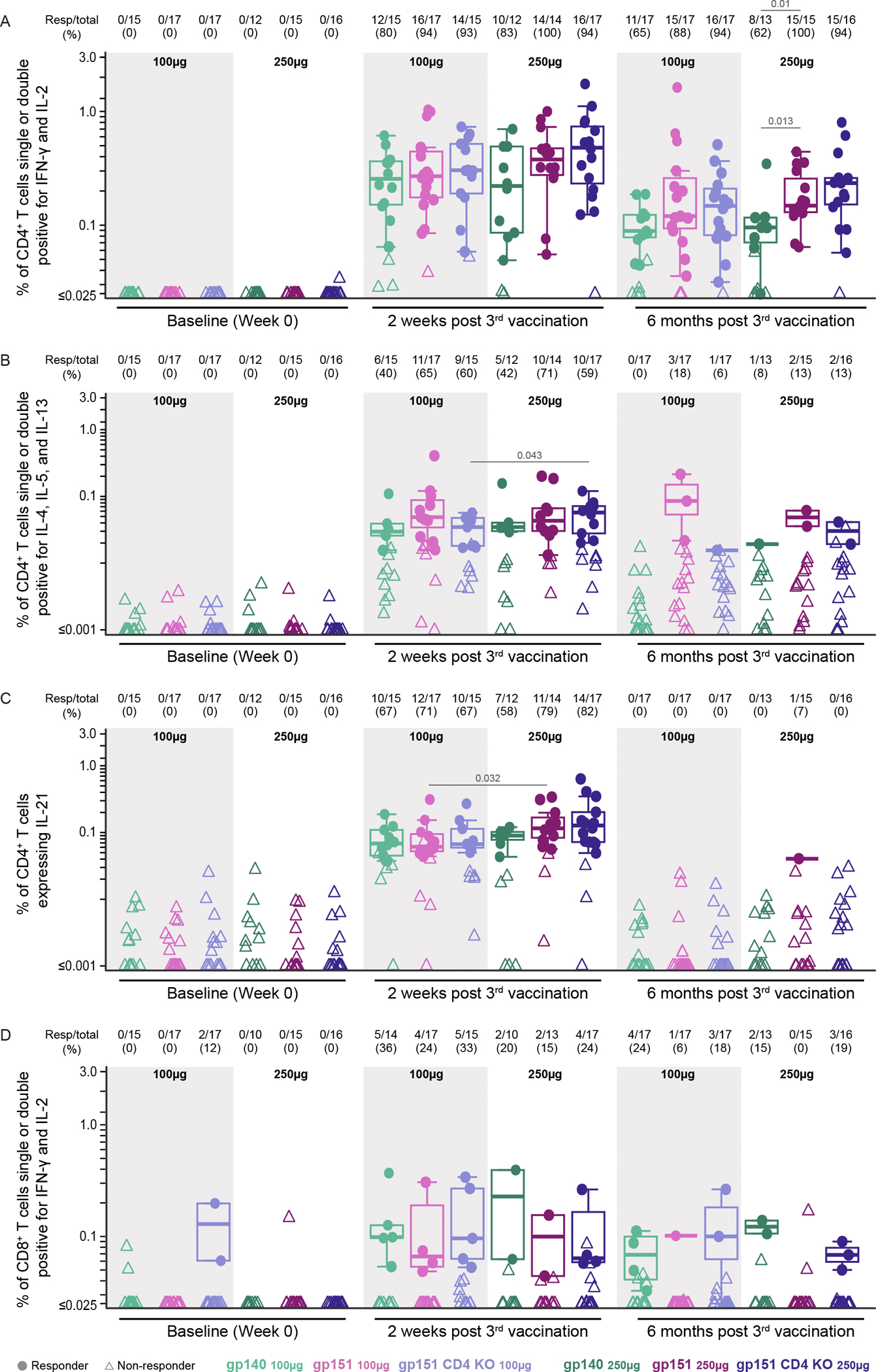
(A to D) CD4+ (A to C) and CD8+ (D) T cell responses were assessed by intracellular cytokine staining. Data are expressed as the percentage of cells producing one or both IFN-γ and IL-2 (A, D); IL-4, IL-5, or IL-13 (B); and IL-21 (C) in the different vaccine groups. CD4+ and CD8+ T cell positive response rates and magnitudes among positive responders to total Env are shown at week 0, 2 weeks after the 3rd vaccination, as well as 6 months after the 3rd vaccination. The proportion of positive responders by group is shown above each panel. Boxplots show the distribution of positive responses in a given vaccine group with medians indicated by mid-lines; the ends of the boxes denote the 25th and 75th percentiles and the whiskers extend to the most extreme data points that are no more than 1.5 times the interquartile range (the height of the box) or if no value meets this criterion, to the data extremes. Circles denote responders; triangles represent non-responders. Significant p-values are shown for comparisons of response rates by Barnard’s test (listed above Resp/total) and magnitudes among positive responders by Wilcoxon test (listed above boxplots) for the following group comparisons: gp140 vs. gp151 at each dose, gp151 vs. gp151 CD4KO at each dose, gp140 100μg vs. gp140 250μg, gp151 100μg vs. gp151 250μg, gp151 CD4KO 100μg vs. gp151 CD4KO 250μg.
No differences in response rates or magnitudes were detected in BG505 MD39.3-specific CD4+ T cell subsets expressing IL-21 or Th2 cytokines across vaccine platforms (gp140 vs. gp151 or gp151 vs. gp151 CD4KO). A dose effect was observed in CD4+ Th2 cell frequencies in the gp151 CD4KO recipients and in CD4+ T cells expressing IL-21 in recipients of the gp151 construct, with higher doses resulting in higher magnitudes among positive responders (medians 0.04 vs. 0.06, p = 0.04 and 0.06 vs. 0.12, p=0.03, respectively) (Fig. 5B and C); no other differences were detected across dose groups at two weeks or six months post 3rd vaccination. Responses determined separately for the gp120 and gp41 subunits show that the majority of the Env-specific T cells were directed toward gp120, with gp41-specific T cells detectable, but at lower magnitude (fig. S11A and B). At six months after the 3rd vaccination, response rates remained high for Env-specific CD4+ T cells expressing IFN-γ or IL-2 together or separately (62 to 100%), although the magnitude was reduced (medians from 0.09 to 0.25% among positive responses), with significantly higher magnitudes in the high dose gp151 vs. gp140 (medians 0.16 vs. 0.10, p=0.01). Response rates for CD4+ Th2 cells and CD4+ T cells expressing IL-21 were markedly reduced (Fig. 5). The latter is to be expected since circulating Tfh cells are only transiently present after vaccination.
We also evaluated if an mRNA vaccine encoding Env trimer could elicit a CD8+ T cell response. We detected BG505 MD39.3-specific CD8+ T cells expressing IFN-γ or IL-2, individually or in combination, in a small percentage of participants in all groups (response rates of 0 to 36%), with median magnitudes between 0.07 and 0.12% among positive responses (Fig. 5D and fig. S11C and D). Despite low frequencies of BG505 MD39.3-reactive CD8+ T cells detected at baseline (0 to 12%), the response rates at 2 weeks after the 3rd vaccination were 15 to 36% (Fig. 5D), suggesting that vaccination induced at least weak Env-specific CD8+ T cell responses in some participants.
Discussion
Stabilized HIV Env trimer immunogens offer the advantage of presenting a more native structure, including multiple epitope regions. The ability to employ the mRNA-LNP vaccine platform to express these Env trimers is a major goal in the pathway to bnAb induction. In this study, we evaluated the safety and immunogenicity in humans of mRNA-LNP vaccines encoding an HIV Env trimer, BG505 M39.3, as both membrane-anchored and soluble forms to guide further HIV vaccine development. We found that the membrane-anchored trimer was superior to the soluble trimer for induction of serum autologous neutralizing antibodies and memory B cells specific for neutralizing non-base epitopes. Antibody and B cell responses were not substantially different between the groups receiving the low dose (100μg) and the high dose (250μg). All trimer immunogens also induced substantial CD4+ T cell responses but only weak or non-detectable CD8+ T cell responses. Neutralizing antibody titers to the autologous BG505/T332N virus were low and strain-specific. It is notable that the response rate for BG505/T332N autologous neutralizing antibodies was higher for the mRNA-encoded membrane-anchored trimers in this study (17/19 in gp151 100μg group, 15/17 in gp151 CD4KO 100μg group, 13/18 in gp151 250μg group, and 17/18 in gp151 CD4KO 250μg group) compared with previous results for soluble BG505 gp140 stabilized trimers formulated with either alum alone (0% or 0/16 participants) (16) or 3M-052-AF+alum (50% or 4/8 participants) (17). This finding indicates that the mRNA membrane-anchored platform elicited immune response rates that are at least comparable, if not potentially superior, to those elicited by an adjuvanted protein.
In addition to comparing the immunogenicity of membrane-anchored and soluble forms of HIV Env trimers, we also evaluated whether including a CD4 binding site knockout (CD4KO) mutation in the membrane-anchored trimer would elicit improved immune responses. However, based on the assays evaluated here, the CD4KO mutation appeared to offer no substantial benefit beyond the mutations of the membrane-anchored MD39.3. Future studies will examine in more detail the induction and binding affinity of serum antibodies directed to potential CD4-binding induced conformations.
The mRNA vaccines in this study were well-tolerated overall, although vaccine-related urticaria was unexpectedly observed in 7 (6.5%) participants, a far higher frequency than reported with COVID-19 mRNA vaccines (20). Of greater concern, five participants experienced chronic urticaria, of whom four had ongoing symptoms at 12 months. As a result, the protocol was amended to provide 4-year follow-up of the individuals with continued symptoms of chronic urticaria to assess the impact on quality of life, and the two remaining participants still reported urticaria at ≥32 months. Moreover, urticaria has also been reported in study participants in another recently conducted phase 1 trial with a different candidate HIV mRNA vaccine (40). Although the immunological basis for the chronic urticaria remains unclear in both HIV mRNA vaccine studies, awareness of these events and careful consideration of exclusion criteria, safety monitoring, and regulatory compliance will be critical in future studies.
Furthermore, mRNA vaccines have the potential to facilitate rapid testing of complex vaccination regimens. Although in HVTN 302 we evaluated a homologous prime-boost immunization scheme, nearly all current strategies for bnAb induction in humans involve vaccination with a series of different immunogens as an approach to overcoming challenges such as low bnAb precursor B cell frequency, low bnAb precursor BCR affinity for wild type HIV Env, and requirements for high SHM or very long heavy chain complementarity determining region 3 sequences. For example, the germline-targeting strategy employs a priming immunogen to induce bnAb precursors that possess genetic properties needed for bnAb development, followed by a series of immunogens of increasing similarity to wild type Env to select for the SHM needed to produce bnAbs, culminating in a polishing step designed to maximize the serum bnAb titer. In addition to the need to deliver multiple immunogens in sequence to induce any one class of bnAbs, it is also likely that multiple classes of bnAbs will need to be induced to cover the high diversity of circulating strains and minimize viral escape, increasing the complexity and iterative nature of HIV vaccine development even further. Therefore, it is expected that developing an optimal bnAb-inducing HIV vaccine regimen will require multiple iterative phase 1 clinical trials, and that the time and cost required to produce many clinical immunogens in an iterative manner could substantially constrain vaccine development and testing. Thus, the mRNA-LNP vaccine platform, with its strong immunogenicity and high speed of production, is promising for HIV vaccine development contingent upon the demonstration of a more favorable safety profile.
We recognize that there are several limitations of our study. The vaccines evaluated in this study were not designed to induce HIV Env bnAbs or even to prime bnAb precursors. As a result, participants elicited serum neutralizing antibodies with minimal breadth and largely restricted to strain-specific glycan holes on the autologous, tier 2 virus. However, we have gained understanding of the immunogenicity and safety profiles of HIV Env trimers delivered by mRNA-LNP, which will inform future application of mRNA-LNPs for HIV vaccines. The study design was also open-label and unblinded, but this facilitated expeditious, iterative decision-making for future product development by allowing rapid access to study samples for immune analyses. In addition, the lack of a control arm limits interpretation of vaccine-induced responses. To address this limitation, baseline samples were analyzed for Env-specific binding antibodies, B cell frequencies, and ex vivo T cell functional phenotypes, all of which indicated no appreciable responses before the first vaccination.
In conclusion, we found that mRNA-LNP delivery of HIV Env elicited robust B and T cell responses with membrane-anchored display improving elicitation of autologous, tier 2 neutralizing sera. Vaccination with mRNA-LNP HIV Env was associated with the unexpected occurrence of chronic urticaria in a minority of participants, as described separately in more detail (24), and should be taken into consideration for the application of mRNA-LNP in future studies. Going forward, it will be important to evaluate the membrane-anchored Env trimer mRNA-LNPs in sequential vaccine regimens designed to prime bnAb precursors and advance maturation toward bnAb induction.
Materials and Methods
Study Design
HVTN 302 was a multicenter, randomized, open-label, phase 1 trial. Eligible participants were adults in overall good health without HIV, aged 18 through 55 years, who had a low likelihood of acquiring HIV. The trial excluded pregnant or breastfeeding individuals, those with current or prior receipt of any investigational HIV vaccine, receipt of any licensed live attenuated vaccine or any mRNA-based SARS-CoV-2 vaccine within 4 weeks of enrollment, receipt of any licensed killed/subunit/inactivated vaccine or any adenoviral-vectored or protein SARS-CoV-2 within 2 weeks of enrollment, history of serious reaction to any vaccine or any component of the study vaccine, or history of idiopathic urticaria within the past year.
Written informed consent was obtained from each study participant. The study protocol (Pro00056475) was reviewed and approved by a single institutional review board (Advarra, Columbia, MD registered with OHRP and FDA under IRB#00000971). Participants enrolled at 10 US clinical research study sites (Birmingham, AL; Boston, MA (2 sites); Philadelphia, PA; Pittsburgh, PA; New York, NY (2 sites); Rochester, NY; Seattle, WA; and Los Angeles, CA).
Eligible participants were enrolled and randomized equally to receive one of the three mRNA-LNP HIV trimer vaccines at study weeks 0, 8, and 24. Enrollment into the 100μg dose groups was initiated prior to the 250μg groups. For each dose level, a sentinel group (n=12) was enrolled and evaluated for safety 2 weeks after the 1st vaccination, prior to enrollment of the remaining participants into each group at that dose level. Study visits with collection of blood for safety and immunologic assessments, concomitant medications, and past medical history were conducted at weeks 0, 1, 2, 8, 9, 10, 24, 25, 26, 32, and 52. Additional collection of PBMC through leukapheresis was done at weeks 10 and 26 for eligible participants at the 100μg dose groups who consented to leukapheresis. The full protocol is available in data file S1, including sample size calculations in Section 6.1.
Statistical Analysis
Individual-level data are presented in data file S2. Safety analyses included all enrolled participants and immunogenicity analyses included only participants with samples available that met assay-specific quality-control criteria. The number and percentage of participants experiencing each reactogenicity symptom were tabulated by severity. 95% confidence intervals (CIs) for proportions were calculated using the score test method (41). Barnard’s tests were used to compare immune response rates and Wilcoxon rank-sum tests were used to compare the distribution of magnitudes among positive responders between vaccine groups of interest. All p-values are two-sided, with p-values less than 0.05 judged statistically significant, with no adjustment for multiple comparisons. Given the desire to maximize power to detect differences among vaccine groups in this phase 1 trial, multiplicity adjustments were not performed. SAS (version 9.4; SAS Institute) and R statistical software (version 4.0.4; R Foundation for Statistical Computing) were used for statistical analysis.
Supplementary Material
MDAR Reproducibility Checklist
Data files S1 and S2
Acknowledgments:
We would like to thank Todd Haight and Carol Marty at Fred Hutch for specimen repository and data management, as well as John Hural for oversight of the HVTN lab operations. We thank the technicians in the McElrath HVTN Endpoints laboratory including Leigh Anne Trushik as the lead ICS technician, Meron Abay as the lead B cell phenotyping technician and Maleia McBride for screening mAbs; and we thank Katharine Schwedhelm for lab management. From Duke University, we would like to thank Yong Lin from the Tomaras HVTN Endpoints laboratory for performing BAMA assays. We thank Lu Zhang, Jaiden Dumas, and Angelina Sharak for Duke BAMA data management. We thank Marcella Sarzotti-Kelsoe for oversight of Duke QAU for Tomaras and Montefiori lab assays under GCLP compliance as well as Tara McNair and Judith Lucas for GCLP Lab operations for BAMA. We thank Nicole Grunenburg for contributing to the protocol design and implementation at the HVTN Clinical Research Sites, and service as medical monitor. We thank Lori Panther from Moderna for protocol support and study document development. We thank Amanda Woodward Davis and Stephen Voght for critical reading of the manuscript and Lisa Donohue for design assistance. Figure 1 was created with BioRender.com. Finally, we thank all study participants, co-investigators and study staff at participating HVTN Clinical Research Sites.
Funding
Funding for this study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health through grants UM1 AI068614 and UM1 AI068635 (to Fred Hutchinson Cancer Center), UM1 AI068618 and UM1 AI069481 (to Fred Hutchinson Cancer Center) (to M.J.M.), UM1 AI069494 (University of Pittsburgh) (to S.A.R.), UM1 AI069534 and P30 AI045008 (University of Pennsylvania) (to I.F.), UM1 Al100663 (Scripps Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery) (to A.B.W, W.R.S., and M.J.M.), UM1 AI144462 (Scripps Consortium for HIV/AIDS Vaccine Development) (to A.B.W, W.R.S., and M.J.M.); the National Center for Advancing Translational Sciences (NCATS) of the U.S. National Institutes of Health through grant UL1 TR001857 (University of Pittsburgh) (to S.A.R.); the Joel Meyers Endowed Chair (to M.J.M.); the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (NAC INV-007522 and INV-008813 to W.R.S.); IAVI (support to D.S.L. and W.R.S.); the IAVI Neutralizing Antibody Center (NAC) to W.R.S.; and Moderna (to S.H., B.L., C.R., K.W.C., and W.R.S.).
Footnotes
Competing interests: W.R.S. is an inventor on a patent for the BG505 MD39 immunogen (Immunogenic trimers, US20240374731A1). C.A.C., A.L., and W.R.S. are inventors on a patent for the BG505 MD39.3 gp151 immunogen (Modified immunogenic proteins, US20230190914A1). C.A.C. is the founder and director of the Vaccine Research Foundation. SRW has received institutional funding from the National Institute of Allergy and Infectious Diseases/National Institutes of Health; and institutional grants or contracts from Sanofi Pasteur, Janssen Vaccines/Johnson & Johnson, AbbVie, Moderna, Pfizer, Vir Biotechnology, and Worcester HIV Vaccine; has participated on data safety monitoring or advisory boards for Janssen Vaccines/Johnson & Johnson and BioNTech; and his spouse holds stock/stock options in Regeneron Pharmaceuticals. KWC, SH, BL, CR, and WRS are employees of Moderna and may hold stock/stock options in the company. SAR has received institutional funding from the National Institute of Allergy and Infectious Diseases/National Institutes of Health; and institutional grants or contracts from Merck and Gilead Sciences. MJM receives institutional funding from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, and she has received institutional grants or contracts from Bill and Melinda Gates Foundation, IAVI, Sanofi Pasteur, Moderna, and Regeneron Pharmaceuticals.
Data and materials availability:
All data associated with this study are in the paper or supplementary materials or are in the public repository available at https://dataverse.harvard.edu/dataverse/hvtn302 (DOI: https://doi.org/10.7910/DVN/Y8OC5L). All individual participant data have been de-identified. The raw participant information data of individuals is protected by privacy laws and ethics regulations. Representative EM maps have been deposited to the Electron Microscopy Data Bank (EMDB) under accession codes EMD-70340, EMD-70341, EMD-70342, EMD-70343, EMD-70344 and EMD-70345.
References and Notes:
- 1.Beyrer C, Tomaras GD, Gelderblom HC, Gray GE, Janes HE, Bekker L-G, Millett G, Pantaleo G, Buchbinder S, Corey L, Is HIV epidemic control by 2030 realistic? Lancet HIV 11, e489–e494 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray GE, Mngadi K, Lavreys L, Nijs S, Gilbert PB, Hural J, Hyrien O, Juraska M, Luedtke A, Mann P, McElrath MJ, Odhiambo JA, Stieh DJ, van Duijn J, Takalani AN, Willems W, Tapley A, Tomaras GD, Hoof JV, Schuitemaker H, Swann E, Barouch DH, Kublin JG, Corey L, Pau MG, Buchbinder S, Tomaka F, I. 705/HPX2008 S. Group, Allagappen J, Andriesen J, Ayres A, Bekker L-G, Borremans C, Brumskine W, Chilengi R, Dubula T, Garrett N, Gelderblom H, Gill K, Hoosain Z, Hosseinipour M, Hutter J, Inambao M, Innes C, Kilembe W, Kotze P, Kotze S, Laher F, Laszlo I, Lazarus E, Malahleha M, Mathebula M, Matoga M, McClennen R, Mda P, Meerts P, Naicker V, Naidoo L, Philip T, Pitsi A, Scheppler L, Sopher C, Takuva SG, Viegas E, Weijtens M, Yuan O, Mosaic HIV-1 vaccine regimen in southern African women (Imbokodo/HVTN 705/HPX2008): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Infect. Dis. (2024), doi: 10.1016/s1473-3099(24)00358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIH, Experimental HIV vaccine regimen safe but ineffective, study finds (2023) (available at https://www.nih.gov/news-events/news-releases/experimental-hiv-vaccine-regimen-safe-ineffective-study-finds).
- 4.Leggat DJ, Cohen KW, Willis JR, Fulp WJ, deCamp AC, Kalyuzhniy O, Cottrell CA, Menis S, Finak G, Ballweber-Fleming L, Srikanth A, Plyler JR, Schiffner T, Liguori A, Rahaman F, Lombardo A, Philiponis V, Whaley RE, Seese A, Brand J, Ruppel AM, Hoyland W, Yates NL, Williams LD, Greene K, Gao H, Mahoney CR, Corcoran MM, Cagigi A, Taylor A, Brown DM, Ambrozak DR, Sincomb T, Hu X, Tingle R, Georgeson E, Eskandarzadeh S, Alavi N, Lu D, Mullen T-M, Kubitz M, Groschel B, Maenza J, Kolokythas O, Khati N, Bethony J, Crotty S, Roederer M, Hedestam GBK, Tomaras GD, Montefiori D, Diemert D, Koup RA, Laufer DS, McElrath MJ, McDermott AB, Schief WR, Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 378, eadd6502 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steichen JM, Phung I, Salcedo E, Ozorowski G, Willis JR, Baboo S, Liguori A, Cottrell CA, Torres JL, Madden PJ, Ma KM, Sutton HJ, Lee JH, Kalyuzhniy O, Allen JD, Rodriguez OL, Adachi Y, Mullen T-M, Georgeson E, Kubitz M, Burns A, Barman S, Mopuri R, Metz A, Altheide TK, Diedrich JK, Saha S, Shields K, Schultze SE, Smith ML, Schiffner T, Burton DR, Watson CT, Bosinger SE, Crispin M, YatesIII JR, Paulson JC, Ward AB, Sok D, Crotty S, Schief WR, Vaccine priming of rare HIV broadly neutralizing antibody precursors in nonhuman primates. Science 384, eadj8321 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottrell CA, Hu X, Lee JH, Skog P, Luo S, Flynn CT, McKenney KR, Hurtado J, Kalyuzhniy O, Liguori A, Willis JR, Landais E, Raemisch S, Chen X, Baboo S, Himansu S, Diedrich JK, Duan H, Cheng C, Schiffner T, Bader DLV, Kulp DW, Tingle R, Georgeson E, Eskandarzadeh S, Alavi N, Lu D, Sincomb T, Kubitz M, Mullen T-M, YatesIII JR, Paulson JC, Mascola JR, Alt FW, Briney B, Sok D, Schief WR, Heterologous prime-boost vaccination drives early maturation of HIV broadly neutralizing antibody precursors in humanized mice. Sci. Transl. Med. 16, eadn0223 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffner T, Phung I, Ray R, Irimia A, Tian M, Swanson O, Lee JH, Lee C-CD, Marina-Zárate E, Cho SY, Huang J, Ozorowski G, Skog PD, Serra AM, Rantalainen K, Allen JD, Baboo S, Rodriguez OL, Himansu S, Zhou J, Hurtado J, Flynn CT, McKenney K, Havenar-Daughton C, Saha S, Shields K, Schultze S, Smith ML, Liang C-H, Toy L, Pecetta S, Lin Y-C, Willis JR, Sesterhenn F, Kulp DW, Hu X, Cottrell CA, Zhou X, Ruiz J, Wang X, Nair U, Kirsch KH, Cheng H-L, Davis J, Kalyuzhniy O, Liguori A, Diedrich JK, Ngo JT, Lewis V, Phelps N, Tingle RD, Spencer S, Georgeson E, Adachi Y, Kubitz M, Eskandarzadeh S, Elsliger MA, Amara RR, Landais E, Briney B, Burton DR, Carnathan DG, Silvestri G, Watson CT, Yates JR, Paulson JC, Crispin M, Grigoryan G, Ward AB, Sok D, Alt FW, Wilson IA, Batista FD, Crotty S, Schief WR, Vaccination induces broadly neutralizing antibody precursors to HIV gp41. Nat. Immunol. 25, 1073–1082 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray R, Schiffner T, Wang X, Yan Y, Rantalainen K, Lee C-CD, Parikh S, Reyes RA, Dale GA, Lin Y-C, Pecetta S, Giguere S, Swanson O, Kratochvil S, Melzi E, Phung I, Madungwe L, Kalyuzhniy O, Warner J, Weldon SR, Tingle R, Lamperti E, Kirsch KH, Phelps N, Georgeson E, Adachi Y, Kubitz M, Nair U, Crotty S, Wilson IA, Schief WR, Batista FD, Affinity gaps among B cells in germinal centers drive the selection of MPER precursors. Nat. Immunol. 25, 1083–1096 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Cottrell CA, Hu X, Ray R, Bottermann M, Villavicencio PM, Yan Y, Xie Z, Warner JE, Ellis-Pugh JR, Kalyuzhniy O, Liguori A, Willis JR, Menis S, Rämisch S, Eskandarzadeh S, Kubitz M, Tingle R, Phelps N, Groschel B, Himansu S, Carfi A, Kirsch KH, Weldon SR, Nair U, Schief WR, Batista FD, mRNA-LNP prime boost evolves precursors toward VRC01-like broadly neutralizing antibodies in preclinical humanized mouse models. Sci. Immunol. 9, eadn0622 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Z, Lin Y-C, Steichen JM, Ozorowski G, Kratochvil S, Ray R, Torres JL, Liguori A, Kalyuzhniy O, Wang X, Warner JE, Weldon SR, Dale GA, Kirsch KH, Nair U, Baboo S, Georgeson E, Adachi Y, Kubitz M, Jackson AM, Richey ST, Volk RM, Lee JH, Diedrich JK, Prum T, Falcone S, Himansu S, Carfi A, YatesIII JR, Paulson JC, Sok D, Ward AB, Schief WR, Batista FD, mRNA-LNP HIV-1 trimer boosters elicit precursors to broad neutralizing antibodies. Science 384, eadk0582 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Wiehe K, Borrow P, Saunders KO, Korber B, Wagh K, McMichael AJ, Kelsoe G, Hahn BH, Alt F, Shaw GM, Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 23, 142–158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu K, Acharya P, Kong R, Cheng C, Chuang G-Y, Liu K, Louder MK, O’Dell S, Rawi R, Sastry M, Shen C-H, Zhang B, Zhou T, Asokan M, Bailer RT, Chambers M, Chen X, Choi CW, Dandey VP, Doria-Rose NA, Druz A, Eng ET, Farney SK, Foulds KE, Geng H, Georgiev IS, Gorman J, Hill KR, Jafari AJ, Kwon YD, Lai Y-T, Lemmin T, McKee K, Ohr TY, Ou L, Peng D, Rowshan AP, Sheng Z, Todd J-P, Tsybovsky Y, Viox EG, Wang Y, Wei H, Yang Y, Zhou AF, Chen R, Yang L, Scorpio DG, McDermott AB, Shapiro L, Carragher B, Potter CS, Mascola JR, Kwong PD, Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 24, 857–867 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams WB, Alam SM, Ofek G, Erdmann N, Montefiori DC, Seaman MS, Wagh K, Korber B, Edwards RJ, Mansouri K, Eaton A, Cain DW, Martin M, Hwang J, Arus-Altuz A, Lu X, Cai F, Jamieson N, Parks R, Barr M, Foulger A, Anasti K, Patel P, Sammour S, Parsons RJ, Huang X, Lindenberger J, Fetics S, Janowska K, Niyongabo A, Janus BM, Astavans A, Fox CB, Mohanty I, Evangelous T, Chen Y, Berry M, Kirshner H, Itallie EV, Saunders KO, Wiehe K, Cohen KW, McElrath MJ, Corey L, Acharya P, Walsh SR, Baden LR, Haynes BF, Vaccine induction of heterologous HIV-1-neutralizing antibody B cell lineages in humans. Cell 187, 2919–2934.e20 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottrell CA, van Schooten J, Bowman CA, Yuan M, Oyen D, Shin M, Morpurgo R, van der Woude P, van Breemen M, Torres JL, Patel R, Gross J, Sewall LM, Copps J, Ozorowski G, Nogal B, Sok D, Rakasz EG, Labranche C, Vigdorovich V, Christley S, Carnathan DG, Sather DN, Montefiori D, Silvestri G, Burton DR, Moore JP, Wilson IA, Sanders RW, Ward AB, van Gils MJ, Mapping the immunogenic landscape of near-native HIV-1 envelope trimers in non-human primates. PLoS Pathog. 16, e1008753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havenar‐Daughton C, Lee JH, Crotty S, Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol. Rev. 275, 49–61 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Houser KV, Gaudinski MR, Happe M, Narpala S, Verardi R, Sarfo EK, Corrigan AR, Wu R, Rothwell RS, Novik L, Hendel CS, Gordon IJ, Berkowitz NM, Cartagena CT, Widge AT, Coates EE, Strom L, Hickman S, Conan-Cibotti M, Vazquez S, Trofymenko O, Plummer S, Stein J, Case CL, Nason M, Biju A, Parchment DK, Changela A, Cheng C, Duan H, Geng H, Teng I-T, Zhou T, O’Connell S, Barry C, Carlton K, Gall JG, Flach B, Doria-Rose NA, Graham BS, Koup RA, McDermott AB, Mascola JR, Kwong PD, Ledgerwood JE, V. 018 S. Team, Safety and immunogenicity of an HIV-1 prefusion-stabilized envelope trimer (Trimer 4571) vaccine in healthy adults: A first-in-human open-label, randomized, dose-escalation, phase 1 clinical trial. Eclinicalmedicine 48, 101477 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn WO, Parks KR, Shen M, Ozorowski G, Janes H, Ballweber-Fleming L, Davis ASW, Duplessis C, Tomai M, Dey AK, Sagawa ZK, Rosa SCD, Seese A, Siddaramaiah LK, Stamatatos L, Lee W-H, Sewall LM, Karlinsey D, Turner HL, Rubin V, Furth S, MacPhee K, Duff M, Corey L, Keefer MC, Edupuganti S, Frank I, Maenza J, Baden LR, Hyrien O, Sanders RW, Moore JP, Ward AB, Tomaras GD, Montefiori DC, Rouphael N, McElrath MJ, Use of 3M-052-AF with Alum adjuvant in HIV trimer vaccine induces human autologous neutralizing antibodies. J. Exp. Med. 221, e20240604 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski G, Georgeson E, Kalyuzhniy O, Willis JR, Kubitz M, Adachi Y, Reiss SM, Shin M, de Val N, Ward AB, Crotty S, Burton DR, Schief WR, Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nat. Commun. 8, 1655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramezani-Rad P, Cottrell CA, Marina-Zárate E, Liguori A, Landais E, Torres JL, Myers A, Lee JH, Baboo S, Flynn C, McKenney K, Salcedo E, Zhou X, Kalyuzhniy O, Georgeson E, Phelps N, Lu D, Eskandarzadeh S, Menis S, Kubitz M, Groschel B, Alavi N, Jackson AM, Lee W-H, Tran AS, Ben-Akiva E, Michaels KK, Diedrich JK, Enemuo CA, Lewis V, Pradhan A, Kasturi SP, Schiffner T, Steichen JM, Carnathan DG, Himansu S, Yates JR, Paulson JC, Ozorowski G, Irvine DJ, Silvestri G, Sok D, Ward AB, Crotty S, Schief WR, Vaccination with an mRNA-encoded membrane-bound HIV Envelope trimer induces neutralizing antibodies in animal models. Sci. Transl. Med., 2025.01.24.634423 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden LR, Sahly HME, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T,C. S. Group, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New Engl J Medicine 384, NEJMoa2035389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH, mRNA-1273 S. Group, An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 383, 1920–1931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH, mRNA-1273 S. Group, Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 383, 2427–2438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu L, McPhee R, Huang W, Bennett H, Pajon R, Nestorova B, Leav B, on behalf of the mRNA-1273 S. Group, A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 39, 2791–2799 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddler SA, Moodie Z, Clark J, Yen C, Allen M, Furch BD, Lu H, Grant S, Mondal K, Anderson M, Maenza J, Lemos MP, Davis ASW, Walsh SR, Sobieszczyk ME, Frank I, Goepfert P, Stephenson KE, Baden LR, Tieu H-V, Keefer MC, McElrath MJ, Kublin JG, Corey L, High Frequency of Chronic Urticaria Following an Investigational HIV-1 BG505 MD39.3 Trimer mRNA Vaccine in a Phase 1, Randomized, Open-Label Clinical Trial (HVTN 302). Ann. Intern. Med. (2025), doi: 10.7326/annals-24-02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner HL, Andrabi R, Cottrell CA, Richey ST, Song G, Callaghan S, Anzanello F, Moyer TJ, Abraham W, Melo M, Silva M, Scaringi N, Rakasz EG, Sattentau QJ, Irvine DJ, Burton DR, Ward AB, Disassembly of HIV envelope glycoprotein trimer immunogens is driven by antibodies elicited via immunization. Sci Adv 7, eabh2791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Leaman DP, Kim AS, de la Peña AT, Sliepen K, Yasmeen A, Derking R, Ramos A, de Taeye SW, Ozorowski G, Klein F, Burton DR, Nussenzweig MC, Poignard P, Moore JP, Klasse PJ, Sanders RW, Zwick MB, Wilson IA, Ward AB, Antibodies to a conformational epitope on gp41 neutralize HIV-1 by destabilizing the Env spike. Nat. Commun. 6, 8167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, de la Peña AT, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR, Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havenar-Daughton C, Carnathan DG, de la Peña AT, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, van der Woude P, Locci M, Le KM, de Taeye SW, Sok D, Mohammed AUR, Huang J, Gumber S, Garcia A, Kasturi SP, Pulendran B, Moore JP, Ahmed R, Seumois G, Burton DR, Sanders RW, Silvestri G, Crotty S, Direct Probing of Germinal Center Responses Reveals Immunological Features and Bottlenecks for Neutralizing Antibody Responses to HIV Env Trimer. Cell Reports 17, 2195–2209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramírez M, Back JW, Koff WC, Julien J-P, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP, HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349, aac4223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mkhize NN, Yssel AEJ, Kaldine H, van Dorsten RT, Davis ASW, Beaume N, Matten D, Lambson B, Modise T, Kgagudi P, York T, Westfall DH, Giorgi EE, Korber B, Anthony C, Mapengo RE, Bekker V, Domin E, Eaton A, Deng W, DeCamp A, Huang Y, Gilbert PB, Gwashu-Nyangiwe A, Thebus R, Ndabambi N, Mielke D, Mgodi N, Karuna S, Edupuganti S, Seaman MS, Corey L, Cohen MS, Hural J, McElrath MJ, Mullins JI, Montefiori D, Moore PL, Williamson C, Morris L, Neutralization profiles of HIV-1 viruses from the VRC01 Antibody Mediated Prevention (AMP) trials. PLOS Pathog. 19, e1011469 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klasse PJ, Ketas TJ, Cottrell CA, Ozorowski G, Debnath G, Camara D, Francomano E, Pugach P, Ringe RP, LaBranche CC, van Gils MJ, Bricault CA, Barouch DH, Crotty S, Silvestri G, Kasturi S, Pulendran B, Wilson IA, Montefiori DC, Sanders RW, Ward AB, Moore JP, Epitopes for neutralizing antibodies induced by HIV-1 envelope glycoprotein BG505 SOSIP trimers in rabbits and macaques. Plos Pathog 14, e1006913 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogal B, McCoy LE, van Gils MJ, Cottrell CA, Voss JE, Andrabi R, Pauthner M, Liang C-H, Messmer T, Nedellec R, Shin M, Turner HL, Ozorowski G, Sanders RW, Burton DR, Ward AB, HIV envelope trimer-elicited autologous neutralizing antibodies bind a region overlapping the N332 glycan supersite. Sci. Adv. 6, eaba0512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogal B, Bianchi M, Cottrell CA, Kirchdoerfer RN, Sewall LM, Turner HL, Zhao F, Sok D, Burton DR, Hangartner L, Ward AB, Mapping Polyclonal Antibody Responses in Non-human Primates Vaccinated with HIV Env Trimer Subunit Vaccines. Cell Rep. 30, 3755–3765.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charles TP, Burton SL, Arunachalam PS, Cottrell CA, Sewall LM, Bollimpelli VS, Gangadhara S, Dey AK, Ward AB, Shaw GM, Hunter E, Amara RR, Pulendran B, van Gils MJ, Derdeyn CA, The C3/465 glycan hole cluster in BG505 HIV-1 envelope is the major neutralizing target involved in preventing mucosal SHIV infection. Plos Pathog 17, e1009257 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antanasijevic A, Sewall LM, Cottrell CA, Carnathan DG, Jimenez LE, Ngo JT, Silverman JB, Groschel B, Georgeson E, Bhiman J, Bastidas R, LaBranche C, Allen JD, Copps J, Perrett HR, Rantalainen K, Cannac F, Yang YR, de la Peña AT, Rocha RF, Berndsen ZT, Baker D, King NP, Sanders RW, Moore JP, Crotty S, Crispin M, Montefiori DC, Burton DR, Schief WR, Silvestri G, Ward AB, Polyclonal antibody responses to HIV Env immunogens resolved using cryoEM. Nat Commun 12, 4817 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubrovskaya V, Tran K, Ozorowski G, Guenaga J, Wilson R, Bale S, Cottrell CA, Turner HL, Seabright G, O’Dell S, Torres JL, Yang L, Feng Y, Leaman DP, Bernat NV, Liban T, Louder M, McKee K, Bailer RT, Movsesyan A, Doria-Rose NA, Pancera M, Hedestam GBK, Zwick MB, Crispin M, Mascola JR, Ward AB, Wyatt RT, Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability. Immunity 51, 915–929.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Peña AT, Julien J-P, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens A-J, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW, Improving the Immunogenicity of Native-like HIV-1 Envelope Trimers by Hyperstabilization. Cell Rep. 20, 1805–1817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crotty S, T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersone L, Walker LSK, T-cell help in the germinal center: homing in on the role of IL-21. Int. Immunol. 36, 89–98 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis JR, Prabhakaran M, Muthui M, Naidoo A, Sincomb T, Wu W, Cottrell CA, Landais E, deCamp AC, Keshavarzi NR, Kalyuzhniy O, Lee JH, Murungi LM, Ogonda WA, Yates NL, Corcoran MM, Phulera S, Musando J, Tsai A, Lemire G, Sein Y, Muteti M, Alamuri P, Bohl JA, Holman D, Himansu S, Leav B, Reuter C, Lin L-A, Ding B, He C, Straus WL, MacPhee KJ, Regadas I, Nyabundi DV, Chirchir R, Anzala A, Kimotho JN, Kibet C, Greene K, Gao H, Beatman E, Benson K, Laddy D, Brown DM, Bronson R, Baptiste J, Gajjala S, Rikhtegaran-Tehrani Z, Benner A, Ramaswami M, Lu D, Alavi N, Amirzehni S, Kubitz M, Tingle R, Georgeson E, Phelps N, Adachi Y, Liguori A, Flynn C, McKenney K, Zhou X, Owuor DC, Owuor S, Kim S-Y, Duff M, Kim JY, Gibson G, Baboo S, Diedrich J, Schiffner T, Shields M, Matsoso M, Santos J, Syvertsen K, Kennedy A, Schroeter M, Vekemans J, Yates J, Paulson JC, Hyrien O, McDermott AB, Maenetje P, Nyombayire J, Karita E, Ingabire R, Edward V, Muturi-Kioi V, Maenza J, Shapiro AE, McElrath MJ, Edupuganti S, Taylor BS, Diemert D, Ozorowski G, Koup RA, Montefiori D, Ward AB, Hedestam GK, Tomaras G, Hunt DJ, Muema D, Sok D, Laufer DS, Andrews SF, Nduati EW, Schief WR, Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science, eadr8382 (2025). [DOI] [PubMed] [Google Scholar]
- 41.Agresti A, Coull BA, Approximate is Better than “Exact” for Interval Estimation of Binomial Proportions. Am Statistician 52, 119–126 (1998). [Google Scholar]
- 42.DAIDS, Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (2017) (available at https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf).
- 43.Montefiori DC, Evaluating Neutralizing Antibodies Against HIV, SIV, and SHIV in Luciferase Reporter Gene Assays. Curr. Protoc. Immunol. 64, 12.11.1–12.11.17 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Turner HL, Jackson AM, Richey ST, Sewall LM, Antanasijevic A, Hangartner L, Ward AB, Protocol for analyzing antibody responses to glycoprotein antigens using electron-microscopy-based polyclonal epitope mapping. STAR Protoc. 4, 102476 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimanius D, Dong L, Sharov G, Nakane T, Scheres SHW, New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng EC, Goddard TD, Pettersen EF, Couch GS, Pearson ZJ, Morris JH, Ferrin TE, UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 32, e4792 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, Chiu Y-L, McElrath MJ, Rosa SCD, Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 323, 39–54 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, Norwood C, Nyhoff LE, Edara VV, Floyd K, Rosa SCD, Ahmed H, Whaley R, Patel SN, Prigmore B, Lemos MP, Davis CW, Furth S, O’Keefe JB, Gharpure MP, Gunisetty S, Stephens K, Antia R, Zarnitsyna VI, Stephens DS, Edupuganti S, Rouphael N, Anderson EJ, Mehta AK, Wrammert J, Suthar MS, Ahmed R, McElrath MJ, Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2, 100354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R, Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alamyar E, Duroux P, Lefranc M-P, Giudicelli V, Immunogenetics, Methods and Applications in Clinical Practice. Methods Mol. Biol. 882, 569–604 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Gupta NT, Heiden JAV, Uduman M, Gadala-Maria D, Yaari G, Kleinstein SH, Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics 31, 3356–3358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gourieroux C, Monfort A, Trognon A, Pseudo Maximum Likelihood Methods: Theory. Econometrica 52, 681 (1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are in the paper or supplementary materials or are in the public repository available at https://dataverse.harvard.edu/dataverse/hvtn302 (DOI: https://doi.org/10.7910/DVN/Y8OC5L). All individual participant data have been de-identified. The raw participant information data of individuals is protected by privacy laws and ethics regulations. Representative EM maps have been deposited to the Electron Microscopy Data Bank (EMDB) under accession codes EMD-70340, EMD-70341, EMD-70342, EMD-70343, EMD-70344 and EMD-70345.


