Abstract
Neurons require high amounts of energy, and mitochondria help to fulfill this requirement. Dysfunctional mitochondria trigger problems in various neuronal tasks. Using the Drosophila neuromuscular junction (NMJ) as a model synapse, we previously reported that Mitochondrial Complex I (MCI) subunits were required for maintaining NMJ function and growth. Here, we report tissue-specific adaptations at the NMJ when MCI is depleted. In Drosophila motor neurons, MCI depletion causes profound cytological defects and increased mitochondrial reactive oxygen species (ROS). But instead of diminishing synapse function, high levels of neuronal mitochondrial ROS trigger a homeostatic signaling process that maintains normal NMJ excitation. We identify molecules mediating this compensatory response. MCI depletion in muscles also enhances local mitochondrial ROS. But high levels of muscle mitochondrial ROS cause destructive responses: synapse degeneration, mitochondrial fragmentation, and impaired neurotransmission. In humans, mutations affecting MCI subunits cause severe neurological and neuromuscular diseases. The tissue-level effects that we describe in the Drosophila system are potentially relevant to forms of mitochondrial pathogenesis.
How do neurons adapt to mitochondrial dysfunction in different tissues? This study shows that Mitochondrial Complex I depletion triggers ROS-driven compensatory signaling in motor neurons that preserves synaptic function, while in muscles, elevated ROS leads to degeneration, revealing tissue-specific responses with implications for understanding mitochondrial diseases in humans.
Introduction
Neurons have vast energy needs. These needs are primarily satisfied by healthy pools of mitochondria [1,2]. Mitochondria generate energy through the action of the ATP synthase complex in the electron transport chain [3,4]. They also perform complementary functions, including maintaining calcium homeostasis [5,6], promoting cell survival [7], triggering reactive oxygen species (ROS) signaling [8], stimulating lipid synthesis [9], and regulating innate immunity [10]. For energy-driven neurons, it is thought that the primary role of mitochondria is to provide ATP. It is less understood how other mitochondrial functions contribute to the regulation of normal neurophysiology. It is also not well understood how neural tissues or synaptic sites cope when they are challenged with a loss of mitochondria. Genetic models can help to address these puzzles.
Mitochondrial Complex I (MCI) (NADH ubiquinone oxidoreductase) is an essential part of the electron transport chain and ATP production, and it comprises dozens of distinct subunits. Much of our understanding about MCI derives from systemic analyses of its assembly. Studies have been performed on Complex I components from diverse organisms, including Neurospora crassa and Drosophila melanogaster. Those studies demonstrate that discrete MCI subunits are ancient; indeed, there are few differences between these MCI models from simple organisms and the corresponding human and bovine orthologs [11–14]. For Drosophila melanogaster, MCI consists of at least 42 distinct subunits; the 14 core MCI subunits are present, as are at least 28 accessory subunits [12].
In humans, MCI dysfunction has been linked to diseases such as Leigh syndrome, mitochondrial myopathy, and encephalomyopathy, as well as forms of stroke [15–18]. On a cellular level, MCI dysfunction can cause the demise of neurons and muscles; these phenotypes are typically attributed to defects in ATP production [19,20]. However, in addition to the ATP production defects, mutations affecting MCI subunit components are also associated with excess mitochondrial ROS. Normally, ROS accumulation can be neutralized by the cellular antioxidant system [21]. But if that system becomes overwhelmed, there can be consequences for cells and organ systems—including progressive neurodegeneration and seizures for the nervous system [22–24]. On the level of synapses, it is possible that MCI loss triggers severe molecular consequences, and it is also possible that excess ROS plays a role.
For a previous paper, we depleted MCI function at the Drosophila neuromuscular junction (NMJ). Our data suggested fundamental synaptic functions for MCI [13]. Here we expand upon that work, mostly taking advantage of RNAi-mediated depletion of the Drosophila homologs of human NDUFS7. We also scrutinize loss-of-function mutants of other MCI subunits and pharmacological inhibition of MCI. Our collective data show that MCI depletion causes Drosophila phenotypes reminiscent of mitochondrial diseases, such as progressive degeneration of muscle and presynaptic cytoskeleton, excess ROS production, loss of mitochondria, and alteration in mitochondrial morphology.
On single-tissue levels, we were surprised to find that there were opposite effects on synapse activity in the presynaptic motor neurons versus the postsynaptic muscles. MCI dysfunction in Drosophila motor neurons causes profound cytological phenotypes, but there are no significant functional phenotypes. This appears to be because neuronal mitochondrial ROS triggers an adaptive response, demonstrated visually by active zone enhancement. This ROS-driven enhancement of active zones occurs through at least two processes: 1) regulation of calcium flux from intracellular stores (ER) and mitochondria and 2) use of glycolysis as an alternative energy source. By contrast, postsynaptic depletion of MCI and the associated elevation of muscle ROS trigger a destructive response: disruption of NMJ morphology and the Dlg-Spectrin scaffold that is critical for normal active zone-receptor apposition. To our knowledge, these cellular and molecular mechanisms of MCI deficiency have not previously been elucidated at synapse-specific or tissue-specific levels.
Results
One RNAi transgene targets two Drosophila homologs of a core MCI subunit, NDUFS7
We previously reported impairments in NMJ synapse development and function when Mitochondrial Complex I (MCI) was depleted [13]. We observed robust synaptic phenotypes when driving a transgenic RNAi line against an MCI subunit, termed UAS-ND-20L[RNAi] (ND-20LHMJ23777) [13]. That RNAi line encodes a short hairpin that matches 21 consecutive nucleotides of the ND-20L gene. Recent work indicates that ND-20L is only sparsely expressed in Drosophila tissues [25–27]. However, the same short hairpin also matches 19 consecutive nucleotides of the ND-20 gene, and ND-20 is ubiquitously expressed [25–27]. ND-20 and ND-20L encode Drosophila homologs of the core NDUFS7 MCI subunit. Because both genes are effectively “on-target” for the RNAi transgene, we term the UAS-ND-20L[RNAi] transgene as UAS-NDUFS7[RNAi] for this study.
We used the GAL4/UAS system to verify that UAS-NDUFS7[RNAi] can diminish MCI function in relevant tissues. We drove its expression using neuronal or muscle GAL4 drivers. By quantitative RT-PCR, message levels of both NDUFS7-encoding genes were significantly diminished in those tissues in larvae (S1A–S1D Fig). ND-20L message levels were down in both neurons and muscle, but variable in neurons, likely due to the low endogenous expression of ND-20L (S1A and S1B Fig). ND-20 message levels were significantly knocked down by the transgene in both tissues (S1C and S1D Fig). We also tested if the transgene could diminish mitochondrial respiration by a Seahorse assay. To acquire sufficient material for the assay, we collected mitochondria from adult muscles (see STAR methods). Driving the UAS-NDUFS7[RNAi] transgene in adult muscle starkly reduced oxygen consumption rate (OCR) in muscle mitochondria across all time points tested (S1E and S1F Fig). These assays confirmed that UAS-NDUFS7[RNAi] targets MCI.
Depletion of MCI affects mitochondrial integrity in multiple Drosophila synaptic tissues
We wanted to understand what was happening to mitochondria to affect synapse function when MCI was depleted. We started by examining mitochondria by microscopy. To visualize them, we expressed a UAS-Mito-GFP transgene [28] in Drosophila tissues. Concurrently, we used tissue-specific GAL4 drivers alone (as controls) or GAL4 drivers + UAS-NDUFS7[RNAi] to deplete MCI function [13]. With these tools, we made qualitative observations of mitochondrial morphology (Fig 1), and we quantified those observations in subsequent analyses.
Fig 1. MCI-depleted flies harbor fewer mitochondria at neuromuscular junctions.
Mitochondrial morphology and trafficking defects in the ventral nerve cord (VNC), distal axons, and boutons. RNAi lines and controls were crossed to a motor neuron driver (D42-GAL4) and a mitochondrial marker (UAS-mito-GFP). (A, A′) control VNC, (B, B′) a magnified section of VNC, (C, C′) proximal (A2) axons, and (D, D′) distal (A5) axons. These tissues exhibit regular mitochondrial clusters in the soma and axons. (E, E′) NDUFS7 knockdown in the VNC, (F, F′) a magnified section of VNC, (G, G′) proximal (A2) axons, and (H, H′) distal (A5) axons. Mitochondria are abnormally clustered from NDUFS7[RNAi] expression in the VNC and distal segments of A5 axons. NDUFS7[RNAi] expression also yields fewer mitochondria in the distal segments when compared to the proximal segments. (I, J) Representative electrophysiological traces showing evoked potentials of mitoGFP, D42-Gal4 × UAS-NDUFS7[RNAi] larvae at the A5 hemisegment of muscle 6/7 synapse. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). Fewer mitochondria at the presynaptic A5 hemisegment did not affect evoked NMJ excitation. (K) Quantification showing EPSP amplitude at NMJ 6/7 in control (D42-Gal4/+; EPSP: 19.99 mV ± 2.53, n = 6) and RNAi-depleted animals (NDUFS7[RNAi]/+; D42-Gal4/+;EPSP: 16.88 mV ± 1.21, n = 9). (L, M) Representative images showing mitochondrial morphology in control and RNAi-depleted animals in muscles. Mitochondria in NDUFS7-depleted larval muscles are clustered compared to the control muscles. (N–Q) NDUFS7[RNAi] expression yields almost no mitochondria in boutons when co-stained with pre- (HRP) or postsynaptic markers (Discs Large, Dlg). (A–H′, L–M, and N–O) Scale bar: 10 µm. (P) Quantification showing the number of mitochondrial clusters at NMJ 6/7 in control (D42-Gal4/+ ; # clusters: 113.6 ± 11.97, n = 7) and RNAi-depleted animals (NDUFS7[RNAi]/+ ; D42-Gal4/+ ; # clusters: 30.33 ± 4.02, n = 6). (Q, R) 3D-rendered image revealing the volume (µm 3) of mitochondria at NMJ 6/7 in RNAi knockdown larvae (NDUFS7[RNAi]/+; D42-Gal4/+; 0.11 ± 0.01 µm 3, n = 16) compared to the driver control animals (D42-Gal4/+; 0.29 ± 0.04 µm 3, n = 14). Insets are large boutons from the same images rotated to display 3D-rendered mitochondrial clusters at these synaptic terminals. Scale bar: 5 µm. (S) Quantification shows a significantly lower mitochondria volume in boutons at NMJ 6/7 in NDUFS7-depleted neurons. ***p < 0.0001 and ***p = 0.0003 for mitochondrial clusters and volume, respectively. Statistical analyses based on Student t test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S1 Data Excel file, tab Fig 1.
In motor neurons, the Mito-GFP signal localized to the neuropil of the VNC (Fig 1A–1A′ and 1B–1B′). In control neurons, the neuropil mitochondria had a filamentous appearance. By contrast, NDUFS7-depleted neurons had punctate and clustered mitochondria. (Fig 1E–1E′ and 1F–1F′). We examined mitochondria in the motor axons that innervate proximal and distal NMJs (Fig 1C–1C′, 1D–1D′, 1G–1G′, and 1H–1H′). The proximal segment A2 axons had abundant mitochondria in all cases (Fig 1C–1C′ and 1G–1G′). However, for the distal segment A5 axons, NDUFS7 depletion elicited an obvious decrease in mitochondria number (Fig 1D–1D′ and 1H–1H′). This A2 versus A5 discrepancy was consistent with prior work by others examining defects in mitochondrial trafficking dynamics: distal sites can show phenotypes more prominently [29,30].
We hypothesized that fewer mitochondria in the A5 axon might correlate with a neurotransmission defect at the NMJ. Yet by NMJ electrophysiology, we found no significant differences in the evoked amplitude compared to the control NMJs in the distal segment A5 (Fig 1I–1K). These data matched our prior examination of MCI at the A2 and A3 segments of the NMJ, where neuronal impairment of MCI was not sufficient on its own to reduce evoked NMJ neurotransmission [13].
In muscle, we observed an array of phenotypes. As with neurons, there were clustered mitochondria when NDUFS7 gene function was depleted (Fig 1L and 1M). Additionally, there was a tissue-level phenotype: NDUFS7-depleted muscles were developed, but they looked disorganized and fragmented, with oblong-shaped nuclei in the muscle syncytia (Fig 1L and 1M). This phenotype could explain why we previously observed that muscle impairment of MCI was sufficient to reduce evoked NMJ neurotransmission [13].
To examine the mitochondria at presynaptic NMJ release sites, we used the motor neuron GAL4 driver to label NMJ boutons with Mito-GFP. For image analysis, we marked the presynaptic membrane boutons with anti-HRP immunostaining. Control NMJs contained abundant and large clusters of mitochondria in synaptic boutons, but by comparison, NDUFS7[RNAi] boutons contained small clusters and few mitochondria (Fig 1N and 1P). We measured the mitochondrial volume in a three-dimensional stack and compared it to the synaptic volume (Fig 1Q–1S). The Mito-GFP signal occupied a sizeable proportion of the bouton volume in controls (~30%), but this value was significantly diminished in NDUFS7-depleted animals (~10%) (Fig 1S). Collectively, our data suggest that the depletion of NDUFS7 by RNAi leads to abnormal mitochondrial clustering in the neuronal cell body and muscle—as well as losses of distal axon and synaptic mitochondria.
Loss of MCI phenocopies loss of Mitofusin
The cell-level NDUFS7-depletion phenotypes were reminiscent of Drosophila mutants impairing mitochondrial dynamics [29,31]. Therefore, we re-examined MCI-depleted mitochondria, this time additionally impairing genes known to mediate mitochondrial fusion and fission. Mitofusin 1 (Mfn1) and Mitofusin 2 (Mfn2) are GTPases that regulate outer mitochondrial membrane fusion [32,33]. The Drosophila gene encoding the Mitofusin homolog is called marf. Dynamin-related protein 1 is a GTPase that regulates mitochondrial fission. In Drosophila, this factor is encoded by the gene drp1 [34]. Previous work reported that defective fusion results in fragmented mitochondria, while defective fission can lead to enlarged mitochondria [35]. We used RNAi-mediated knockdown constructs for each of these genes.
As before, we observed that wild-type motor neurons had filamentous and oval mitochondria, while NDUFS7-depleted neurons had fewer and smaller clustered mitochondria in the ventral nerve cord (VNC) (Fig 2A) and axons (Fig 2B). Knockdown of the fusion gene marf phenocopied NDUFS7 loss, revealing small mitochondria in motor neurons, while knockdown of the fission gene drp1 yielded filamentous mitochondria (Fig 2A). Simultaneously depleting motor neurons of marf and NDUFS7 by RNAi did not show any additive defect in mitochondrial appearance in the VNC and axons (Fig 2A and 2B). This result could mean that the genes share a common process to regulate mitochondrial fusion. By contrast, depleting drp1 and NDUFS7 by RNAi simultaneously yielded punctate mitochondria. This result likely means that the punctate NDUFS7 mitochondrial phenotypes (potential fusion phenotypes) are epistatic to drp1 loss (Fig 2A and 2B).
Fig 2. Loss of NDUFS7 in motor neurons phenocopies a marf depletion.
Mitochondrial morphology and trafficking defects in the ventral nerve cord and distal axons. To label neuronal mitochondria, UAS-[RNAi] lines and controls were crossed to a motor neuron driver (D42-Gal4) and a mitochondrial marker (UAS-mitoGFP). (A) Ventral nerve cord (VNC): UAS-mitoGFP and drp1[RNAi] exhibit normal mitochondrial organization, NDUFS7[RNAi] and marf[RNAi] exhibit clustered mitochondria, NDUFS7[RNAi]; marf[RNAi] and NDUFS7[RNAi]; drp1[RNAi] doubles exhibit clustered mitochondria in the soma. The fluorescent images were subsequently skeletonized to measure mitochondrial branch length. (B) Comparison of a proximal axonal segment in A2 and a distal segment in A5. Distal segments of A5 axons in NDUFS7[RNAi] and marf[RNAi] contain many fewer mitochondria than proximal segments. Knocking down NDUFS7[RNAi] and marf[RNAi] together does not show an additive effect. (A, B) Scale bar: 10 µm. (C, D) Histogram showing mitochondrial branch length (µm) and number (µm 2 area of bouton) in VNC and axons of the third instar larvae in the indicated genotypes. (E–L) Representative images of the A2 hemisegment of muscle 6/7 NMJs in (E) UAS-mito-GFP, D42-Gal4/+, (F) UAS-Sod2/+; UAS-mito-GFP, D42-Gal4/+, (G) NDUFS7[RNAi]/+; UAS-mito-GFP, D42-Gal4/+, (H) NDUFS7[RNAi]/UAS-Sod2; UAS-mito-GFP, D42-Gal4/+, (I) UAS-mito-GFP, D42-Gal4/marf[RNAi], (J) UAS-Sod2/+; UAS-mito-GFP, D42-Gal4/marf[RNAi], (K) NDUFS7[RNAi]/+; UAS-mito-GFP, D42-Gal4/marf[RNAi], and (L) NDUFS7[RNAi]/UAS-Sod2; UAS-mito-GFP, D42-Gal4/marf[RNAi] larvae immunostained with antibodies against HRP (magenta) and GFP (mito-GFP:green) to label neurons and mitochondria. NDUFS7[RNAi]- and marf[RNAi]-depleted animals harbor fewer mitochondria at the terminals as compared to control animals. Transgenic UAS-Sod2 did not rescue mitochondrial clustering defects. (E–L) Scale bar: 5 µm. (M) Histograms showing quantification of mitochondrial clusters at the NMJs in the indicated genotypes. ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S1 Data Excel file, tab Fig 2.
We measured mitochondrial branch length from skeletonized images of the mitochondria (Skeletonize3D, ImageJ plugin, data in Tables A, B, and C in S1 Tables). Control and drp1 knockdown showed normal mitochondrial branch length, but knockdown using NDUFS7[RNAi] or marf[RNAi]—or knockdowns using combinations of each—exhibited short branch length (Fig 2C and Table A in S1 Tables).
To quantify mitochondria in axons, we counted Mito-GFP-positive puncta in distal A5 motor axons labeled by anti-GFP. Control axons and drp1-depleted axons contained abundant mitochondria (Fig 2D and Table A in S1 Tables). By contrast, any gene manipulation or combination targeting NDUFS7 or marf by RNAi resulted in diminished numbers of mitochondrial clusters (Fig 2D and Table A in S1 Tables).
We extended the analysis to NMJ terminals. We counted Mito-GFP clusters in presynaptic boutons apposed by postsynaptic densities, labeled by anti-Discs Large 1 (Dlg1) (Fig 2E–2M and Table D in S1 Tables). The results matched our earlier observations (Fig 1N–1S). Control NMJs and drp1-depleted NMJs contained numerous mitochondrial clusters per bouton (Fig 2E and 2M). However, NDUFS7-depleted boutons contained few mitochondria, and this was phenocopied by marf[RNAi] (Fig 2E–2M and Table D in S1 Tables). Collectively, these results suggest that Drosophila NDUFS7 (and hence MCI) contributes to normal mitochondrial fusion, likely in conjunction with the Mitofusin homolog, Marf.
Mitochondrial ROS contribute to synaptic phenotypes
Several studies have demonstrated that Complex I loss results in high levels of mitochondrial ROS [36–40]. This means that excess ROS could be contributing to the cytological and mitochondrial fusion phenotypes that we have described.
We checked if we could observe mitochondrial ROS (superoxide) in living Drosophila tissue and if ROS levels corresponded to Complex I function (S1 Fig). We used a commercially available fluorescent mitochondrial superoxide indicator, MitoSOX (ThermoFisher, STAR methods) [41–43]. With MitoSOX, we observed mitochondrial superoxide in many tissues by fluorescence microscopy. There was a baseline level of ROS in controls (S2A, S2E, S2I, and S2J Fig and Table E in S1 Tables), and the level was greatly increased in NDUFS7-deficient motor neuron cell bodies and muscle (S2B, S2F, S2I, and S2J Fig and Table E in S1 Tables).
Next, we tested if ROS scavengers could reverse the high MitoSOX fluorescence levels in NDUFS7-deficient tissues. We fed a pharmacological scavenger, N-Acetyl Cysteine Amide (NACA) [44–46] to Drosophila larvae (STAR methods). We also used a transgene, UAS-Sod2 [47], to express a superoxide dismutase enzyme. Both successfully diminished the high levels of mitochondrial ROS that resulted from NDUFS7 depletion at the NMJ, and both worked in muscle and neurons (S2C, S2D, and S2G–S2J and Table E in S1 Tables). We also tested a related idea: if genetic Sod2 knockdown by RNAi could phenocopy loss of MCI by enhancing MitoSOX levels in these same tissues. It did: Transgenic UAS-Sod2[RNAi] enhanced MitoSOX signal in all tissues and subcompartments, including the VNC (S3A–S3C Fig and Table F in S1 Tables), the motor neuron axons (S3D–S3F Fig and Table F in S1 Tables), the synaptic boutons (S3G–S3I Fig and Table F in S1 Tables), and the muscle (S3J–S3L Fig and Table F in S1 Tables).
Next, we tested if these same ROS scavengers could reverse mitochondrial phenotypes caused by loss of MCI. Co-expressing UAS-Sod2 or rearing larvae with NACA suppressed the mitochondrial morphology defects in the VNC of NDUFS7-depleted animals; it also restored axonal loss of mitochondria (S4A–S4D Fig and Table B in S1 Tables). To test an additional MCI manipulation, we knocked down Drosophila ND-30 (homologous to human NDUFS3) in motor neurons. As with NDUFS7, depleting ND-30 in motor neurons yielded punctate mitochondria in the VNC, but the addition of UAS-Sod2 restored a wild-type, filamentous mitochondrial morphology (S4A and S4C Fig). Similarly, loss of ND-30 gene function in neurons depleted A5 axonal mitochondria and this phenotype was also reversed by UAS-Sod2 transgenic expression (S4B and S4D Fig and Table B in S1 Tables). In contrast to UAS-Sod2, neither UAS-Sod1 nor UAS-Catalase worked to reverse mitochondrial morphology defects (S5 Fig and Table C in S1 Tables). SOD1 (cytosol) and Catalase (peroxisome) localize to different compartments than SOD2 (mitochondrial matrix). Therefore, these results could indicate that a scavenger needs to access the proper compartment for rescue.
Because of the links between MCI and mitochondrial fusion, we considered whether a marf loss of function could also yield high levels of neuronal ROS (S6 Fig). It did: both marf and NDUFS7 loss-of-function conditions showed high levels of mitochondrial superoxide in motor neuron cell bodies (S6A–S6D, S6A′–S6D′ and S6M Fig and Table E in S1 Tables) in motor axons (S6E–S6H, S6E′–S6H′ and S6N Fig and Table E in S1 Tables); and at NMJ sites (S6I–S6L, S6I′–S6L′, and S6O Fig and Table E in S1 Tables). These ROS phenotypes were not confined to genetic manipulations. We made similar observations when MCI was impaired pharmacologically by feeding rotenone to developing larvae (S6 Fig and Table E in S1 Tables). In the case of rotenone, the amount of mitochondrial ROS in the tissues was high, but it was generally not increased as much as with the genetic manipulations (S6M–S6O Fig and Table E in S1 Tables).
Finally, because the distal A5 motor axons accumulated high levels of ROS when subjected to these insults, we examined them for synaptic vesicle trafficking defects. We immunostained for Cysteine String Protein (CSP), a DNAJ-like co-chaperone and synaptic vesicle-associated protein (CSP). NDUFS7 depletion caused aberrant accumulation of CSP in the A5 motor axons; and this defect was suppressed by motor neuron transgene overexpression of UAS-Sod2 or by feeding animals with NACA (S7A–S7L Fig and Table G in S1 Tables). However, this phenotype was not suppressed by motor neuron overexpression of UAS-Sod1 or UAS-Catalase (S8 Fig and Table H in S1 Tables).
ROS scavengers did not reverse all mitochondrial abnormalities. For example, expressing UAS-Sod2 in the UAS-NDUFS7[RNAi] or UAS-marf[RNAi]-depletion backgrounds did not restore mitochondrial clusters to motor neuron terminals (Fig 2E–2M and Table D in S1 Tables). For the remainder of the study, we used scavengers as complementary tools to test which MCI-loss phenotypes were likely due to mitochondrial ROS.
Loss of MCI subunits impairs synaptic cytoskeletal stability
ROS can modulate the cytoskeleton, either through redox modification of cytoskeletal proteins or by altering pathways that regulate cytoskeletal organization [48]. To test whether the mitochondrial defects and abnormal accumulation of ROS were associated with the altered synaptic cytoskeleton, we labeled synaptic boutons with an anti-Futsch antibody (Fig 3). Futsch is a Drosophila MAP1B homolog that associates with microtubules [49].
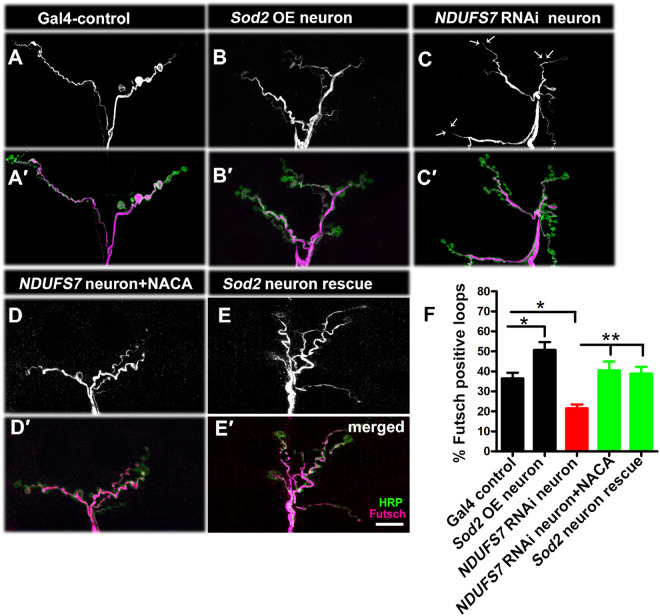
Fig 3. NDUFS7 depletion in motor neurons affects synapse stability.
Representative confocal images of NMJ synapses at muscle 6/7 of (A, A′) D42-Gal4 control, (B, B′) UAS-Sod2 overexpression (C, C′) D42-Gal4-driven NDUFS7[RNAi] (NDUFS7[RNAi]/+; D42-Gal4/+), (D, D′) NDUFS7 knockdown with NACA rescue (NDUFS7[RNAi]/+; D42-Gal4/+ with NACA), (E, E′) NDUFS7 knockdown with UAS-Sod2 (UAS-NDUFS7[RNAi]/UAS-Sod2; D42-Gal4/+). Each condition was double immunolabeled with 22C10 (anti-Futsch, magenta) and anti-HRP (green) antibodies. The motor neuron-depleted NDUFS7[RNAi] larvae showed a decrease in the number of Futsch-positive loops as compared to the Gal4 control. Futsch-positive loops were significantly restored to the control number when NDUFS7[RNAi] knockdown larvae were raised in media containing NACA or when genetically expressing UAS-Sod2 in the UAS-NDUFS7[RNAi] knockdown background. Scale bar: 10 µm. (F) Histograms showing the percentage of Futsch-positive loops in the indicated genotypes. *p = 0.01 (Gal4 control vs. Sod2 OE neuron), *p = 0.0008 (Gal4 control vs. NDUFS7[RNAi] neuron), **p = 0.001 (NDUFS7[RNAi] neuron vs. NDUFS7[RNAi] neuron with NACA) and **p = 0.0005 (NDUFS7[RNAi] neuron vs. Sod2 neuron rescue). Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S1 Data Excel file, tab Fig 3.
In motor neuron Gal4-control and UAS-Sod2 overexpression larvae, Futsch organized in periodic loops, as expected from previous characterizations [49] (Fig 3A and 3B and Table G in S1 Tables). But in NDUFS7-depleted larvae, the anti-Futsch staining showed a significant reduction in microtubule loops (Fig 3C and Table G in S1 Tables). These NDUFS7 phenotypes were suppressed by motor neuron overexpression of UAS-Sod2 or by raising animals on food containing 0.5 mM NACA (Fig 3D and 3E and Table G in S1 Tables). However, they were not significantly restored by neuronal overexpression of UAS-Sod1 or UAS-Catalase (S9 Fig and Table H in S1 Tables). These data indicate that loss of MCI regulates cytoskeletal architecture due to excessive accumulation of mitochondrial ROS in neurons.
Modest neurotransmission phenotypes after motor neuron-specific loss of MCI or Marf
Given the cytological phenotypes after neuronal MCI loss, it was puzzling that there seemed to be little-to-no electrophysiological consequence at NMJs ([13] and Fig 1J). We probed this finding, this time depleting motor neurons of marf and/or NDUFS7 gene function. We recorded spontaneous miniature excitatory postsynaptic potentials (mEPSP) and excitatory postsynaptic potentials (EPSP).
Phenotypes were normal-to-mild (S10 Fig). For both NDUFS7[RNAi] and marf[RNAi], there were small, but statistically significant decreases in mEPSP amplitude (S10A–S10C and S10G Fig and Table I in S1 Tables). But for NDUFS7[RNAi], evoked events (EPSP) and calculated quantal content (QC) were at control levels (S10A, S10B, S10H, and S10I Fig and Table I in S1 Tables). For neuronal marf[RNAi], those measures were near-normal, with a slight decrease in EPSP amplitude (S10C and S10H Fig and Table I in S1 Tables) and a slight increase in QC (S10I Fig and Table I in S1 Tables). Finally, for a double NDUFS7[RNAi] + marf[RNAi] knockdown condition in neurons, we observed a small, but statistically significant decrease in EPSP amplitude (S10E and S10H Fig and Table I in S1 Tables).
Synaptic phenotypes caused by mitochondrial dysfunction might be masked until synapses are challenged with extreme conditions, like high-frequency stimulation [50,51]. Therefore, we challenged neuronally NDUFS7-depleted Drosophila NMJs in several ways (S11 Fig). First, we lowered recording saline [Ca2+] to 0.15 mM, which is roughly one order of magnitude lower than physiological calcium. In low calcium, the motor neuron-driven NDUFS7[RNAi] NMJs had slightly smaller evoked potentials compared to driver controls, but the numerical reduction was not statistically significant (S11A–S11C Fig and Table J in S1 Tables). Next, we lowered extracellular [Ca2+] even further, to 0.1 mM, which yielded a mix of successful EPSP firing events and failures. Failure analyses revealed a decrease in failure rate at the neuronally depleted NDUFS7[RNAi] NMJs compared to control animals (S11D Fig and Table J in S1 Tables). This was slightly surprising because it potentially demonstrates an increased probability of release. Higher failure rates in 0.1 mM calcium were restored when NDUFS7-depleted larvae were raised in a media containing NACA or genetically expressing Sod2 in motor neurons (S11D Fig and Table J in S1 Tables), indicating that presynaptic release probability could be related to mitochondrial ROS levels.
Finally, we checked if forms of short-term neuroplasticity were affected by NDUFS7 loss in motor neurons. For two different extracellular [Ca2+] conditions (0.4–1.5 mM), we did not observe any significant changes in paired-pulse ratios (S11E–S11J Fig and Table J in S1 Tables). Likewise, we did not note depreciation of evoked neurotransmission over the course of high-frequency stimulus trains in high calcium (S11K–S11P Fig). Collectively, these data suggest that there might be small effects on NMJ physiology due to loss of MCI or defective mitochondrial fusion and ROS production. But the aggregate data also indicate that neuronal mitochondrial defects alone do not drastically affect NMJ neurotransmission.
Loss of MCI in neurons controls the level and distribution of the active zone to stabilize synaptic strength
We wondered how the NMJ synapse might evade severe dysfunction—and even show resilience during a failure analysis—despite loss of mitochondria in the motor neurons and synaptic terminals. One possibility is that NDUFS7 loss/MCI impairment could trigger a form of functional homeostatic compensation of the NMJ. Another idea is that the mitochondrial ATP generated is superfluous at the NMJ—and that any energy-intensive functions that mitochondria support could be redundantly covered by glycolysis. These models are not mutually exclusive, and for any scenario, mitochondrial ROS downstream of defective MCI could be a candidate signal. Recent findings have demonstrated that ROS intermediates, mitochondrial distribution, and mitochondrial trafficking all affect development of the Drosophila NMJ [48,52,53].
To probe this idea, we imaged the presynaptic active zone apparatus in neuronally depleted NDUFS7[RNAi] flies. Third-instar larval active zones showed a decrease in active zone protein Bruchpilot (BRP) puncta density per unit area in NDUFS7-depeleted NMJs compared to control NMJs (Fig 4A–4C and 4G–4I and Table K in S1 Tables). But they also showed a robust enhancement phenotype: a 40% increase in active zone (BRP) immunofluorescence signal per unit area, compared to control by laser scanning confocal microscopy (Fig 4A–4C, 4F, 4H, and 4I and Table K in S1 Tables). This result was intriguing because NMJ active zone enhancements (or changes in active zone sub-structure) have been proposed by other labs to be molecular correlates of forms of homeostatic plasticity and potentiation of neurotransmitter release [54–58]. This result matched the possibility that the NMJs evade severe dysfunction through a form of synaptic homeostasis.
Fig 4. Neuronal ROS (nROS) controls active zone material levels at NMJs.
(A) Representative images of the A2 hemisegment of muscle 6/7 NMJs in UAS-mito-GFP, D42-Gal4/+, (B) UAS-Sod2/+; UAS-mito-GFP, D42-Gal4/+, (C) NDUFS7[RNAi]/+; UAS-mito-GFP, D42-Gal4/+, (D) NDUFS7[RNAi]/+; UAS-mito-GFP, D42-Gal4/+ with NACA, and (E) NDUFS7[RNAi]/UAS-Sod2; UAS-mito-GFP, D42-Gal4/+ larvae immunostained with antibodies against the active zone scaffold Bruchpilot (BRP:fire-LuT) to label the active zones. BRP levels are upregulated at the NMJs in NDUFS7-depleted flies, while overexpression of ROS scavenger gene Sod2 in the neuron or feeding the larvae with N-Acetyl l-cysteine amide (NACA) restores BRP to the control level. (A–E) Scale bar: 2.5 µm. (F–G) Histograms showing quantification of BRP intensity (F) and density (G) per µm 2 area of bouton at muscle 6/7 in the genotypes mentioned above. At least 8 NMJs of each genotype were used for quantification. ***p < 0.0001. Error bars denote mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (H, H′ and I, I′) Representative confocal images of muscle 6/7 NMJs in the (H, H′) control (D42-Gal4/+) and (I, I′) motor neuron Gal4-driven NDUFS7[RNAi] (NDUFS7[RNAi]/+; D42-Gal4/+) immunostained with antibodies against Bruchpilot (BRP: magenta) and GluRIII (green) to label a glutamate receptor subunit. (H, H′ and I, I′) Scale bar: 2.5 µm. There are no significant changes of GluRIII-BRP apposed clusters. At least 8 NMJs of each genotype were used for quantification (J–P). Representative traces, quantification of mEPSPs, EPSPs, and quantal content in the indicated genotypes. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). EPSPs amplitudes were maintained in NDUFS7-depleted flies due to increased levels of active zone material (e.g., BRP); however, NMJs with Sod2-rescued NDUFS7[RNAi] in neurons showed diminished evoked release when compared with NDUFS7[RNAi]. Minimum 8 NMJs recordings of each genotype were used for quantification. *p < 0.05, ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars denote the standard error of the mean. (Q) Representative images of the A2 hemisegment of muscle 6/7 NMJs in UAS-mito-GFP, D42-Gal4/+, (R) UAS-Sod2/+; UAS-mito-GFP, D42-Gal4/+, (S) NDUFS7[RNAi]/+; UAS-mito-GFP, D42-Gal4/+, and (T) NDUFS7[RNAi]/UAS-Sod2; UAS-mito-GFP, D42-Gal4/+ larvae immunostained with antibodies against HRP (magenta) and GFP (mito-GFP:green) to label neurons and mitochondria. NDUFS7-depleted and Sod2-rescued NDUFS7[RNAi] animals harbor fewer mitochondria at the terminals than control animals. (Q–T) Scale bar: 5 µm. (U) Histograms showing quantification of mitochondrial clusters in the above-indicated genotypes. (V–X) Schematic illustration (drawn on bioRender) showing ROS (magenta) levels, BRP (gray) and mitochondria (red) number in the indicated genotypes. At least 8 NMJs of each genotype were used for quantification. ***p < 0.0001. Error bars represent mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Raw data for this figure are available in the S1 Data Excel file, tab Fig 4.
As an independent test, we impaired MCI pharmacologically. To do this, we raised larvae on 50 µM rotenone-spiked food; and we also incubated wild-type fillet preparations with 500 µM of rotenone for extended time. For both cases, we observed significant increases in BRP protein at the presynaptic active zones (S12A–S12U Fig and Table L in S1 Tables). For the extended incubation, the fillet preparations required sufficient rotenone incubation time (six hours) and an intact motor nerve to show the active zone enhancement (S12U Fig and Table L in S1 Tables). This result suggested that downstream of MCI depletion, a compensatory delivery of active zone material required either substantial trafficking time and/or fully intact neuroanatomy.
Next, we checked if the enhanced active zone signal was triggered by excess mitochondrial ROS in motor neurons. Indeed, we found that the NDUFS7-depletion active zone enhancements were fully reversed by ROS scavengers, either by raising larvae in food containing NACA or by neuronally expressing UAS-Sod2 (Fig 4D–4G and Table K in S1 Tables). However, they were not reversed by overexpressing UAS-Sod1 or UAS-Catalase (S13A–S13G Fig and Table M in S1 Tables).
Finally, we assessed synapse function. As with our prior recordings, evoked postsynaptic potentials at the NMJ were not significantly changed by NDUFS7 depletion in motor neurons. But interestingly, scavenging mitochondrial ROS in the NDUFS7[RNAi] neuronal depletion background with UAS-Sod2 unmasked a small deficit in NMJ excitation, compared to controls (Fig 4J–4P and Table K in S1 Tables). As before, neither UAS-Sod1 overexpression nor UAS-Catalase overexpression unmasked this deficit in NMJ excitation (S13N–S13P Fig and Table M in S1 Tables).
These data could mean that mitochondrial ROS is helping to maintain synaptic activity. Neuronal expression of UAS-Sod2 did not restore mitochondrial clusters to the NMJ after NDUFS7 gene function depletion (Fig 4Q–4U and Table K in S1 Tables; like Fig 2H), meaning that the synaptic sites were still deficient in mitochondria. And as expected, neuronal expression of UAS-Sod1 and UAS-Catalase also failed to mitochondrial clusters to the NMJ (S13Q–S13W Fig and Table M in S1 Tables). Collectively, our data support a model in which neuronal ROS (nROS) triggers active zone enhancement and functional compensation when MCI is limiting (Fig 4V–4X and Table K in S1 Tables).
Neuronal MCI subunits stabilize synaptic strength in conjunction with intracellular calcium signaling proteins
Recent work described a mechanism for local calcium uptake into mitochondria that drives ATP production to maintain synaptic function [59]. The process is governed by the mitochondrial calcium uniporter (MCU) and its accessory EF-hand MICU proteins [59]. Beyond this role for mitochondrial calcium, there are also known roles for core synaptic functions like vesicle cycling [60,61].
To test if mitochondrial or neuronal calcium could be involved in maintaining synapse function at the NMJ, we acquired genetic reagents to examine depressed MCU function in conjunction with depressed MCI. We also used pharmacological reagents to inhibit release of intracellular sources of calcium, like those from the Ryanodine Receptor (RyR) and the IP3 receptor (IP3R) of the endoplasmic reticulum (ER) [62], as well as a genetic reagent that we previously used at the NMJ to deplete IP3 signaling (UAS-IP3-sponge) (Fig 5A–5D). For this set of experiments, we used the NDUFS7 neuronal knockdown condition as a sensitized genetic background (Fig 5E–5T and Table N in S1 Tables). We also used a pan-neuronal driver (elaV-Gal4) to facilitate Drosophila genetic stock construction instead of a motor neuronal driver. We previously observed similar NMJ effects either for motor neuronal or pan-neuronal depletion of MCI [13].
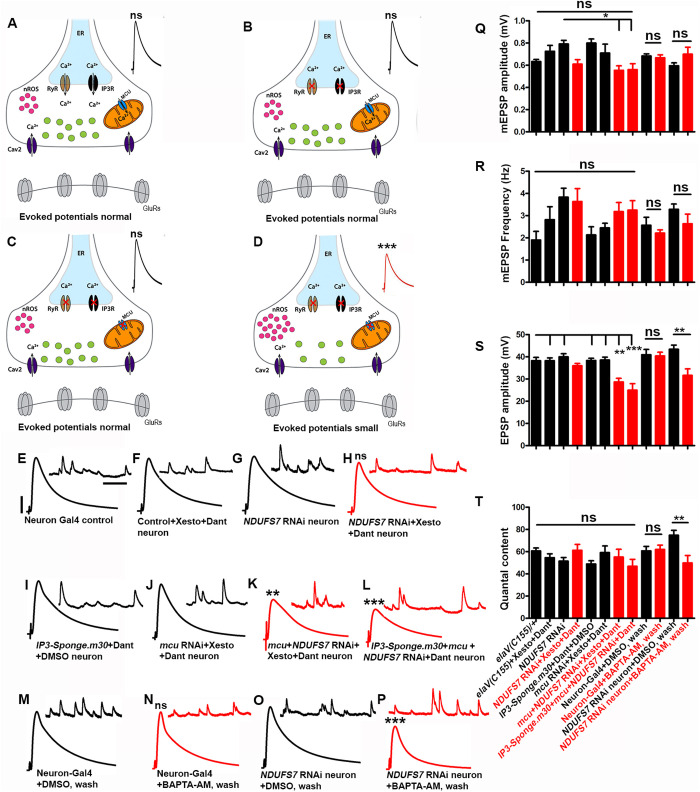
Fig 5. Loss of an MCI subunit necessitates ER-mediated calcium release to maintain evoked neurotransmission at the NMJs.
(A–D) Schematics (drawn on Adobe Illustrator) illustrating the role of IP3 receptor (IP3R), Ryanodine receptor (RyR) in the endoplasmic reticulum, mitochondrial calcium uniporter Complex (MCU), Cav2 calcium channel, and synaptic vesicles at the presynaptic nerve terminal. The IP3 and Ryanodine receptors were blocked pharmacologically (red X symbols) by using the IP3R antagonist Xestospongin C or neuronally expressing UAS-IP3-Sponge and RyR antagonist Dantrolene, while mcu[RNAi] was used to block the Mitochondrial calcium uniporter complex (red X). (E–P) Representative traces of mEPSPs and EPSPs in (E) pan-neuronal Gal4 control (elaV(C155)-Gal4/+), (F) pan-neuronal Gal4 control (elaV(C155)-Gal4/+) with an acute application (10 min) of 20 µM Xestospongin C and 10 µM Dantrolene, (G) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+), (H) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4/+; NDUFS7[RNAi]/+) with 20 µM Xestospongin C and 10 µM Dantrolene, (I) pan-neuronal Gal4-driven UAS-IP3-Sponge.m30 with an acute application of 10 µM Dantrolene (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+; UAS-IP3-Sponge.m30/+), (J) pan-neuronal Gal4-driven mcu[RNAi] with 20 µM Xestospongin C and 10 µM Dantrolene (elaV(C155)-Gal4)/+; mcu[RNAi]/+), (K) pan-neuronal mcu[RNAi] + NDUFS7[RNAi] with 20 µM Xestospongin C and 10 µM Dantrolene and(elaV(C155)-Gal4)/+; mcu[RNAi]/NDUFS7[RNAi]) and (L) pan-neuronal UAS-IP3-Sponge.m30 + mcu[RNAi] + NDUFS7[RNAi] with 10 µM Dantrolene (elaV(C155)-Gal4/+;NDUFS7[RNAi]/mcu[RNAi];UAS-IP3-Sponge.m30/+), (M) Pan-neuronal Gal4 (elaV(C155)-Gal4/+) with DMSO, (N) pan-neuronal Gal4 control (elaV(C155)-Gal4/+) with 20 µM BAPTA-AM, (O) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+) with DMSO and (P) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+) with 20 µM BAPTA-AM. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). Note that EPSP amplitudes were reduced in pan-neuronal Gal4-driven mcu[RNAi] + NDUFS7[RNAi] with an acute exposure of 20 µM Xestospongin C, 10 µM Dantrolene or UAS-IP3-sponge.m30 + mcu[RNAi] + NDUFS7[RNAi] with 10 µM Dantrolene and pan-neuronal Gal4-driven NDUFS7[RNAi] with 20 µM BAPTA-AM. (Q–T) Histograms showing average mEPSPs, EPSPs amplitude, and quantal content in the indicated genotypes. A minimum of 8 NMJs recordings of each genotype were used for quantification. **p < 0.05 (EPSP and QC: NDUFS7[RNAi] neuron + DMSO, wash vs. NDUFS7[RNAi] neuron + BAPTA-AM, wash), *p < 0.05, **p = 0.001, ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S1 Data Excel file, tab Fig 5.
We observed no significant differences in EPSP amplitudes when we impaired mcu function neuronally (Table N in S1 Tables). Similarly, we did not observe deficits in baseline synaptic activity by blocking RyR and IP3R, alone or in conjunction with NDUFS7[RNAi] (Fig 5E–5H and 5Q–5T and Table N in S1 Tables). However, when we concurrently impaired a combination of NDUFS7, mcu, and those ER calcium store channels, we observed marked decreases in evoked amplitude (Fig 5K, 5L, and 5S and Table N in S1 Tables). These results are consistent with a model in which mitochondrial calcium uptake, MCU activation, and ER (store) calcium efflux combine to stabilize synaptic strength.
If this idea were correct, then it should also be possible to chelate cytoplasmic calcium in a neuronal NDUFS7[RNAi] background and reveal neurotransmission defects. Direct application of the membrane-permeable chelator BAPTA-AM, followed by a wash to remove chelator residing in the saline, had no significant effect on neurotransmission parameters versus the mock-treated baseline control (DMSO carrier + wash). But in the NDUFS7[RNAi] background, BAPTA-AM + wash significantly diminished evoked potentials, compared to mock-treated (DMSO + wash) NMJs. (Fig 5M–5T and Table N in S1 Tables).
To check if these effects on neurotransmission correlated with effects on active zone protein accumulation, we conducted anti-BRP immunostaining experiments (S14 Fig). As before, neuronal knockdown of NDUFS7 gene function triggered a marked, compensatory increase in presynaptic active zone material that was readily apparent by confocal microscopy (S14A, S14C, and S14I Fig and Table O in S1 Tables). But this increase was reversed when combined with mcu gene function knockdown and pharmacological blockade of store calcium release channels (S14G–S14I Fig and Table O in S1 Tables). Together, our data indicate that loss of MCI subunits in neurons sensitizes synapses to decreases in intracellular calcium.
A combination of mitochondria, glycolysis, and the TCA Cycle stabilizes NMJ function
Neuronal calcium handling and MCU play roles in NMJ stability that are uncovered by loss of MCI at the NMJ. Downstream of calcium handling, a logical hypothesis is that synaptic energy would play a role [59]. If this were the case, would mitochondria be the sole energy source? Alternatively, in the absence of full mitochondrial function, could glycolysis theoretically substitute, as a homeostatic (or redundant) means for staving off synapse dysfunction?
We tested these ideas by limiting glycolysis as an energy source in two ways: (1) swapping out sucrose and trehalose in our recording saline in favor of 2-deoxy-d-glucose (a nonglycolytic sugar; (Fig 6A); (2) addition of lonidamine (LDA) to the saline to acutely inhibit hexokinase (Fig 6A). Control recordings with these conditions showed little effect on baseline physiology (Fig 6A, 6D, 6F, and 6H–6J and Table P in S1 Tables). However, when Mitochondrial Complex I was impaired neuronally through NDUFS7[RNAi] in combination with inhibition of glycolysis, there was a drop in evoked neurotransmission (Fig 6C, 6E, 6G, and 6H–6J and Table P in S1 Tables). This correlated with a failure to increase active zone material after NDUFS7 gene knockdown (Fig 6K–6Q and Table P in S1 Tables). These results match the idea that a combination of mitochondrial function or glycolysis can work to maintain normal levels of NMJ output.
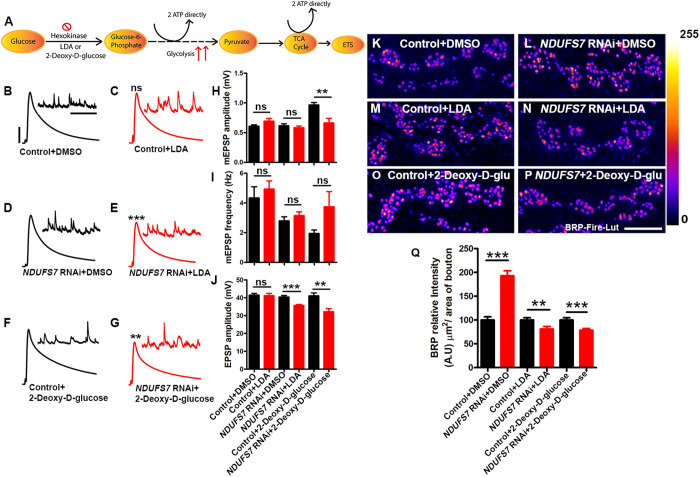
Fig 6. Loss of an MCI subunit necessitates glycolysis to regulate levels of active zone materials and stabilize synaptic strength.
(A) Schematic illustrations showing steps of glucose metabolism and ATP production during glycolysis and TCA cycle in the cell. (B–G) Representative traces of mEPSPs and EPSPs in (B) pan-neuronal Gal4 control (elaV(C155)-Gal4/+ with DMSO), (C) pan-neuronal Gal4 control (elaV(C155)-Gal4/+) with an acute application (30 min) of 150 µM Lonidamine (LDA) (D) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with DMSO), (E) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+) with 150 µM Lonidamine, (F) pan-neuronal Gal4 control (elaV(C155)-Gal4/+) and HL3 containing 2-Deoxy-d-glucose and (G) pan-neuronal Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+) with recording saline containing 2-Deoxy-d-glucose as the sugar source. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). The EPSPs amplitudes were reduced in pan-neuronal Gal4-driven NDUFS7[RNAi] with an acute exposure of 150 µM Lonidamine (LDA) or pan-neuronal Gal4-driven NDUFS7[RNAi] in 2-Deoxy-d-glucose for 30 min. There was also a reduction in mEPSP amplitudes in saline containing 2-Deoxy-d-glucose in pan-neuronal Gal4-driven NDUFS7-depleted larvae compared to the control animals. (H–J) Histograms showing average mEPSPs, EPSPs amplitude, and frequencies in the indicated genotypes. A minimum of 8 NMJ recordings of each genotype were used for quantification. **p = 0.003 (mEPSP amplitude: elaV(C155)-Gal4/+2-Deoxy-d-glucose vs elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with 2-Deoxy-d-glucose), **p=0.002 (EPSP amplitude: elaV(C155)-Gal4/+ with 2-Deoxy-d-glucose vs. elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with 2-Deoxy-d-glucose),***p=0.0001 (EPSP: elaV(C155)-Gal4/+ with LDA vs elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with LDA); ns, not significant. Statistical analysis is based on the Student t test for pairwise sample comparison. Error bars represent mean ± s.e.m. (K–P) Representative images of the A2 hemisegment of muscle 6/7 NMJs in the above-indicated genotypes immunostained with antibodies against the active zone scaffold Bruchpilot (BRP:fire-LuT) to label the active zones. The BRP levels downregulated at the NMJs in pan-neuronal Gal4-driven NDUFS7[RNAi] either incubated with 150 µM LDA or in HL3 containing 2-Deoxy-d-glucose for 30 min. (K–P) Scale bar: 5 µm. (Q) Histograms showing quantification of BRP intensity in µm 2 area of bouton at muscle 6/7 in the genotypes mentioned above. At least 8 NMJs of each genotype were used for quantification.***p < 0.0001 (BRP levels: elaV(C155)-Gal4/+ with DMSO vs elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with DMSO),**p=0.008 (BRP levels: elaV(C155)-Gal4/+ with LDA vs. elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with LDA), ***p=0.0005 (BRP levels: elaV(C155)-Gal4/+ with 2-Deoxy-d-glucose vs elaV(C155)-Gal4)/+; NDUFS7[RNAi]/+ with 2-Deoxy-d-glucose). Error bars denote mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Raw data for this figure are available in the S1 Data Excel file, tab Fig 6.
We continued this line of investigation genetically. We acquired RNA interference-based transgenes to target five genes involved in Drosophila glycolysis or subsequent ATP generation in the Citric Acid (TCA) Cycle: hexokinase A (hex-A), hexokinase C (hex-C), Citrate (Si) Synthase I, Isocitrate dehydrogenase (Idh), and Succinyl-coenzyme A synthetase α subunit 1 (Scsα1). We knocked down these genes neuronally, either alone or in combination with NDUFS7[RNAi] (S15 and S16 Figs). Neuronal impairment of hex-C had no effect on baseline neurotransmission, but hex-A impairment reduced it (S15A–S15E Fig and Table Q in S1 Tables). Impairment of the TCA Cycle enzymes on their own had little-to-no effect on baseline neurotransmission (S15A and S15F–S15H and Table Q in S1 Tables). However, concurrent impairment of NDUFS7 and most of these genes significantly blunted neurotransmission, with the exception being hex-C (S15I–S15N Fig and Table Q in S1 Tables). Collectively, the data suggest that MCI works in conjunction with—or redundantly to—alternative energy-generating pathways to support normal levels of neurotransmission (S15O–S15Q Fig). In the case of the hexokinases, Drosophila hex-A seems more important for this process than hex-C.
We also quantified active zone material accumulation. The results mirrored the neurotransmission tests: as before, neuronal NDUFS7[RNAi] impairment elicited enhanced active zone material (S16A, S16B, and S16M and Table R in S1 Tables). On their own, impairment of glycolysis or TCA Cycle genes had variable effects active zone material (S16C–S16G and S16M Fig and Table R in S1 Tables). But concurrent neuronal impairment of NDUFS7 and any of the glycolysis or TCA Cycle genes reversed active zone enhancement (S16H–S16M Fig and Table R in S1 Tables).
MCI subunits in muscle are required for proper synapse development
We previously reported that impairments of MCI diminish Drosophila NMJ growth [13]. The roles of MCI in specific tissues for this developmental process were unclear. For the present study, we tested for tissue-specific roles of MCI in NMJ development. To visualize NMJ boutons, we co-stained larval fillets with anti-Horseradish Peroxidase (HRP) a presynaptic membrane marker, and anti-Discs Large (Dlg), a postsynaptic density marker [63,64].
On a coarse level, MCI loss in muscle (BG57-Gal4 > UAS-NDUFS7[RNAi]) caused a severe reduction in average bouton size, a decrease in bouton number, a notable decrease in Dlg expression, and a bouton “clustering” phenotype (Fig 7A and 7B), reminiscent of what we previously reported [13]. To quantify these observations, we measured bouton number, muscle area, and branch number per muscle in the third-instar larval NMJ synapses (Table S in S1 Tables). We found that NDUFS7 muscle knockdown resulted in a significant reduction in all these parameters compared to controls (Fig 7J–JL, 7U, and 7V and Table S in S1 Tables).
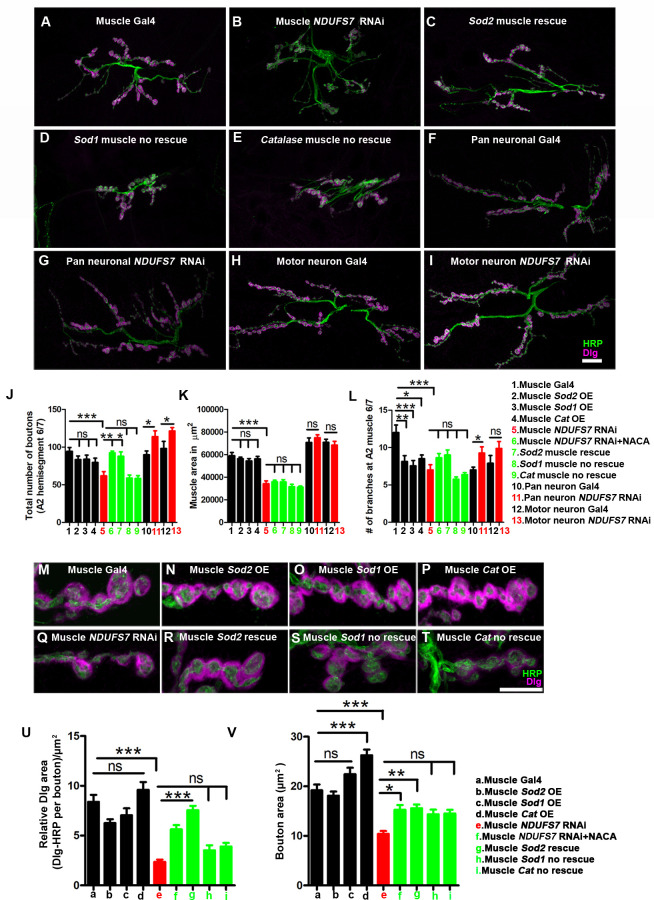
Fig 7. NDUFS7 in muscles is required to promote normal synapse growth.
(A–I) Representative confocal images of NMJ synapses at muscle 6/7 of (A) Muscle-Gal4 control (BG57-Gal4/+), (B) Muscle Gal4-driven NDUFS7[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+), (C) Sod2 muscle rescue (UAS-Sod2/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), (D) Sod1 muscle nonrescue (UAS-Sod1/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), (E) Catalase muscle nonrescue (UAS-Cat/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), (F) Pan-neuronal Gal4 control (elaV(C155)-Gal4)/+), (G) elaV(C155)-Gal4-driven NDUFS7[RNAi] (elaV(C155)-Gal4/+; NDUFS7[RNAi]/+), (H) Motor neuron Gal4 control (D42-Gal4/+), and (I) D42-Gal4-driven NDUFS7[RNAi] (NDUFS7[RNAi]/+; D42-Gal4/+) double immunolabeled with Dlg (magenta) and HRP (green) antibodies. (A–I) Scale bar: 10 µm. The NMJ morphological defects in NDUFS7[RNAi] were restored upon co-expression of UAS-Sod2 in muscle; however, it did not rescue with UAS-Sod1 or UAS-Catalase transgene. (J–L) Histograms show the number of boutons, muscle area, and average NMJ length at muscle 6/7 of A2 hemisegment in the indicated genotypes. *p < 0.05, *p = 0.006 (# of boutons: Muscle NDUFS7[RNAi] vs. Sod2 muscle rescue), **p = 0.0002 (# of boutons: Muscle NDUFS7[RNAi] vs. Muscle NDUFS7[RNAi] +NACA), *p = 0.009 (# of branches: Muscle Gal4 vs. Muscle Sod2 OE), ***p = 0.002 (# of branches: Muscle Gal4 vs. Muscle Sod1 OE), *p = 0.008 (# of branches: Muscle Gal4 vs. Muscle cat OE), ***p = 0.001 (Muscle Gal4 vs. Muscle NDUFS7[RNAi]), ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple and Student t tests for pairwise comparison. Error bars represent mean ± s.e.m. (M–T) Representative confocal images of boutons at the third instar larval NMJ synapse in (M) Muscle-Gal4 control (BG57-Gal4/+), (N) Muscle Gal4-driven UAS-Sod2 (UAS-Sod2/+; BG57-Gal4/+), (O) UAS-Sod1 (UAS-Sod1/+; BG57-Gal4/+), (P) UAS-Catalase (UAS-Catalase/+; BG57-Gal4/+) (Q) Muscle NDUFS7[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4 (R) Sod2 muscle rescue (UAS-Sod2/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), (S) Sod1 muscle rescue (UAS-Sod1/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), and (T) Catalase muscle rescue (UAS-Cat/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4) animals double immunolabeled with anti-HRP (green) and anti-Dlg (magenta) antibodies. (M–T) Scale bar: 5 µm. Note that the gross morphology of SSRs and immunoreactivity of Dlg were reduced in NDUFS7[RNAi] animals. As mentioned, phenotypes were restored to wild-type level when NDUFS7[RNAi]-depleted flies were reared in NACA or by genetically expressing Sod2 transgene in muscle. (U, V) Histograms showing normalized synaptic fluorescence of Dlg and bouton area in the indicated genotypes. *p < 0.01, **p < 0.001, ***p < 0.0001; ns, not significant. Error bars represent mean ± s.e.m. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple and Student t tests for pairwise comparison. Raw data for this figure are available in the S1 Data Excel file, tab Fig 7.
In contrast to muscle knockdown, pan-neuronal or motor neuron-specific knockdown of NDUFS7 showed slight NMJ overgrowth phenotypes (Fig 7F–7L and Table S in S1 Tables). As was the case with the active zone enhancement associated with neuronal NDUFS7 knockdown (Fig 4), this NMJ overgrowth could represent a developmental mechanism to stave off dysfunction caused by missing neuronal mitochondria. Taken together, our NMJ immunostaining results indicated to us that blunted NMJ growth due to MCI loss was likely due to muscle MCI dysfunction.
We tested if the NMJ undergrowth phenotypes could be due to the increased levels of mitochondrial ROS we had observed in muscle (mROS) (S2F Fig). If that idea were correct, then the undergrowth phenotypes should be reversed if mitochondrial mROS were scavenged. Consistent with this idea, bouton number and synaptic undergrowth phenotypes were fully restored to wild-type levels when the NDUFS7[RNAi] muscle knockdown animals also had UAS-Sod2 transgenically expressed in the muscles (Fig 7C, 7J–7L, and 7R and Table S in S1 Tables). They were also restored to wild-type levels when muscle NDUFS7 knockdown animals were raised on food containing the antioxidant N-acetyl cysteine amide (NACA) (Fig 7J–7L and Table S in S1 Tables). By contrast, none of these NMJ growth parameters were restored to wild-type levels if scavengers UAS-Sod1 or UAS-Catalase were misexpressed in the muscle (Fig 7D–7E and 7J–7L and Table S in S1 Tables).
Loss of MCI and mROS in muscle disorganize NMJ postsynaptic densities
The muscle Discs Large (Dlg)-Spectrin network functions as an organizing scaffold for synaptic assembly [64,65]. Dysregulation of this network can lead to an aberrant muscle subsynaptic reticulum (SSR) [64]. Therefore, one possible target of excess mROS in the absence of MCI function is Dlg. Dlg is the fly homolog of PSD-95/SAP97/PSD-93, and it is a member of the membrane-associated guanylate kinase (MAGUK) family of NMJ scaffolding proteins [64]. It is present both within presynaptic boutons and in the portion of the SSR closest to the bouton.
Using the same antibodies detailed above (anti-Dlg and anti-HRP), we used a high magnification to examine at the postsynaptic densities closely. By confocal immunofluorescence, Dlg area was significantly reduced when NDUFS7 was depleted postsynaptically by[RNAi] (Fig 7M–7U and Table S in S1 Tables). To quantify the relative Dlg area, we measured Dlg area with respect to HRP (Relative Dlg area = Dlg area minus HRP area) in type 1b boutons at muscle 6/7 of the A2 hemisegment (Fig 7U). Compared with the control synapses, NDUFS7 knockdown resulted in a significant reduction in the relative Dlg area (Fig 7M–7U and Table S in S1 Tables). Consistently, the relative α-Spectrin area was also reduced when NDUFS7 was depleted in muscle (S17A–S17F Fig and Table G in S1 Tables).
Next, we scavenged mROS to check how that affected the postsynaptic densities. The relative Dlg and α-Spectrin areas were restored to wild-type levels when animals were grown in a media containing NACA (NDUFS7[RNAi]/+; BG57-Gal4/+ with NACA) or genetically expressing UAS-Sod2 in the muscle (UAS-Sod2/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4) (Dlg data: Fig 7R and 7U and Table S in S1 Tables; α-Spectrin data: S17D–S17F Fig and Table G in S1 Tables). By contrast, Dlg levels were not restored while expressing other scavengers in the muscle, encoded by UAS-Sod1 and UAS-Catalase transgenes (Fig 7S–7U and Table S in S1 Tables). We conclude that depletion of MCI subunits in the muscle disables postsynaptic density formation via the formation of mitochondrial ROS intermediates.
The postsynaptic density (PSD-95/Dlg) has been shown to cluster glutamate receptors at the SSR [66]. Our results raised the possibility that glutamate receptor clusters could be disrupted when NDUFS7 was depleted in the muscle (Fig 8). To test this idea, we simultaneously immunostained NMJs with antibodies against BRP (neuron, presynaptic active zone) and glutamate receptor clusters (muscle). In controls, these pre and postsynaptic structures were directly apposed to one another (Fig 8A and 8G). But when NDUFS7 gene function was depleted, we observed “missing” GluRIIA and GluRIII receptor clusters (Fig 8B, 8F, 8H, and 8K and Table G in S1 Tables), i.e., BRP puncta without apposed glutamate receptors. These lack of apposition phenotypes were fully reversed by raising the larvae with NACA or genetically expressing UAS-Sod2 in the muscle (Fig 8D–8F and 8I–8K and Table G in S1 Tables). However, as with prior tests, these phenotypes were not reversed by UAS-Sod1 or UAS-Catalase overexpression (S18 Fig and Table H in S1 Tables). Together, our data indicate that loss of MCI subunit in the muscle (NDUFS7) disrupts several aspects of the postsynaptic density organization, and these disruptions are likely due to the accumulation of mitochondrial ROS in the muscle.
Fig 8. NDUFS7 subunit in muscle affects the organization of GluRs cluster in Drosophila.
Representative confocal images of boutons at the third instar larval NMJ synapse for (A) Muscle-Gal4 control (BG57-Gal4/+), (B) Muscle NDUFS7[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+), (C) Muscle Gal4-driven UAS-Sod2 (UAS-Sod2/+; BG57-Gal4/+), (D) NDUFS7[RNAi]/+; BG57-Gal4/+NACA), and (E) Sod2 muscle rescue (UAS-Sod2/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4) animals immunolabeled with active zone marker BRP (green) and anti-GluRIII (magenta) antibodies. Scale bar: 5 µm. Note that GluRIII apposed clusters with BRP are missing in the NDUFS7[RNAi] knockdown animals (marked in arrow) compared to control. These phenotypes were restored to normal when NDUFS7[RNAi] knockdown flies were reared in media containing NACA or genetically expressing Sod2 transgene in muscle. (F) Histograms showing quantification of the number of missing BRP-GluRIII apposed puncta per bouton in the indicated genotypes. (G–J) Similar phenotypes were observed when analyzed for BRP-GluRIIA apposed clusters in boutons. (K) Histograms showing quantification of the number of missing BRP-GluRIIA apposed puncta per bouton in the indicated genotypes. ***p < 0.0001. Error bars represent mean ± s.e.m. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple comparisons. Raw data for this figure are available in the S1 Data Excel file, tab Fig 8.
Loss of MCI subunits in muscle diminishes evoked NMJ neurotransmission through postsynaptic mROS
Mitochondria and ROS influence presynaptic vesicle release and plasticity at synapses. This has been shown in diverse model systems like flies, mice, and worms [52,53,67–69]. In our prior work, we identified an NMJ neurotransmission defect when MCI function is impaired [13], but based on our current study, that defect does not seem like it is dependent upon MCI’s neuronal functions (Figs 1 and 4–6). Therefore, we turned to analyzing postsynaptic muscle MCI and mROS to check if these parameters influenced neurotransmission.
We performed sharp electrode electrophysiological recordings of miniature and excitatory postsynaptic potentials (mEPSP and EPSP) at NMJ muscle 6, hemisegment A2. We also used average mEPSP and EPSP values to estimate QC for each NMJ. For the most part, mEPSP values remained steady (Fig 9A–9E and Table T in S1 Tables), with some exceptions. The starkest phenotypes came in terms of evoked amplitudes (Fig 9A–9D and 9F and Table T in S1 Tables), sometimes due to changes in QC (Fig 9G) or combinatorial changes in both mEPSP (Fig 9E) and QC (Fig 9G).
Fig 9. Loss of MCI subunits affects synaptic transmission via the formation of excess ROS in the muscle.
(A) Representative traces of mEPSPs and EPSPs in muscle-Gal4 control (BG57-Gal4/+), muscle Gal4-driven NDUFS7[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+), muscle Catalase nonrescue (2× muscle-Gal4>UAS-Catalase/NDUFS7[RNAi]: UAS-Catalase/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), muscle Sod1 nonrescue (2× muscle-Gal4>UAS-Sod1/NDUFS7[RNAi]: UAS-Sod1/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), muscle Sod2 rescue (2× muscle-Gal4>UAS-Sod2/NDUFS7[RNAi]: UAS-Sod2/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4) animals. Note that EPSP amplitudes were reduced in NDUFS7[RNAi], and the phenotype was restored to wild-type levels by expressing Sod2 transgene in the muscle but not with Sod1 and Catalase transgenes. (B) Representative traces of mEPSPs and EPSPs in muscle Gal4 control (BG57-Gal4/+) larvae raised on 10% EtOH, NDUFS7 muscle[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+) raised on 10% EtOH, NDUFS7 muscle[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+) raised on 0.5mM curcumin, NDUFS7 muscle[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+) raised on 0.5mM NACA, (C) Representative traces of mEPSPs and EPSPs in ND-30epgy/Df mutants, ND-30epgy/Df mutants raised on 0.5 mM NACA and UAS-Sod2 muscle rescued ND-30epgy/Df mutant (UAS-Sod2/+; ND-30 (Df), BG57-Gal4/ND-30epgy) animals. The EPSPs amplitudes were restored to wild-type when muscle depleted NDUFS7[RNAi] larvae were raised in food containing NACA or by UAS-Sod2 muscle overexpression in NDUFS7[RNAi] depleted animals. (D) Representative traces of mEPSPs and EPSPs in muscle-Gal4 control (BG57-Gal4/+) larvae raised on DMSO, muscle Gal4 (BG57-Gal4/+) larvae raised on 50 µM rotenone (complex I inhibitor), UAS-Catalase (UAS-Catalase/+; BG57-Gal4/+), UAS-Sod1 (UAS-Sod1/+; BG57-Gal4/+), and UAS-Sod2 (UAS-Sod2/+; BG57-Gal4/+) muscle overexpression animals raised on 50 µM rotenone. The EPSP amplitudes were restored in rotenone raised larvae overexpressing UAS-Sod2 in muscle (UAS-Sod2/+; BG57-Gal4/+), likely due to its free radical scavenging activity. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). (E–G) Histograms showing average mEPSPs, EPSPs amplitude, and quantal content in the indicated genotypes. Minimum 8 NMJs recordings of each genotype were used for quantification. (H) Histogram representing crawling behavior (in cm) of the larvae in the indicated genotypes. Knocking down NDUFS7[RNAi] or ND-30[RNAi] in muscle and ND-30epgy/Df mutants showed a severe defect in crawling behavior. The abnormal crawling behavior was rescued by expressing a Sod2 transgene in the muscle or rearing the larvae in a media containing NACA. Moreover, neuronally depleting NDUFS7 did not show any notable change in crawling defects. Minimum 10 animals were analyzed for crawling behavioral analysis. *p < 0.05 (mEPSP amplitude: Muscle NDUFS7[RNAi] + 10% EtOH vs. Muscle NDUFS7[RNAi] + 0.5 mM NACA),**p = 0.006 (mEPSP amplitude: ND-30 mutant vs. N-30 mutant + 0.5 mM NACA), *p = 0.0004 (mEPSP amplitude: Muscle-Gal4 + 0.5% DMSO vs. Muscle Cat OE + 50 µM rotenone),*p = 0.039 (mEPSP amplitude: Muscle Gal4 + 50mM rotenone vs. Muscle Sod2 OE + 50 µM rotenone), *p = 0.001 (EPSP amplitude: Muscle NDUFS7[RNAi] vs. Sod2 muscle rescue),**p = 0.003 (EPSP amplitude: Muscle Gal4 + 10% EtOH vs. Muscle NDUFS7[RNAi] + 0.5mM curcumin), **p = 0.0004 (EPSP amplitude: Muscle NDUFS7[RNAi] + 10% EtOH vs. Muscle NDUFS7[RNAi] + 0.5mM NACA), *p = 0.004 (EPSP amplitude: Muscle Gal4 + 0.5% DMSO vs. Muscle Gal4 + 50 µM rotenone), *p = 0.015 (EPSP amplitude: Muscle-Gal4 + 0.5% DMSO vs. Muscle cat OE + 50 µM rotenone), **p = 0.015 (EPSP amplitude: Muscle-Gal4 + 0.5% DMSO vs. Muscle Sod1 OE + 50 µM rotenone), **p = 0.001 (QC: ND-30 mutant vs. Sod2 muscle rescue), **p = 0.003 (QC: Muscle Gal4 + 50 µM rotenone vs. Muscle Sod2 OE + 50 µM rotenone), **p = 0.0002 (Distance crawled: Muscle Gal4 vs. Muscle ND-30[RNAi]), ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple and Student t test for pairwise comparison. Raw data for this figure are available in the S1 Data Excel file, tab Fig 9.
Specifically, evoked synaptic vesicle release (EPSP) was significantly reduced when NDUFS7 was depleted in muscles by RNAi (Fig 9A and 9F and Table T in S1 Tables). That reduction was reversed after scavenging mitochondrial ROS in the muscle. Indeed, transgenic muscle-driven Sod2 expression suppressed the NDUFS7[RNAi] phenotype, but expression of the Catalase and Sod1 did not (Fig 9A and 9F and Table T in S1 Tables). We also tested if feeding a ROS scavenger to developing larvae would reverse the same neurotransmission defect. Carrier feeding alone (10% EtOH) did not affect evoked neurotransmission, nor did it influence the neurotransmission loss caused by NDUFS7[RNAi] (Fig 9B and 9F and Table T in S1 Tables). Feeding larvae 0.5 mM NACA successfully reversed the phenotype, but the nonspecific additive curcumin had no effect (Fig 9B and 9F and Table T in S1 Tables).
We checked different MCI manipulations. First, we examined hemizygous ND-30EY03664/Df genetic mutants. As with muscle-driven NDUFS7[RNAi], the ND-30 mutant NMJs had blunted evoked neurotransmission, but this defect was successfully reversed by ROS scavengers (Fig 9C and Table T in S1 Tables). We also impaired MCI pharmacologically, by feeding larvae 50 µM rotenone (or 0.5% DMSO carrier control), similar to conditions we previously published [13]. As with the prior study, rotenone blunted neurotransmission (Fig 9D, 9F, and 9G and Table T in S1 Tables), but this effect was ameliorated by a genetic background overexpressing UAS-Sod2 in the muscle (Fig 9D, 9F, and 9G and Table T in S1 Tables). By contrast, overexpressions of UAS-Catalase and UAS-Sod1 were not effective at reversing the effect of rotenone (Fig 9D, 9F, and 9G and Table T in S1 Tables).
Finally, we performed behavioral experiments on NDUFS7[RNAi] and ND-30[RNAi] and mutant animals. Consistent with the electrophysiological recordings, MCI muscle-depleted and mutant animals showed severe defects in crawling ability. The crawling behavior was rescued by expressing the UAS-Sod2 transgene in the muscle or raising the larvae in a media containing NACA (Fig 9H and Table U in S1 Tables). By contrast, neuronal depletion of NDUFS7 in larvae did not show any significant crawling defects (Fig 9H and Table U in S1 Tables). Together, our data suggest that excess mitochondrial ROS accumulation in muscle (mROS) diminishes baseline synaptic physiology when MCI activity is lost, and it also triggers aberrant crawling behavior.
Discussion
We uncovered novel aspects of NMJ synapse biology controlled by Mitochondrial Complex I (MCI). Impairment of MCI causes profound cytological phenotypes in synaptic tissues (Figs 1–3) [13]. By examining mitochondria directly, we discovered shared phenotypes between MCI loss and loss of the Drosophila Mitofusin, Marf (Fig 2). Additionally, with MCI loss, we noted an enhancement of mitochondrial ROS, S2 Fig), consistent with prior work [36–40].
Unexpectedly, these perturbations spur functionally opposite responses in presynaptic neurons versus postsynaptic muscles. In motor neurons, MCI loss and mitochondrial ROS appear to trigger a compensatory response, where the underlying cytological problems are offset by an increase in active zone material, resulting in normal levels of evoked excitation (Fig 4). This process requires known intracellular calcium signaling components (Fig 5). It also appears to require energy stores because loss of glycolysis—which may function as a supplemental energy source to mitochondria—abrogates the presynaptic compensation (Fig 6). By contrast, in the muscle, MCI loss and mitochondrial ROS trigger a destructive response, where there is a disassembly of the postsynaptic density (Fig 7). This disassembly correlates with mis-apposition of pre and postsynaptic structures (Fig 8) and defects in neurotransmission and locomotion (Fig 9).
Disruption of mitochondrial dynamics, ROS, and physiological responses in Drosophila
Energy is needed for normal levels of synaptic transmission [2]. Intuitively, a loss of synaptic mitochondria should blunt transmission because transmission requires energy. Consistently, several labs have previously implicated mitochondrial dynamics in Drosophila synapse function, including mitochondrial fission (Dynamin-related protein 1, Drp1 [51]), fusion (Mitofusin/dMarf [31]), trafficking (Miro and Milton [50,70,71]), or quality control (Pink and Parkin [72–75]). Additionally, it has been established from model organisms such as flies, worms, and mice that any misregulation in mitochondrial distribution could affect synaptic activity [51,59,76,77]. Adding to that work, we uncovered synaptic transmission and developmental phenotypes after depletion of MCI at the NMJ [13] potentially related to defective mitochondrial fusion (this study).
Additionally, ROS has previously been studied in the context of mitochondrial dysfunction [78]. Excess ROS can trigger mitochondrial calcium uptake and subsequently trigger apoptosis or degeneration of neurons or neural support cells [79,80]. MCI deficiency elevates ROS levels, and this process can promote the fragmentation of mitochondria in cells like fibroblasts [81].
Numerous papers using Drosophila as a model have examined potential connections between MCI, ROS, aging, and physiology. The consequences of MCI loss and the induction of ROS are context dependent. They are not always in obvious agreement, either from tissue to tissue or from study to study. For example, one analysis demonstrated that reverse electron transfer (RET) at MCI increases ROS production, and this occurs with the normal aging process [82]. Consistently, inhibition of RET decreases ROS and increases life span; this result would suggest that RET-induced ROS hurts survival [82]. However, a different study reported the opposite finding under stress-inducing conditions. For that scenario, inhibition of RET decreases ROS production and decreases life span—while activation of RET enhances ROS and extends life span. Those findings would suggest that ROS is protective under some conditions [83]. Effects are not limited to Complex I manipulation. Drosophila deficient for the Complex V subunit bellweather gene function demonstrate enhanced mitochondrial ROS; and downstream of this increased ROS, they have broadly enhanced levels of active zone material (BRP) in adult brains [84]. Genetically boosting mitochondrial function in adults has the opposite effect—attenuated BRP accumulation in the fly brain [84]. These findings closely match our larval motor neuron observations.
On the molecular level there are signaling puzzles uncovered by prior Drosophila work. For example, the unfolded protein response (UPR) is a key mediator when MCI function in lost. Neuronal impairment of the NDUFS1 (MCI subunit) homolog ND-75 elicits a spectrum of movement and seizure phenotypes, and it also activates the UPR on the cellular level [85]. All those Drosophila neuronal phenotypes can be reversed when yeast ND1 is exogenously added to the system, which restores NADH dehydrogenase function (while not restoring ATP production) [85]. Those findings suggest a damaging role in adult neurons for the MCI-deficient ROS-UPR axis. By contrast, another paper examined ND-75 disruption in adult muscle—and for this tissue, researchers found that elevated ROS levels trigger the UPR activation ultimately to preserve function [86]. That suggests a protective in in muscles for the ROS-UPR axis [86].
Our study expands upon these results and supports the idea that MCI has distinct roles at synaptic sites. Lack of MCI in larval motor neurons causes loss of mitochondria at synaptic terminals (Fig 1). This defect is linked to defective mitochondrial fusion (Fig 2), which is known to maintain mitochondrial integrity [87,88]. By contrast, lack of MCI in larval muscle appears to disassemble the postsynaptic density, which hurts neurotransmission (Figs 7–9).
A novel form of presynaptic homeostatic plasticity triggered by ROS?
ROS has been linked to short-term synaptic plasticity in Drosophila [53], as well as long-term potentiation (LTP) in mammals [89–91]. Here, we uncovered a role for ROS in augmentation of active zone material when MCI is impaired in neurons. This finding could be considered a form of homeostatic plasticity: an increase in active zone components likely drives potentiated release to compensate for defective baseline synaptic transmission, which would be expected after the loss of an energy source like mitochondria.
Homeostatic augmentations of active zone material have been reported at the Drosophila NMJ. For example, rab3 mutants have an increase in active zone material to offset decreased synapse growth [92]. Additionally, ROS has been shown to be an obligate signal in Drosophila to maintain fundamental properties in both pre and postsynaptic compartments, including at the NMJ [53]. In our study, we observe an increase in active zone intensity (Fig 4), and this increase could overlap with mechanisms uncovered in those prior studies.
Alternatively, our results could be consistent with a form of homeostatic plasticity at the Drosophila NMJ called Presynaptic Homeostatic Potentiation (PHP). PHP is initiated when the activity of postsynaptic glutamate receptors is impaired. This decreases quantal size. The synapse detects the impairment, and muscle-to-nerve signaling drives an increase in presynaptic glutamate release [93,94]. This happens in part through an increase in influx of calcium into the neuron through CaV2 voltage-gated calcium channels [93,95–98]. PHP coincides with an increase in the size of the readily releasable pool (RRP) of synaptic vesicles [99–102] and apparent increases in active zone protein content or organization [54,56,103]. The general model is that these modifications drive the neuron to release more glutamate, offsetting the initial synaptic challenge.
Could the excess mitochondrial ROS that is caused by the loss of Complex I be triggering the same or overlapping homeostatic mechanisms? It is possible. Quenching presynaptic ROS by expression of Sod2 or by feeding flies NACA led to a reversion of active zone material back down to the control levels (Fig 4E). Under ideal conditions, control levels of active zone material would support normal neurotransmission (Fig 4A and 4J). However, when combined with presynaptic NDUFS7 loss (already depleting synaptic mitochondria; Figs 1O and 2G), there is a loss in neurotransmission capacity (Fig 4M).
The molecular underpinnings of this mitochondrial-loss-induced homeostatic plasticity are unknown, but prior work offers ideas. One possibility is that Sod2 changes the redox status of the entire cell, which could potentially affect active zone components [89]. Another possibility is that ROS could alter the release of calcium from intracellular stores such as ER; and in turn, this could induce calcium signaling to mitochondria, which would contribute to the formation of ATP and subsequent vesicle fusion [59,60]. Consistent with this idea, we observed a reduced synaptic strength at the terminals after simultaneous blockade of calcium release and import from ER stores to mitochondria through genetic or pharmacological manipulations (Fig 5). The link would not have to be a direct one. Indeed, a recent study indicated that activity-driven mitochondrial calcium uptake does not depend on the ER as a source of calcium to maintain normal synaptic strength [59]. Future studies can refine conclusions about presynaptic calcium dynamics downstream of MCI disruption. This is possible through reagents in the Drosophila neurogenetic toolkit like compart-targeted genetic-encoded calcium indicators [104].
Loss of MCI in muscle and subsequent ROS accumulation diminish synaptic excitation
Little is known about what role postsynaptic ROS plays in regulating synaptic plasticity at the NMJ. It is unlikely that ROS is an abstract signal that triggers wholesale destruction. It likely has specific targets. There are clues from previous work. For example, ROS signaling plays direct roles in the activity-dependent structural plasticity of motor neurons and postsynaptic dendrites [53]. Additionally, postsynaptic ROS plays critical roles in dendritic development. In Drosophila, ROS appears to act as a developmental plasticity signal to regulate the size of the dendritic arbors. [105].
It is slightly surprising that the NMJ fails to compensate for a lack of MCI function in the muscle. This is because the NMJ employs multiple retrograde signals to stabilize function [106]. One hypothesis is that MCI deficiency and ROS interfere with important muscle-to-nerve signals normally required for maintaining NMJ setpoint function. Some of these signals are required to correct acute challenges (minutes) to NMJ function. In this context, some of the best characterized retrograde signals are: Bone Morphogenetic Protein (BMP) signaling [107–109]; the Insomniac (adaptor)-Cul3 (ubiquitin ligase)-Peflin (substrate) signaling complex [110]; and the endosomal recycling molecules class II PI3 kinase (PI3K) and Rab11 [111]. Additionally, the field has uncovered instructive signaling molecules from the muscle that maintain robust neurotransmission, including: muscle-secreted Semaphorin-2b [112]; and Target of Rapamycin (TOR) [113,114]. Future work can address whether these or similar targets are substrates of negative ROS regulation in the muscle.
The roles of ROS in muscle are less understood in terms of synapse regulation and function. In this study, we showed that excess ROS is sufficient to affect the organization of postsynaptic densities (Fig 7), ionotropic glutamate receptor clusters (Fig 8), and spectrin cytoskeleton (S11 Fig) in the muscle. The correlated uncoupling of pre and postsynaptic structures seems to be responsible for neurotransmission defects (Fig 9), and it likely coincides with broader structural instability [65,115]. These phenotypes are reminiscent of other Drosophila mutant phenotypes showing a degenerative NMJ (e.g., [65,115–117]). Consistent with a model of degeneration, we found behavioral defects in locomotion (Fig 9).
Despite the profound defects that result from muscle depletion of MCI, we were able to reverse many of those structural and functional problems by culturing animals in an antioxidant-rich media (0.5 mM NACA) or through muscle expression of UAS-Sod2. This is an interesting finding for future work because it points to mitochondrial ROS as a signal of interest in the manifestation of synapse dysfunction. In subsequent work, we will take advantage of the Drosophila neurogenetic toolkit to identify specific targets.
Limitations and future directions: Linking mitochondrial ROS and synapse function
There are fundamental questions that remain to be answered in future work. Many of these deal with ROS. First, we have not identified the specific ROS molecule(s) responsible for the synaptic phenotypes we have observed. Our data point to mitochondrial matrix ROS because UAS-Sod2 overexpression ameliorates MCI loss phenotypes, while UAS-Catalase and UAS-Sod1 do not. Additionally, UAS-Sod2[RNAi] phenocopies the ROS accumulation associated with MCI loss.
If the relevant ROS molecule is signaling from the mitochondrial matrix, this could mean superoxide (O2•−) or hydrogen peroxide (H2O2)—or less likely, another species, like hydroxyl radicals (•OH). The function of superoxide dismutase enzymes is to convert superoxide into hydrogen peroxide [118]. Because SOD2 is mitochondrial and it has a mollifying effect on MCI dysfunction in our system, we would hypothesize that mitochondrial matrix superoxide (O2•−) is the likely culprit for the synaptic phenotypes we observe. This idea would explain why other antioxidants like SOD1, and Catalase do not help. SOD1 accumulates mainly in the cytoplasm [119,120], while Catalase accumulates mainly in peroxisomes [121,122]. NACA, which is the amide form of the antioxidant N-acetylcysteine, is membrane permeable; as such, it could accumulate in mitochondria [123]. We have not affirmatively demonstrated sub-compartment mitochondrial localization of NACA in our system. However, recent work by other groups is consistent with a reductive function of NACA for mitochondria [124,125].
Even though our data point to mitochondrial ROS, the mechanistic connection between matrix O2•− and the synapse is not clear. Additionally, we do not know the relevant subcellular site(s) where ROS would need to accumulate to have its effects on the synapse. An intuitive idea is that the affected mitochondria and ROS would be near the synapse itself. For the NMJ, this is possible both in neurons and muscle. Yet our data do not neatly match that model. For example, six hours of rotenone exposure to the NMJ can enhance active zone accumulation, but only if the motor nerve is intact (S12 Fig). This finding suggests that the signaling downstream of mitochondrial ROS in neurons needs both time and space to work.
Concerning the muscle, mitochondrial ROS seems to have a damaging effect directly on the postsynaptic density. This effect hurts synaptic function. If the relevant site of mitochondrial ROS in muscle happens to be closer to the NMJ than the relevant site in neurons, then the damaging effects from the muscle could partly a function of proximity. Determining if it matters whether mitochondrial ROS is physically near the NMJ could inform the possible signaling modalities that govern mitochondrial regulation of synapse function.
Resource availability
Lead contact
The lead contact for this study is Dr. C. Andrew Frank (andy-frank@uiowa.edu).
Materials availability
Dr. C. Andrew Frank’s laboratory uses the fruit fly Drosophila melanogaster as a model organism. Consistent with ethical conduct of research, the lab maintains Drosophila stocks that it generates and publishes, as well as useful precursors and derivatives of those stocks. These stocks are available to researchers who make requests to Dr. Frank (andy-frank@uiowa.edu)—or to the appropriate original source—and they are shipped promptly.
Data tables
Summary data are in Tables A–U in the S1 Tables file. Raw data are housed in Excel file tables. The S1 Data Excel file contains raw data for the main figures. The S2 Data Excel file contains raw data for the supplemental figures.
STAR methods
Please see Table V in the S1 Tables file for detailed information about the reagents and materials described below.
Experimental model details
Drosophila husbandry.
Drosophila melanogaster was cultured on a standard cornmeal media containing molasses and yeast prepared according to the Bloomington Drosophila Stock Center (BDSC, Bloomington, IN, USA) recipe. Fruit fly husbandry was performed according to standard practices [126]. Both male and female larvae were used for this study. Larvae were raised at 25 °C in humidity-controlled and light-controlled Percival DR-36VL incubators (Geneva Scientific). For drug feeding experiments in larvae, the control, RNAi, and mutant animals were grown throughout life (egg laying to analysis) in media containing the relevant drug. Those drugs were 0.5 mM curcumin (Sigma Aldrich), 0.5 mM N-acetyl cysteine amide (NACA) (Sigma Aldrich), or 50 µM rotenone (Sigma Aldrich).
Drosophila stocks.
w1118 was used as a nontransgenic wild-type stock [127]. The GAL4 drivers used in this study were elaVC155-Gal4 [128], BG57-Gal4 [64], and D42-Gal4 [129]. For the neuronal experiments, a switch from D42-Gal4 (motor neuron) to elaVC155-Gal4 (pan-neuronal) was made between Figs 4 and 5 for practicality of genetic stock building with several markers in the latter experiments. The core neuronal results remained unchanged.
Several UAS-RNAi and genetic mutant lines were obtained from the Bloomington Drosophila Stock Center or directly from researchers who produced them (Table V in S1 Tables includes the Bloomington Line (BL) numbers). We verified the effectiveness of UAS-ND-20L[RNAi] (ND-20LHMJ23777, now termed UAS-NDUFS7[RNAi]) in this study. Other mutant and transgenic lines have been characterized in prior studies. These include: UAS-IP3-sponge (UAS-IP3-Sponge.m30 shared by Dr. Masayuki Koganezawa’s lab) [126,130]; UAS-Catalase [131,132]; UAS-Sod1 [133]; UAS-Sod2 [47]; UAS-Sod2[RNAi] [134]; Df(3L)ED4288 [135]; ND-30epgy [136]; UAS-ND-30[RNAi] [12]; UAS-mitoGFP [137]; UAS-marf[RNAi] [31]; UAS-drp1[RNAi] [138]; UAS-CalX[RNAi] [139]; UAS-mcu[RNAi] [140,141]; UAS-hexokinase A[RNAi] [142]; UAS-hexokinase C[RNAi] [142]; UAS-idh[RNAi] [143]; UAS-Cit.(Si)-Syn[RNAi] [144]; UAS-Scsα[RNAi] [145].
Method details
Immunohistochemistry.
Wondering third instar larvae were dissected and fixed on a sylgard Petri plate in ice-cold HL3 and fixed in 4% paraformaldehyde in PBS for 30 min or in Bouin’s fixative for 2 min as described earlier [146]. Briefly, larvae were washed with PBS containing 0.2% Triton X-100 (PBST) for 30 min, blocked for an hour with 5% normal goat serum in PBST, and incubated overnight in primary antibodies at 4 °C followed by washes and incubation in secondary antibodies. Monoclonal antibodies: anti-Dlg (4F3), anti-DGluRIIA (8B4D2), anti-CSP (ab49), anti-Synapsin (3C11), anti-Futsch (22C10), anti-Bruchpilot (nC82), and anti-α-Spectrin (3A9) were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, IA, USA) and were used at 1:30 dilution. Rabbit anti-GFP (Abcam) was used at 1:200 dilutions. Anti-GluRIII (1:100) ([147]; Table V in S1 Tables) was gifted by Aaron DiAntonio (Washington University, St. Louis, USA). Fluorophore-coupled secondary antibodies Alexa Fluor 488, Alexa Fluor 568, or Alexa Fluor 647 (Molecular Probes, ThermoFisher Scientific) were used at 1:400 dilution. Alexa 488 or 647 and Rhodamine conjugated anti-HRP were used at 1:800 and 1:600 dilutions, respectively. The larval preparations were mounted in VECTASHIELD (Vector Laboratories, USA) and imaged with a laser scanning confocal microscope (LSM 710; Carl Zeiss). All the images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA).
Confocal imaging, quantification, and morphometric analysis.
Samples were imaged using a Carl Zeiss scanning confocal microscope equipped with 63×/1.4 NA oil immersion objective using separate channels with four laser lines (405, 488, 561, and 637 nm) at room temperature. The stained NMJ boutons were counted using anti-Synapsin or anti-HRP co-stained with anti-Dlg on muscle 6/7 of A2 hemisegment, considering each Synapsin or HRP punctum to be a bouton. At least 8 NMJs were used for bouton number quantification. For fluorescence quantifications of GluRs, Dlg, BRP, α-Spectrin, HRP, and CSP, all genotypes were immunostained in the same tube with identical reagents, then mounted and imaged in the same session. Z-stacks were obtained using identical settings for all genotypes with z-axis spacing between 0.2 and 0.5 μm and optimized for detection without saturation of the signal.
ROS and rotenone incubation assay.
Larvae were dissected in ice-cold calcium-free HL3 to label ROS in neurons. ROS levels were detected in mitochondria by incubating live preparation in 1× Schneider’s media with MitoSOX Red (Molecular Probes, ThermoFisher Scientific) fluorogenic dye at 1:200 dilutions for 20–30 min. Briefly, larvae were washed with HL3, mounted in VECTASHIELD (Vector Laboratories, USA), and immediately imaged in a laser scanning confocal microscope (LSM 710; Carl Zeiss). To study the effect of DMSO and rotenone (Sigma Aldrich) in BRP, larvae were dissected in HL3 and incubated in 1× Schneider’s media containing either 500 µM of rotenone or DMSO. After every 30 min, the old media was replaced with fresh media containing rotenone or DMSO. The above preparations were fixed with 4% paraformaldehyde, stained with anti-nc82 antibodies, mounted and imaged in a confocal microscope.
Electrophysiology and pharmacology.
All dissections and recordings were performed in modified HL3 saline [148] containing 70 mM NaCl, 5 mM KCl, 10 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 4.2 mM trehalose, 5 mM HEPES, and 0.5 mM CaCl2 (unless otherwise noted), pH 7.2. Neuromuscular junction sharp electrode (electrode resistance between 20 and 30 MΩ) recordings were performed on muscles 6/7 of abdominal segments A2 and A3 in wandering third-instar larvae as described [126]. Recordings were performed on a Leica microscope using a 10× objective and acquired using an Axoclamp 900A amplifier, Digidata 1440A acquisition system, and pClamp 10.7 software (Molecular Devices). Electrophysiological sweeps were digitized at 10 kHz and filtered at 1 kHz. Data were analyzed using Clampfit (Molecular Devices) and MiniAnalysis (Synaptosoft) software. Miniature excitatory postsynaptic potentials (mEPSPs) were recorded in the absence of any stimulation and motor axons were stimulated to elicit excitatory postsynaptic potentials (EPSPs).
Larval crawling assay.
Vials containing third instar larvae were analyzed for this assay. Vials were poured with 4 ml of 20% sucrose solution and left for 10 min to let the larvae float on top. Floating third instar animals were poured into a petri dish and washed gently twice with deionized water in a paintbrush. A minimum of 10 larvae of each genotype were analyzed on a 2% agarose gel in a petri dish with gridline markings 1 cm on graph paper. The larvae were acclimatized in the petri dish before videotaping. The average distance crawled (in centimeters) by larvae was calculated based on the average number of gridlines passed in 30 s [146].
Real-time PCR (RT-PCR).
Total RNA was extracted from 10 larval muscle fillets or 20 larval brains for each experiment using TRIzol (GIBCO) according to the manufacturer’s instructions. Concentration and purity of total RNA were measured using a NanoDrop. 1 μg of total RNA was then subjected to DNA digestion using DNase I (Ambion). This was immediately followed by reverse transcription using the iScript Reverse Transcription Supermix (1708841, Bio-Rad). qPCR was performed using the StepOnePlus instrument (ThermoFisher Scientific) and SYBR Green Supermix (172-5270, Bio-Rad) by following the manufacturer’s instructions. The Step One Software analyzed the qPCR, and the relative expression level was presented as the ratio of the target gene to the internal standard gene, rp49. Each sample was analyzed in triplicate. At least three independent biological repeats were obtained for each experiment. The following primers were used for quantitative PCR (qPCR) analysis. For the ND-20L gene, forward: 5′ CATGCCGGTGTACGATTACC 3′ and reverse: 5′ CGTCCCCAGTTTAGCAGGTC 3′ primers are used. Similarly, for analyzing the ND-20 transcript, forward: 5′ GAAGTGGCCCAAAATCTGCC 3′ and reverse: 5′ GAGCAGATCGTCCAGTCTGG 3′ primers were used, respectively. All data for the RT-PCR are presented as mean ± standard deviation.
Seahorse analysis for OCR measurement.
The Seahorse analysis was carried out as previously described [149] with some modifications. Briefly, mitochondria were isolated from the thoracic muscles of 20 adult Drosophila melanogaster using a differential centrifugation method. Thoraces were homogenized in cold isolation buffer, followed by a 300×g spin to remove debris and a subsequent 3,000×g spin to pellet mitochondria. The final mitochondrial pellet was resuspended in mitochondrial isolation buffer, and protein concentration was quantified using a BCA assay. Isolated mitochondria from Drosophila melanogaster thoracic muscles were resuspended in Agilent Seahorse XF assay medium supplemented with glucose (10 mM), pyruvate (1 mM), and glutamine (2 mM), pH 7.4. For the Seahorse assay, ~5–10 µg of isolated mitochondria were added to each well of a Seahorse XF Cell Culture Microplate. After centrifugation (3,000×g for 20 min at 4 °C), the plate was then loaded into the Seahorse XFeMini Analyzer, and the mitochondrial respiration profile was measured. The OCR data were normalized to protein concentration. All data for the Seahorse assay are presented as mean ± standard deviation.
Rendering schematic cartoons, figures, and tables.
For this study, the schematic cartoons for Fig 4 were drawn for this study using BioRender software. The schematic cartoons for Fig 5 were drawn using Adobe Illustrator. All final Figures or Supplemental Figures were saved as TIF files and sized in Adobe Photoshop. Tables were generated in Microsoft Word.
Quantification and statistical analysis
Quantification of mitochondrial branch length.
Controls and RNAi-depleted animals were analyzed using mitochondrial marker Mito-GFP, D42 driver in cell bodies from larvae VNC. All images were processed using ImageJ software. Z-stacks of individual neurons were merged. The Mito-GFP signal was enhanced by adjusting brightness and contrast. The binary masks were created using Image>Adjust>Threshold, Method:Otsu, and Background:Dark. The branches were generated using Process>Binary>Skeletonized [150]. These skeletonized images were analyzed, and branch length of the individual cluster was manually calculated using ImageJ tools.
Imaging quantifications.
Maximum intensity projections were used for quantitative image analysis with the ImageJ software (National Institutes of Health) analysis toolkit. Boutons from muscle 4 or type Ib terminal boutons on the muscle 6/7 of A2 hemisegment from at least six NMJ synapses were used for quantification using Image J software. Student t test for pairwise and one-way ANOVA with post-hoc Tukey’s test for multiple comparisons was used for statistical analysis, using GraphPad Prism Software. Specific p-value and tests are noted in the figures and figure legends and supplemental files and shown in graphs as follows: * p < 0.05, ** p < 0.001, and *** p < 0.0001. The data are presented as mean ± s.e.m. For quantification of Futsch loops, third instar larval preparations were double immunostained with HRP, 22C10 and images were captured in Zeiss LSM710 confocal microscope. Only NMJs of muscles 6/7 of A2 hemisegment were used for quantification. The images were digitally magnified using ImageJ software and the total number of HRP-positive boutons was manually counted in each image. Futsch positive loops, which are co-localized with HRP, were included in this analysis. Images with incomplete loops and diffused staining were not included in the count [151].
Electrophysiological analysis.
Average mEPSP, EPSP, and QC were calculated for each genotype by dividing EPSP amplitude by mEPSP amplitude. Muscle input resistance (Rin) and resting membrane potential (Vrest) were monitored during each experiment. Recordings were rejected if the Vrest was above −60 mV, and Rin was less than 5 MΩ. Pharmacological agents were bath applied in recording saline at the final concentrations indicated in the text, figures, and tables. The agents included Xestospongin C (Abcam), Dantrolene (Tocris), and BAPTA-AM (Sigma Aldrich). Failure analysis was performed in HL3 solution containing 0.1 mM CaCl2, which resulted in failures in about half of the stimulated responses in wild-type larvae. A total of 30 trials (stimulations) were performed at each NMJ in all genotypes. The failure rate was obtained by dividing the total number of failures by the total number of trials (100). High-frequency (10 Hz) recordings were performed at a calcium concentration of 2 mM and paired-pulse recordings (10 Hz) were performed at calcium concentrations of 0.4 mM and 1.5 mM, respectively. Paired-pulse ratios were calculated as the EPSP amplitude of the second response divided by the first response (EPSP2/EPSP1).
Supporting information
(A and B) Quantitative RT-PCR showing transcript levels of ND-20L in Gal4 controls and pan-neuronal and muscle Gal4-driven UAS-NDUFS7[RNAi]. Compared to pan-neuronal Gal4 control (elaV(C155)-Gal4/+), elaV(C155)-Gal4-driven UAS-NDUFS7[RNAi] (elaV(C155)-Gal4/+; NDUFS7[RNAi]/+) led to ~25% reduction in ND-20L transcript level in neurons. The muscle Gal4-driven UAS-NDUFS7[RNAi] showed ~50% reduction in ND-20L transcript levels (UAS-NDUFS7[RNAi]/+; BG57-Gal4/+) compared to Gal4 control (BG57-Gal4/+) in the muscle. Error bars represent mean ± standard deviation p = 0.354, *p = 0.015. Statistical analysis based on Student t test for pairwise comparisons. (C and D) Quantitative RT-PCR showing transcript levels of ND-20 in Gal4 controls and pan-neuronal and muscle Gal4-driven UAS-NDUFS7[RNAi]. Compared to pan-neuronal Gal4 control (elaV(C155)-Gal4/+), elaV(C155)-Gal4-driven UAS-NDUFS7[RNAi] (elaV(C155)-Gal4/+; UAS-NDUFS7[RNAi]/+) led to ~70% reduction in ND-20 transcript level in neurons. The muscle Gal4-driven UAS-NDUFS7[RNAi] showed ~60% reduction in ND-20 transcript levels (UAS-NDUFS7[RNAi]/+; BG57-Gal4/+) compared to Gal4 control (BG57-Gal4/+) in the muscle. Error bars represent mean ± standard deviation ***p = 0.001, ***p = 0.0003. Statistical analysis based on Student's t test for pairwise comparisons. (E) Histogram showing OCR in the mitochondria isolated from the thoracic region in muscle Gal4 control and muscle-depleted UAS-NDUFS7[RNAi] animals. The muscle depletion of NDUFS7 message resulted in a ~80% reduction in oxygen consumption compared to the Gal4 control. (F) OCR in muscle Gal4 control and muscle-depleted UAS-NDUFS7[RNAi] are plotted on a 60-min time scale. Raw data for this figure are available in the S2 Data Excel file, tab S1 Fig.
(TIF)
(A–D) Representative confocal images of the ventral nerve cord (VNC) at the third instar larval brain in (A) mito-GFP, D42-Gal4/+, (B) UAS-NDUFS7[RNAi]/+; mito-GFP, D42-Gal4/+, (C) UAS-NDUFS7[RNAi]/+; mito-GFP, D42-Gal4/+ with NACA, and (D) UAS-NDUFS7[RNAi]/UAS-Sod2; mito-GFP, D42-Gal4/+ labeled with superoxide indicator MitoSOX (magenta) in live animals. UAS-NDUFS7[RNAi]-depleted larvae supplemented with NACA or co-expressing Sod2 in neurons showed a significant correction in mitochondrial ROS levels compared to the NDUFS7[RNAi] knockdown tissues. (E–H) Confocal images of third instar larval body wall muscle 6 at A2 hemisegment in the indicated genotypes. Muscles depleted of MCI function by UAS-NDUFS7[RNAi] had ROS corrected back down to normal levels when the animals were supplemented with NACA or when there was co-expression of a UAS-Sod2 transgene. Scale bar: 5 μm. (I and J) Histogram showing the relative intensity of mitoSOX in VNC and body wall muscle in (E) UAS-mitoGFP/+; BG-57-Gal4/+, (F) UAS-NDUFS7[RNAi]/UAS-mitoGFP; BG57-Gal4/+, (G) UAS-NDUFS7[RNAi]/UAS-mitoGFP; BG-57-Gal4/+, and (H) UAS-NDUFS7[RNAi]/UAS-Sod2; BG57-Gal4/+ labeled with superoxide indicator MitoSOX (magenta) in live animals. ***p < 0.0001; ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S2 Fig.
(TIF)
(A, -A′ and B, B′) Representative confocal images of the ventral nerve cord (VNC) at the third instar larval brain in the genotypes (A, A′) UAS-mito-GFP, D42-Gal4/+ and (B, B′) UAS-mito-GFP, D42-Gal4/Sod2[RNAi] labeled with superoxide indicator MitoSOX (magenta) and mito-GFP (green) in live tissue. (C) Histogram showing the relative intensity of MitoSOX in the VNC of indicated genotypes. ***p = 0.0003 (VNC: control versus Sod2[RNAi]). (D, D′ and E, E′) Representative confocal images of the axon at the third instar larval fillet in (D, D′) UAS-mito-GFP, D42-Gal4/+ and (E, E′) UAS-mito-GFP, D42-Gal4/UAS-Sod2[RNAi] labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live tissue. (F) Histogram showing the relative intensity of MitoSOX in axons in the indicated genotypes. *p = 0.020 (Axon: control versus Sod2 RNAi). (G, G′ and H, H′) Representative confocal images of a third instar bouton of synapse 6/7 in the A2 hemisegment in (G, G′) UAS-mito-GFP, D42-Gal4/+, and (H, H′) UAS-mito-GFP, D42-Gal4/Sod2[RNAi] labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live tissue. (I) Histogram showing the relative intensity of MitoSOX at boutons in the indicated genotypes. ***p < 0.0001 (boutons: control versus Sod2 RNAi). (J, J′ and K, K′) Representative confocal images of the third instar 6/7 muscle of A2 hemi segment in (J-J′) UAS-mito-GFP/+; BG57-Gal4/+, and (K, K′) UAS-mito-GFP/+; BG57-Gal4/Sod2[RNAi] labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live animals. (L) Histogram showing the relative intensity of mitoSOX in 6/7 muscle in the indicated genotypes. ***p = 0.0001 (6/7 muscles: control versus Sod2 [RNAi]). The depletion of Sod2 by RNAi results in the abnormal accumulation of reactive oxygen species (ROS) in neurons and muscles. Scale bar: 10 μm (A, A′–E, E′) and 5 μm (G, G′ and K, K′). Statistical analysis based on Student’s t test for pairwise comparison. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S3 Fig.
(TIF)
UAS-NDUFS7[RNAi], UAS-NDUFS7[RNAi] co-expressing UAS-Sod2, ND-30[RNAi], and ND-30[RNAi] co-expressing UAS-Sod2 and controls were crossed to a motor neuron driver line (D42-Gal4, UAS-mitoGFP) to label neuronal mitochondria. (A) Ventral nerve cord (VNC): UAS-mitoGFP exhibits normal mitochondrial organization, UAS-NDUFS7[RNAi] and ND-30 RNAi exhibit clustered mitochondria. However, when UAS-NDUFS7[RNAi] animals were raised in a media containing NACA or when there was co-expression of UAS-Sod2 in UAS-NDUFS7[RNAi]- and ND-30[RNAi]-depleted animals, there was a restoration of normal mitochondrial organization. The respective fluorescent images were skeletonized to measure mitochondrial branch length (organization). (B) Comparison of a proximal axonal segment in A2 and a distal segment in A5. Distal segments of A5 axons in UAS-NDUFS7[RNAi] contain fewer mitochondria than proximal segments. Scale bar: 5 μm. Mitochondrial distribution was significantly suppressed back to control values when UAS-NDUFS7[RNAi] was raised in media containing NACA or genetically over-expressing UAS-Sod2 in the UAS-NDUFS7[RNAi] and ND-30[RNAi] backgrounds in neurons. (C and D) Histogram showing mitochondrial branch length (μm) and number in the indicated genotypes. ***p < 0.0001, ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S4 Fig.
(TIF)
Mitochondrial morphology and distribution are affected in the ventral nerve cord and distal axons. Drosophila stocks of UAS-NDUFS7[RNAi], UAS-NDUFS7[RNAi] co-expressing UAS-Catalase or UAS-Sod1, as well as controls,were crossed to a motor neuron driver (D42-Gal4, UAS-mitoGFP) to label neuronal mitochondria. (A) Ventral nerve cord (VNC): UAS-mitoGFP, UAS-Cat, and UAS-Sod1 exhibit normal mitochondrial organization. By contrast, NDUFS7[RNAi] exhibits clustered mitochondria. Co-expression of UAS-Cat or UAS-Sod1 in the NDUFS7[RNAi] background does not restore normal mitochondrial organization. The respective fluorescent images were skeletonized to measure mitochondrial branch length (organization). Scale bar: 10 μm. (B) Comparison of a proximal axonal segment in A2 and a distal segment in A5. Distal segments of A5 axons in NDUFS7[RNAi] contain fewer mitochondria than proximal segments. Scale bar: 5 μm. Mitochondrial distribution was not restored to control values when NDUFS7[RNAi] was coexpressed with UAS-Cat or UAS-Sod1 in neurons. (C and D) Histogram showing mitochondrial branch length (μm) and number in the indicated genotypes. ***p < 0.0001, ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m.
(TIF)
(A, A′–D, D′) Representative confocal images of the ventral nerve cord (VNC) at the third instar larval brain in (A, A′) mito-GFP, D42-Gal4/+, (B, B′) mito-GFP, D42-Gal4/marf[RNAi], (C, C′) mito-GFP, D42-Gal4/+ with 25 μM rotenone (ROT), and (D, D′) UAS-NDUFS7[RNAi]/+; mito-GFP, D42-Gal4/+ labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live animals. (E, E′–H, H′) Representative confocal images of the axon at the third instar larval fillet in (E, E′) mito-GFP, D42-Gal4/+, (F, F′) mito-GFP, D42-Gal4/marf[RNAi], (G, G′) mito-GFP, D42-Gal4/+ with 25 μM ROT and (H, H′) UAS-NDUFS7[RNAi]/+; mito-GFP, D42-Gal4/+ labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live animals. (I, I′–L, L′). Representative confocal images of the third instar bouton of A2 hemisegment in (II’) mito-GFP, D42-Gal4/+, (J, J′) mito-GFP, D42-Gal4/marf[RNAi], (K, K′) mito-GFP, D42-Gal4/+ with 25 μM ROT and (L-L’) UAS-NDUFS7[RNAi]/+; mito-GFP, D42-Gal4/+ labeled with superoxide indicator MitoSOX (magenta) and mitoGFP (green) in live animals. The depletion of marf by RNAi or blocking MCI activity induces abnormal accumulation of ROS in neurons. Scale bar: 10 μm. (M-O) Histogram showing the relative intensity of MitoSOX in VNC, axon and boutons in the indicated genotypes ***p < 0.0001; **p < 0.001; **p = 0.002 (Bouton: control versus marf[RNAi]), *p < 0.05. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S6 Fig.
(TIF)
Representative confocal images of the proximal (A2) and distal (A5) axons of larvae in (A, A′ and B, B′) Gal4 control, (C, C′ and D, D′) UAS-Sod2/+; D42-Gal4/+, (E, E′ and F, F′) UAS-NDUFS7[RNAi]/+; D42-Gal4/+, (G, G′ and H, H′) UAS-NDUFS7[RNAi]/+; D42-Gal4/+ with NACA, and (I, I′ and J, J′) UAS-NDUFS7[RNAi]/UAS-Sod2; D42-Gal4/+. Axons were double immunolabeled with CSP (magenta) and HRP (green) antibodies. Motor neuron-depleted UAS-NDUFS7[RNAi] larval axons showed abnormal accumulation of CSP at a more distal hemisegment (A5) compared to Gal4 controls. Scale bar: 10 μm. CSP aggregates were cleared when UAS-NDUFS7[RNAi] animals were raised in media containing NACA or genetically expressing UAS-Sod2 in neurons. (K) Schematic illustration showing VNC, axons and body wall muscle in a third instar larvae (L) Histogram showing the percentage of axons with abnormal accumulation in the indicated genotypes. ***p = 0.0008 (control: normal versus accumulation), *p = 0.0008 (UAS-NDUFS7[RNAi]: normal versus accumulation), *p = 0.0179 (Sod2 OE: normal versus accumulation), *p = 0.0179 (UAS-NDUFS7[RNAi] with NACA: normal versus accumulation), *p = 0.0004 (UAS-NDUFS7[RNAi] with Sod2 OE: normal versus accumulation), **p = 0.0046 (UAS-NDUFS7[RNAi] versus UAS-NDUFS7[RNAi] with NACA: accumulation) and ***p = 0.0006 (UAS-NDUFS7[RNAi] versus UAS-NDUFS7[RNAi] with Sod2 OE: accumulation). Statistical analysis was based on Fisher’s exact test to differentiate two distinct phenotypes in the same sample. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S7 Fig.
(TIF)
Representative confocal images of the proximal (A2) and distal (A5) axons of larvae in (A, A′, B, B′) Gal4 control: UAS-mitoGFP, D42-Gal4, (C, C′, D, D′) NDUFS7[RNAi]/+; UAS-mitoGFP, D42-Gal4/+, (E, E′, F, F′) UAS-Cat/+; UAS-mitoGFP, D42-Gal4/+, (G, G′, H, H′) UAS-Sod1/+; UAS-mitoGFP, D42-Gal4/+, (I, I′, J, J′) NDUFS7[RNAi]/UAS-Cat; UAS-mitoGFP, D42-Gal4/+, and (J, J′, K, K′) NDUFS7[RNAi]/UAS-Sod1; UAS-mitoGFP, D42-Gal4/+. Axons were double immunolabeled with CSP (magenta) and HRP (green) antibodies. Motor neuron-depleted NDUFS7[RNAi] larval axons showed abnormal accumulation of CSP in axons at the more distal hemisegment (A5) compared to Gal4 controls. Scale bar: 10 μm. CSP aggregates were not cleared when NDUFS7[RNAi] animals were concurrently expressing UAS-Cat or UAS-Sod1 in neurons. (M) Histogram showing the percentage of axons with abnormal accumulation in the indicated genotypes. ***p < 0.0001 (control: normal vs. accumulation), ***p < 0.0001 (NDUFS7[RNAi]: normal vs. accumulation), ***p < 0.0001 (Cat OE: normal vs. accumulation), ***p < 0.0001 (Sod1 OE: normal vs. accumulation), ***p < 0.0001 (NDUFS7[RNAi]+Cat OE: normal vs. accumulation), ***p < 0.0001 (NDUFS7[RNAi]+Sod1 OE: normal vs. accumulation), p = 0.911 (NDUFS7[RNAi] vs. NDUFS7[RNAi]+Cat OE: accumulation) and p = 1.00 (NDUFS7[RNAi] vs. NDUFS7[RNAi]+Sod1 OE: accumulation). Statistical analysis was based on Fisher’s exact test to differentiate two distinct phenotypes in the same sample. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S8 Fig.
(TIF)
Representative confocal images of NMJ synapses at muscle 6/7 of (A, A′) UAS-mitoGFP, D42-Gal4 control, (B, B′) UAS-Cat overexpression (UAS-Cat/+; UAS-mitoGFP, D42-Gal4/+), (C, C′) UAS-Sod1 overexpression (UAS-Sod1/+; UAS-mitoGFP, D42-Gal4/+), (D, D′) UAS-mitoGFP, D42-Gal4-driven NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; UAS-mitoGFP, D42-Gal4/+), (E, E′) NDUFS7 knockdown with UAS-Cat (UAS-NDUFS7[RNAi]/UAS-Cat; UAS-mitoGFP, D42-Gal4/+) and (F, F′) NDUFS7 knockdown with UAS-Sod1 (UAS-NDUFS7[RNAi]/UAS-Sod1; UAS-mitoGFP, D42-Gal4/+). Each condition was double immunolabeled with 22C10 (anti-Futsch, magenta) and anti-HRP (green) antibodies. The motor neuron-depleted NDUFS7[RNAi] larvae showed a decrease in the number of Futsch-positive loops as compared to the Gal4 control. Neither UAS-Cat nor UAS-Sod1 restored Futsch-positive loops in the UAS-NDUFS7[RNAi] background. Scale bar: 5 μm. (G) Histograms showing the percentage of Futsch-positive loops in the indicated genotypes. p = 0.659 (Control versus UAS-Cat), p = 0.505 (Control versus UAS-Sod1), p < 0.0001 (Control versus UAS-NDUFS7[RNAi]), p = 0.071 (UAS-NDUFS7[RNAi] versus UAS-NDUFS7[RNAi] + UAS-Cat) and p = 0.039 (UAS-NDUFS7[RNAi] versus UAS-NDUFS7[RNAi] + UAS-Sod1). Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S9 Fig.
(TIF)
(A–F) Representative electrophysiological traces of mEPSPs and EPSPs in (A) motor-neuron Gal4 control D42-Gal4/+, (B) UAS-NDUFS7[RNAi]/+; D42-Gal4/+, (C) UAS-marf[RNAi]/D42-Gal4, (D) UAS-Sod2 OE/+; UAS-marf[RNAi]/D42-Gal4, (E) UAS-NDUFS7[RNAi]/+; UAS-marf[RNAi]/D42-Gal4, and (F) UAS-NDUFS7[RNAi]/UAS-Sod2; UAS-marf[RNAi]/D42-Gal4. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). Note that mEPSP and EPSP amplitudes were reduced in motor neuron Gal4-driven UAS-marf[RNAi] and UAS-marf[RNAi] + UAS-NDUFS7[RNAi] animals. (G–I) Histograms showing average mEPSPs, EPSPs, and quantal content in the indicated genotypes. A minimum of 8 NMJ recordings of each genotype were used for quantification. *p < 0.05 (mEPSP amplitude: control vs. UAS-NDUFS7[RNAi]), *p = 0.007 (QC: control vs. UAS-marf[RNAi]),*p = 0.026 (QC: UAS-marf[RNAi] vs. UAS-marf[RNAi] + Sod2 OE), **p < 0.001, ***p < 0.0001, ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars represent mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S10 Fig.
(TIF)
(A and B) Representative traces of EPSPs and mEPSPs in (A) motor neuron-Gal4 control (D42-Gal4/+) and (B) motor neuron-Gal4 driven UAS-NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; D42-Gal4/+) at 0.15 mM extracellular Ca2+ concentration. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mv (1 mV). (C) Quantification of EPSPs in the indicated genotypes. A very low extracellular calcium concentration mildly affects EPSPs in NDUFS7[RNAi] compared to control larvae. (D) Failure analysis at 0.1 mM Ca2+ for the following conditions: Gal4 control (D42-Gal4/+), Sod2 overexpression (UAS-Sod2/+; D42-Gal4/+), D42-Gal4-driven UAS-NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; D42-Gal4/+), UAS-NDUFS7[RNAi]/+; D42-Gal4/+ with NACA, and UAS-NDUFS7[RNAi]/UAS-Sod2; D42-Gal4/+. The number of failures was counted per hundred trials in each genotype. There is a marked decrease in failure rate in motor neurons driving UAS-NDUFS7[RNAi]. The synaptic failure rates were restored back up to baseline levels when UAS-NDUFS7[RNAi]-depleted flies were reared in the media containing NACA or genetic expression of Sod2 in motor neurons. (E and F) Representative paired-pulse EPSP traces at 0.4 mM extracellular Ca2+ in the indicated genotypes. Scale bars for EPSPs are x = 50 ms and y = 10 mV. No change in paired-pulse ratio was observed in motor neuron-depleted UAS-NDUFS7[RNAi] animals at 0.4 mM Ca2+. (G) Quantification of paired-pulse ratio (EPSP2/EPSP1) in motor neuron-Gal4 control (D42-Gal4/+) and (B) motor neuron-Gal4 driven UAS-NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; D42-Gal4/+) larvae. (H and I) Representative paired-pulse EPSP traces at 1.5 mM extracellular Ca2+ in the indicated genotypes. Scale bars for EPSPs are x = 50 ms and y = 10 mV. No change in paired-pulse ratio was observed in motor neuron-depleted UASNDUFS7[RNAi] animals, even at higher calcium concentrations. (J) Quantification of paired ratio (EPSP2/EPSP1) in motor neuron-Gal4 control (D42-Gal4/+) and (B) motor neuron-Gal4 driven UAS-NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; D42-Gal4/+) larvae at higher extracellular calcium. (K and L) Representative EPSP recordings of 100 stimuli at 3 mM extracellular Ca2+ during a 10 Hz stimulus train in the indicated genotypes. Scale bars for EPSPs are x = 1,000 ms and y = 10 mV. (M) Quantifying EPSP amplitudes in the indicated stimulus number in the genotypes mentioned above. (N and O) Representative high-frequency recordings of 6,000 stimuli at 2 mM extracellular Ca2+ during a 10 Hz stimulus train in the indicated genotypes. Scale bars for EPSPs are x = 1,000 ms and y = 10 mV. (P) Quantification of the EPSP amplitudes in the indicated stimulus number in the genotypes mentioned above. No significant depletion in vesicle pools was observed when UAS-NDUFS7[RNAi]-depleted animals were subjected to high-frequency nerve stimulation for 10 min. p-values are indicated in the figure, ns; they are not significant. Statistical analysis based on Student's t test. Error bars signify mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S11 Fig.
(TIF)
(A) Representative images of the A2 hemisegment of muscle 6/7 NMJs in (A) w1118 + DMSO, 1 hour, nerve severed, (B) w1118 + DMSO, 1 hour, nerve intact, (C) w1118 + 500 μM rotenone (ROT), 1 hour, nerve severed, (D) w1118 + ROT, 1 hour, nerve intact, (E) w1118 + DMSO, 2 hours, nerve severed, (F) w1118 + DMSO, 2 hours, nerve intact, (G) (w1118 + 500 μM ROT, 2 hours, nerve severed), (H) (w1118 + ROT, 2 hours, nerve intact), (I) (w1118 + DMSO, 4 hours, nerve severed), (J) (w1118 + DMSO, 4 hours, nerve intact), (K) (w1118 + 500 μM ROT, 4 hours, nerve severed), (L) (w1118 + ROT, 4 hours, nerve intact), (M) (w1118 + DMSO, 6 hours, nerve severed), (N) (w1118 + DMSO, 6 hours, nerve intact), (O) (w1118 + 500 μM ROT, 6 hours, nerve severed), (P) (w1118 + 500 μM ROT, 6 hours, nerve intact), (Q) (w1118 + DMSO, 8 hours feeding, intact larvae), (R) (w1118 + 25 μM ROT, 8 hours feeding, intact larvae), (S) (w1118 + DMSO, embryo to 3rd instar), and (T) (w1118 + 25 μM ROT, embryo to 3rd instar) larvae immunostained with antibodies against the active zone scaffold Bruchpilot (BRP:fire-LuT) to label the active zones. Scale bar: 5 μm. Note that the incubation or feeding larvae with rotenone elevate BRP levels at the NMJs in w1118 + 500 μM ROT, 6 hours nerve intact and w1118 + 25 μM ROT, embryo to 3rd instar compared to control nerve severed larvae. (U) Histograms show the quantification of BRP intensity in μm2 of bouton area at muscle 6/7 in the indicated genotypes. At least 8 NMJs of each genotype were used for quantification. ***p = 0.0003 (w1118 + DMSO, 1 hour, nerve severed vs. w1118 + DMSO, 1 hour, nerve intact), ***p < 0.0001, **p = 0.0019 (w1118 + DMSO, embryo to 3rd instar vs. w1118 + 25 μM ROT, embryo to 3rd instar). Error bars signify the standard error of the mean. Statistical analysis is based on the Student’s t test for pairwise comparison among the samples. Raw data for this figure are available in the S2 Data Excel file, tab S12 Fig.
(TIF)
Representative images of the A2 hemisegment of muscle 6/7 NMJs in (A) UAS-mitoGFP, D42-Gal4/+, (B) UAS-Cat/+; UAS-mitoGFP, D42-Gal4/+, (C) UAS-Sod1/+; UAS-mitoGFP, D42-Gal4/+, (D) NDUFS7[RNAi]/+; UAS-mitoGFP, D42-Gal4/+, (E) NDUFS7[RNAi]/UAS-Cat; UAS-mitoGFP, D42-Gal4/+, and (F) NDUFS7[RNAi]/UAS-Sod1; UAS-mitoGFP, D42-Gal4/+ larvae immunostained with antibodies against the active zone scaffold Bruchpilot (BRP:fire-LuT) to label the active zones. BRP levels are upregulated at the NMJs in NDUFS7-depleted flies, while overexpression of ROS scavenger genes Cat or Sod1 in the neuron fails to restore BRP to the control level. (A–F) Scale bar: 2.5 μm. (F and G) Histograms showing quantification of (F) BRP intensity and (G) density per μm2 area of bouton at muscle 6/7 for the genotypes mentioned above. At least 8 NMJs of each genotype were used for quantification. ***p < 0.0001. Error bars denote mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (H–P) Representative electrophysiological traces and quantifications of mEPSPs, EPSPs and quantal content in the indicated genotypes. Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). EPSP amplitudes were maintained in NDUFS7-depleted flies, likely due to the induction of BRP. A minimum of 7 NMJs recordings of each genotype were used for quantification. mEPSP amplitude: **p = 0.002 (Control versus UAS-Cat), *p = 0.009 (Control versus UAS-Sod1), *p = 0.018 (Control versus NDUFS7[RNAi]); Quantal content: **p = 0.0009 (Control versus UAS-Cat), *p = 0.004 (Control versus UAS-Sod1), *p = 0.010 (Control versus NDUFS7[RNAi]); ns, not significant. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Error bars denote the standard error of the mean. (Q) Representative images of the A2 hemisegment of muscle 6/7 NMJs in UAS-mitoGFP, D42-Gal4/+, (R) UAS-Cat/+; UAS-mitoGFP, D42-Gal4/+, (S) UAS-Sod1/+; UAS-mitoGFP, D42-Gal4/+, (T) NDUFS7[RNAi]/+; UAS-mitoGFP, D42-Gal4/+, (U) NDUFS7[RNAi]/UAS-Cat; UAS-mitoGFP, D42-Gal4/+ and (V) NDUFS7[RNAi]/UAS-Sod1; UAS-mitoGFP, D42-Gal4/+ larvae immunostained with antibodies against HRP (magenta) and GFP (mito-GFP:green) to label neurons and mitochondria. NDUFS7-depleted and Cat- or Sod1-expressing NDUFS7[RNAi] animals harbor fewer mitochondria at the terminals than control animals. (Q–V) Scale bar: 5 μm. (W) Histograms showing quantification of mitochondrial clusters in the above-indicated genotypes. At least 8 NMJs of each genotype were used for quantification. ***p < 0.0001. Error bars represent mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Raw data for this figure are available in the S2 Data Excel file, tab S13 Fig.
(TIF)
Representative images of the A2 hemisegment of muscle 6/7 NMJs in (A) elaV(C155)/+ with DMSO, (B) elaV(C155)/+ with Xestospongin C (Xesto) and Dantrolene (Dant), (C) elaV(C155)/+ ; UAS-NDUFS7[RNAi]/+ with DMSO, (D) elaV(C155)/ + ; UAS-NDUFS7[RNAi]/+ with Xesto and Dant, (E) elaV(C155)/+; UAS-IP3-sponge.m30/+, (F) elaV(C155)/+; UAS-mcu[RNAi]/+, (G) elaV(C155)/+; UAS-mcu[RNAi]/UAS-NDUFS7[RNAi] with Xesto and Dant and (H) elaV(C155)/+; UAS-mcu[RNAi]/UAS-NDUFS7[RNAi]/+; UAS-IP3-sponge.m30/+ with Dant. Larvae were immunostained with antibodies against the active zone scaffold Bruchpilot (BRP:fire-LuT) to label the active zones. BRP levels are upregulated at the NMJs in UAS-NDUFS7[RNAi] with DMSO and UAS-NDUFS7[RNAi] with Xesto and Dant flies. However, genetically inhibiting ER calcium release and calcium import to the mitochondria reduces BRP levels below the control level. (A–H) Scale bar: 5 μm. (I) Histograms showing quantification of BRP intensity in the μm2 area of bouton at muscle 6/7 in the above genotypes. At least 8 NMJs of each genotype were used for quantification. ***p < 0.0001 **p = 0.0002, (BRP levels: elaV(C155)/+ with DMSO vs. elaV(C155)/+; UAS-NDUFS7[RNAi]/+ with Xesto and Dant, Error bars denote mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Raw data for this figure are available in the S2 Data Excel file, tab S14 Fig.
(TIF)
(A–N) Representative electrophysiological traces of Gal4 driver control (elaV(C155)-Gal4/+) or experimental genotypes tested for combinatorial effects when losing NDUFS7 gene function and/or the function of glycolysis or TCA cycle genes, including: hexokinase A (hex-A), hexokinase C (hex-C), Citrate (Si) Synthase I, Isocitrate dehydrogenase (Idh), and Succinyl-coenzyme A synthetase α subunit 1 (Scsa1). Scale bars for EPSPs (mEPSP) are x = 50 ms (1,000 ms) and y = 10 mV (1 mV). (O–Q) Data histograms and statistical analyses for these same genotypes (knockdowns all in the elaV(C155)-Gal4/ + genetic background; see Table Q in the S1 Tables file for full genotypes), including quantal size (mEPSP), evoked excitation (EPSP) and quantal content. Error bars denote mean ± s.e.m. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. *p < 0.05, **p < 0.01, ***p < 0.001. Raw data for this figure are available in the S2 Data Excel file, tab S15 Fig.
(TIF)
(A–L) Representative images of the A2 hemisegment of muscle 6/7 NMJs, examining active zone material through anti-Bruchpilot (BRP) immunostaining. Analysis for Gal4 driver control (elaV(C155)-Gal4/+) or experimental genotypes tested for combinatorial effects of losing NDUFS7 gene function and/or the function of glycolysis or TCA cycle genes, including hexokinase A (hex-A), hexokinase C (hex-C), Citrate (Si) Synthase I, Isocitrate dehydrogenase (Idh), and Succinyl-coenzyme A synthetase α subunit 1 (Scsa1). Scale bar: 5 μm. See Table R in the S1 Tables file for full genotypes. (M) Histograms showing quantification of BRP intensity in the μm2 area of bouton at muscle 6/7 in the above genotypes. At least 8 NMJs of each genotype were used for quantification. Error bars signify mean ± s.e.m. Raw data for this figure are available in the S2 Data Excel file, tab S16 Fig.
(TIF)
(A–E) Representative confocal images of third instar larval NMJs in (A) Muscle-Gal4 control (BG57-Gal4/+), (B) Muscle Gal4-driven UAS-Sod2 (UAS-Sod2/+; BG57-Gal4/+), (C) Muscle UAS-NDUFS7[RNAi] (UAS-NDUFS7[RNAi]/+; BG57-Gal4/+), (D) Sod2 muscle rescue (UAS-Sod2/UAS-NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), and (E) (UAS-NDUFS7[RNAi]/+; BG57-Gal4/+ with NACA). Synapses are immunostained with anti-HRP (green) and α-Spectrin (magenta) antibodies. Scale bar: 5 μm. (N) Histogram showing relative α-Spectrin area in the indicated genotypes. Compared with controls, UAS-NDUFS7[RNAi]/ + ; BG57-Gal4/+) NMJs show a significant reduction in α-Spectrin area, which is restored upon muscle overexpression of a Sod2 transgene or feeding larvae with NACA. ***p < 0.0001; ns, not significant. Error bars signify mean ± s.e.m. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple comparisons. Raw data for this figure are available in the S2 Data Excel file, tab S17 Fig.
(TIF)
The NDUFS7 subunit in muscle affects the organization of the GluR cluster in Drosophila. Representative confocal images of boutons at third instar larval NMJ synapse in (A) Muscle-Gal4 control (BG57-Gal4/+), (B) Muscle Gal4-driven UAS-Cat (UAS-Cat/+; BG57-Gal4/+), (C) Muscle Gal4-driven UAS-Sod1 (UAS-Sod1/+; BG57-Gal4/+), (D) Muscle NDUFS7[RNAi] (NDUFS7[RNAi]/+; BG57-Gal4/+), (E) Cat muscle rescue (UAS-Cat/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4), and (F) Sod1 muscle rescue (UAS-Sod1/NDUFS7[RNAi]; BG57-Gal4/BG57-Gal4) animals immunolabeled with active zone marker BRP (magenta) and anti-GluRIII (green) antibodies. Scale bar: 2.5 μm. Note that GluRIII clusters apposed by BRP are missing in the NDUFS7-depleted NMJs, as well as the Cat and Sod1 non-rescued NMJs (marked in arrow). (G) Histograms showing quantification of the number of missing BRP-GluRIII apposed puncta per bouton in the indicated genotypes. ***p < 0.0001. Error bars represent mean ± s.e.m. Statistical analysis based on one-way ANOVA with post-hoc Tukey’s test for multiple comparisons. Raw data for this figure are available in the S2 Data Excel file, tab S18 Fig.
(TIF)
Tables A–U: summary data for graphs in all figures and supplemental figures. Table V: detailed information about reagents and materials for items described in the STAR methods section.
(PDF)
(XLSX)
(XLSX)
Acknowledgments
We acknowledge the Developmental Studies Hybridoma Bank (University of Iowa, USA) for antibodies used in this study and the Bloomington Drosophila Stock Center (Indiana University, Bloomington, USA) for fly stocks. We thank members of the Frank lab for their helpful comments and discussions in this study.
Abbreviations
- BL
Bloomington Line
- BDSC
Bloomington Drosophila Stock Center
- BMP
bone morphogenetic protein
- CSP
cysteine string protein
- EPSP
excitatory postsynaptic potentials
- ER
endoplasmic reticulum
- IP3R
IP3 receptor
- LTP
long-term potentiation
- MAGUK
membrane-associated guanylate kinase
- MCU
mitochondrial calcium uniporter
- MCI
mitochondrial complex I
- mEPSP
miniature excitatory postsynaptic potentials
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- NACA
N-acetyl cysteine amide
- NMJ
neuromuscular junction
- OCR
oxygen consumption rate
- PHP
presynaptic homeostatic potentiation
- QC
quantal content
- RET
reverse electron transfer
- ROS
reactive oxygen species
- RRP
readily releasable pool
- RyR
ryanodine receptor
- SSR
subsynaptic reticulum
- STAR
structured, transparent, accessible reporting
- TOR
target of rapamycin
- UPR
unfolded protein response
- VNC
ventral nerve cord
Data Availability
All relevant data are within the paper and its Supporting information files. Quantified data are plotted and displayed in the main and supplemental figures. Summary data of the figures are displayed in Tables A–U in S1 Tables. Reagents and sources for generating the data are shown in Table V in S1 Tables. Raw data for all figures are in supplemental Excel files. The S1 Data Excel file contains raw data for the main figures. The S2 Data Excel file contains raw data for the supplemental figures.
Funding Statement
B.M. was supported in part by NIH/NINDS (https://www.ninds.nih.gov) grants to C.A.F. (NS085164, NS130108, and NS136753). Collectively, the work was supported by those same NIH/NINDS grants, as well as funds from the University of Iowa’s Carver College of Medicine (https://medicine.uiowa.edu) to C.A.F. Funding supporting the work executed by S.A.B. and X.W. was from an NIH/NINDS (https://www.ninds.nih.gov) R01 grant NS128040 to X.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chamberlain KA, Sheng Z-H. Mechanisms for the maintenance and regulation of axonal energy supply. J Neurosci Res. 2019;97(8):897–913. doi: 10.1002/jnr.24411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32(26):8940–51. doi: 10.1523/JNEUROSCI.0026-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neupane P, Bhuju S, Thapa N, Bhattarai HK. ATP synthase: structure, function and inhibition. Biomol Concepts. 2019;10(1):1–10. doi: 10.1515/bmc-2019-0001 [DOI] [PubMed] [Google Scholar]
- 4.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992;284(Pt 1):1–13. doi: 10.1042/bj2840001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faria-Pereira A, Morais VA. Synapses: the brain’s energy-demanding sites. Int J Mol Sci. 2022;23(7):3627. doi: 10.3390/ijms23073627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandecasteele G, Szabadkai G, Rizzuto R. Mitochondrial calcium homeostasis: mechanisms and molecules. IUBMB Life. 2001;52(3–5):213–9. doi: 10.1080/15216540152846028 [DOI] [PubMed] [Google Scholar]
- 7.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–66. doi: 10.1016/j.neuron.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35(9):505–13. doi: 10.1016/j.tibs.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastaniotis AJ, Autio KJ, Kerätär JM, Monteuuis G, Mäkelä AM, Nair RR, et al. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(1):39–48. doi: 10.1016/j.bbalip.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 10.Banoth B, Cassel SL. Mitochondria in innate immune signaling. Transl Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte M, Sousa R, Videira A. Inactivation of genes encoding subunits of the peripheral and membrane arms of neurospora mitochondrial complex I and effects on enzyme assembly. Genetics. 1995;139(3):1211–21. doi: 10.1093/genetics/139.3.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia CJ, Khajeh J, Coulanges E, Chen EI-J, Owusu-Ansah E. Regulation of mitochondrial complex I biogenesis in drosophila flight muscles. Cell Rep. 2017;20(1):264–78. doi: 10.1016/j.celrep.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallik B, Frank CA. Roles for mitochondrial complex I subunits in regulating synaptic transmission and growth. Front Neurosci. 2022;16:846425. doi: 10.3389/fnins.2022.846425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma LK, Lu J, Bai Y. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr Med Chem. 2009;16(10):1266–77. doi: 10.2174/092986709787846578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J-L, Mukda S, Chen S-D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–75. doi: 10.1016/j.redox.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin SE, Mesterman R, Robinson B, Rodenburg RJ, Smeitink J, Tarnopolsky MA. Leigh syndrome associated with mitochondrial complex I deficiency due to novel mutations In NDUFV1 and NDUFS2. Gene. 2013;516(1):162–7. doi: 10.1016/j.gene.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 17.Goto Y. Mitochondrial encephalomyopathy. Neuropathology. 2000;20 Suppl:S82–4. doi: 10.1046/j.1440-1789.2000.00304.x [DOI] [PubMed] [Google Scholar]
- 18.Shoffner JM. Mitochondrial myopathy diagnosis. Neurol Clin. 2000;18(1):105–23. doi: 10.1016/s0733-8619(05)70180-8 [DOI] [PubMed] [Google Scholar]
- 19.Burman JL, Itsara LS, Kayser E-B, Suthammarak W, Wang AM, Kaeberlein M, et al. A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech. 2014;7(10):1165–74. doi: 10.1242/dmm.015321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. 2003;111(3):303–12. doi: 10.1172/JCI17741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–53. doi: 10.1159/000485089 [DOI] [PubMed] [Google Scholar]
- 22.Folbergrová J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12(1):35–40. doi: 10.1016/j.mito.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459(2):277–89. doi: 10.1007/s00424-009-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curcio R, Frattaruolo L, Marra F, Pesole G, Vozza A, Cappello AR, et al. Two functionally different mitochondrial phosphate carriers support Drosophila melanogaster OXPHOS throughout distinct developmental stages. Biochim Biophys Acta Mol Cell Res. 2024;1871(1):119615. doi: 10.1016/j.bbamcr.2023.119615 [DOI] [PubMed] [Google Scholar]
- 26.Krause SA, Overend G, Dow JAT, Leader DP. FlyAtlas 2 in 2022: enhancements to the Drosophila melanogaster expression atlas. Nucleic Acids Res. 2022;50(D1):D1010–5. doi: 10.1093/nar/gkab971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 2018;46(D1):D809–15. doi: 10.1093/nar/gkx976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17(4):2057–68. doi: 10.1091/mbc.e05-06-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misgeld T, Schwarz TL. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron. 2017;96(3):651–66. doi: 10.1016/j.neuron.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol. 2014;204(7):1087–98. doi: 10.1083/jcb.201312123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandoval H, Yao C-K, Chen K, Jaiswal M, Donti T, Lin YQ, et al. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife. 2014;3:e03558. doi: 10.7554/eLife.03558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Y, Shi X, Boopathy S, McDonald J, Smith AW, Chao LH. Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane. Elife. 2020;9:e50973. doi: 10.7554/eLife.50973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. MBoC. 2009;20(15):3525–32. doi: 10.1091/mbc.e09-03-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–56. doi: 10.1091/mbc.12.8.2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–46. doi: 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breuer ME, Koopman WJ, Koene S, Nooteboom M, Rodenburg RJ, Willems PH, et al. The role of mitochondrial OXPHOS dysfunction in the development of neurologic diseases. Neurobiol Dis. 2013;51:27–34. doi: 10.1016/j.nbd.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 37.Berger I, Hershkovitz E, Shaag A, Edvardson S, Saada A, Elpeleg O. Mitochondrial complex I deficiency caused by a deleterious NDUFA11 mutation. Ann Neurol. 2008;63(3):405–8. doi: 10.1002/ana.21332 [DOI] [PubMed] [Google Scholar]
- 38.Formosa LE, Muellner-Wong L, Reljic B, Sharpe AJ, Jackson TD, Beilharz TH, et al. Dissecting the roles of mitochondrial complex I intermediate assembly complex factors in the biogenesis of complex I. Cell Rep. 2020;31(3):107541. doi: 10.1016/j.celrep.2020.107541 [DOI] [PubMed] [Google Scholar]
- 39.Guerrero-Castillo S, Baertling F, Kownatzki D, Wessels HJ, Arnold S, Brandt U, et al. The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab. 2017;25(1):128–39. doi: 10.1016/j.cmet.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 40.Stroud DA, Surgenor EE, Formosa LE, Reljic B, Frazier AE, Dibley MG, et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature. 2016;538(7623):123–6. doi: 10.1038/nature19754 [DOI] [PubMed] [Google Scholar]
- 41.Kauffman ME, Kauffman MK, Traore K, Zhu H, Trush MA, Jia Z, et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React Oxyg Species (Apex). 2016;2(5):361–70. doi: 10.20455/ros.2016.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103(41):15038–43. doi: 10.1073/pnas.0601945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little AC, Kovalenko I, Goo LE, Hong HS, Kerk SA, Yates JA, et al. High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions. Commun Biol. 2020;3(1):271. doi: 10.1038/s42003-020-0988-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martelli F, Hernandes NH, Zuo Z, Wang J, Wong C-O, Karagas NE, et al. Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies. eLife. 2022;11. doi: 10.7554/elife.73812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martelli F, Zhongyuan Z, Wang J, Wong C-O, Karagas NE, Roessner U, et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc Natl Acad Sci U S A. 2020;117(41):25840–50. doi: 10.1073/pnas.2011828117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penugonda S, Mare S, Lutz P, Banks WA, Ercal N. Potentiation of lead-induced cell death in PC12 cells by glutamate: protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol Appl Pharmacol. 2006;216(2):197–205. doi: 10.1016/j.taap.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 47.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003;278(48):47365–9. doi: 10.1074/jbc.m307700200 [DOI] [PubMed] [Google Scholar]
- 48.Oswald MCW, Garnham N, Sweeney ST, Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592(5):679–91. doi: 10.1002/1873-3468.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roos J, Hummel T, Ng N, Klämbt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26(2):371–82. doi: 10.1016/s0896-6273(00)81170-8 [DOI] [PubMed] [Google Scholar]
- 50.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47(3):379–93. doi: 10.1016/j.neuron.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 51.Verstreken P, Ly CV, Venken KJT, Koh T-W, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47(3):365–78. doi: 10.1016/j.neuron.2005.06.018 [DOI] [PubMed] [Google Scholar]
- 52.Milton VJ, Jarrett HE, Gowers K, Chalak S, Briggs L, Robinson IM, et al. Oxidative stress induces overgrowth of the Drosophila neuromuscular junction. Proc Natl Acad Sci U S A. 2011;108(42):17521–6. doi: 10.1073/pnas.1014511108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oswald MC, Brooks PS, Zwart MF, Mukherjee A, West RJ, Giachello CN, et al. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. Elife. 2018;7:e39393. doi: 10.7554/eLife.39393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Böhme MA, McCarthy AW, Grasskamp AT, Beuschel CB, Goel P, Jusyte M, et al. Rapid active zone remodeling consolidates presynaptic potentiation. Nat Commun. 2019;10(1):1085. doi: 10.1038/s41467-019-08977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goel P, Dufour Bergeron D, Böhme MA, Nunnelly L, Lehmann M, Buser C, et al. Homeostatic scaling of active zone scaffolds maintains global synaptic strength. J Cell Biol. 2019;218(5):1706–24. doi: 10.1083/jcb.201807165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gratz SJ, Goel P, Bruckner JJ, Hernandez RX, Khateeb K, Macleod GT, et al. Endogenous tagging reveals differential regulation of Ca2+ channels at single active zones during presynaptic homeostatic potentiation and depression . J Neurosci. 2019;39(13):2416–29. doi: 10.1523/JNEUROSCI.3068-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong H, Zhao K, Huang S, Huang S, Yao A, Jiang Y, et al. Structural remodeling of active zones is associated with synaptic homeostasis. J Neurosci. 2020;40(14):2817–27. doi: 10.1523/JNEUROSCI.2002-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dannhäuser S, Mrestani A, Gundelach F, Pauli M, Komma F, Kollmannsberger P, et al. Endogenous tagging of Unc-13 reveals nanoscale reorganization at active zones during presynaptic homeostatic potentiation. Front Cell Neurosci. 2022;16:1074304. doi: 10.3389/fncel.2022.1074304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashrafi G, de Juan-Sanz J, Farrell RJ, Ryan TA. Molecular tuning of the axonal mitochondrial Ca2+ uniporter ensures metabolic flexibility of neurotransmission. Neuron. 2020;105(4):678-687.e5. doi: 10.1016/j.neuron.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Datta S, Jaiswal M. Mitochondrial calcium at the synapse. Mitochondrion. 2021;59:135–53. doi: 10.1016/j.mito.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 61.Marland JRK, Hasel P, Bonnycastle K, Cousin MA. Mitochondrial calcium uptake modulates synaptic vesicle endocytosis in central nerve terminals. J Biol Chem. 2016;291(5):2080–6. doi: 10.1074/jbc.M115.686956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paradis M, Kucharowski N, Edwards Faret G, Maya Palacios SJ, Meyer C, Stümpges B, et al. The ER protein Creld regulates ER-mitochondria contact dynamics and respiratory complex 1 activity. Sci Adv. 2022;8(29):eabo0155. doi: 10.1126/sciadv.abo0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benson TE, Voigt HF. Neuron labeling by extracellular delivery of horseradish peroxidase in vivo: a method for studying the local circuitry of projection and interneurons at physiologically characterized sites. J Neurosci Methods. 1995;57(1):81–91. doi: 10.1016/0165-0270(94)00131-y [DOI] [PubMed] [Google Scholar]
- 64.Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, et al. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17(4):627–40. doi: 10.1016/s0896-6273(00)80196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175(3):491–503. doi: 10.1083/jcb.200607036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen K, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3:1. doi: 10.1186/1741-7007-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Accardi MV, Daniels BA, Brown PMGE, Fritschy J-M, Tyagarajan SK, Bowie D. Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun. 2014;5:3168. doi: 10.1038/ncomms4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doser RL, Amberg GC, Hoerndli FJ. Reactive oxygen species modulate activity-dependent AMPA receptor transport in C. elegans. J Neurosci. 2020;40(39):7405–20. doi: 10.1523/JNEUROSCI.0902-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng J-J, Lin S-H, Liu Y-T, Lin H-C, Li T-N, Yao C-K. A circuit-dependent ROS feedback loop mediates glutamate excitotoxicity to sculpt the Drosophila motor system. Elife. 2019;8:e47372. doi: 10.7554/eLife.47372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147(4):893–906. doi: 10.1016/j.cell.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136(1):163–74. doi: 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441(7097):1162–6. doi: 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- 73.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441(7097):1157–61. doi: 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- 74.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105(5):1638–43. doi: 10.1073/pnas.0709336105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang J-W, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103(28):10793–8. doi: 10.1073/pnas.0602493103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang H-C, Hsu J-M, Yen C-P, Chao C-C, Chen R-H, Pan C-L. Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons. Proc Natl Acad Sci U S A. 2015;112(28):8768–73. doi: 10.1073/pnas.1501831112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obashi K, Okabe S. Regulation of mitochondrial dynamics and distribution by synapse position and neuronal activity in the axon. Eur J Neurosci. 2013;38(3):2350–63. doi: 10.1111/ejn.12263 [DOI] [PubMed] [Google Scholar]
- 78.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–95. doi: 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 79.Esterberg R, Linbo T, Pickett SB, Wu P, Ou HC, Rubel EW, et al. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J Clin Inves. 2016;126(9):3556–66. doi: 10.1172/jci84939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–71. doi: 10.1016/j.redox.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653–8. doi: 10.1073/pnas.0511154103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rimal S, Tantray I, Li Y, Pal Khaket T, Li Y, Bhurtel S, et al. Reverse electron transfer is activated during aging and contributes to aging and age-related disease. EMBO Rep. 2023;24(4):e55548. doi: 10.15252/embr.202255548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graham C, Stefanatos R, Yek AEH, Spriggs RV, Loh SHY, Uribe AH, et al. Mitochondrial ROS signalling requires uninterrupted electron flow and is lost during ageing in flies. Geroscience. 2022;44(4):1961–74. doi: 10.1007/s11357-022-00555-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fei L, Liang Y, Kintscher U, Sigrist SJ. Coupling of mitochondrial state with active zone plasticity in early brain aging. Redox Biol. 2025;79:103454. doi: 10.1016/j.redox.2024.103454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Granat L, Knorr DY, Ranson DC, Hamer EL, Chakrabarty RP, Mattedi F, et al. Yeast NDI1 reconfigures neuronal metabolism and prevents the unfolded protein response in mitochondrial complex I deficiency. PLoS Genet. 2023;19(7):e1010793. doi: 10.1371/journal.pgen.1010793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155(3):699–712. doi: 10.1016/j.cell.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaw JM, Winge DR. Shaping the mitochondrion: mitochondrial biogenesis, dynamics and dysfunction. Conference on Mitochondrial Assembly and Dynamics in Health and Disease. EMBO Rep. 2009;10(12):1301–5. doi: 10.1038/embor.2009.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esparza-Moltó PB, Romero-Carramiñana I, Núñez de Arenas C, Pereira MP, Blanco N, Pardo B, et al. Generation of mitochondrial reactive oxygen species is controlled by ATPase inhibitory factor 1 and regulates cognition. PLoS Biol. 2021;19(5):e3001252. doi: 10.1371/journal.pbio.3001252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huddleston AT, Tang W, Takeshima H, Hamilton SL, Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol. 2008;99(3):1565–71. doi: 10.1152/jn.00659.2007 [DOI] [PubMed] [Google Scholar]
- 91.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103(1):382–91. doi: 10.1152/jn.90906.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Müller M, Pym ECG, Tong A, Davis GW. Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron. 2011;69(4):749–62. doi: 10.1016/j.neuron.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52(4):663–77. doi: 10.1016/j.neuron.2006.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19(6):1237–48. doi: 10.1016/s0896-6273(00)80415-8 [DOI] [PubMed] [Google Scholar]
- 95.Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61(4):556–69. doi: 10.1016/j.neuron.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller M, Davis GW. Transsynaptic control of presynaptic Ca²⁺ influx achieves homeostatic potentiation of neurotransmitter release. Curr Biol. 2012;22(12):1102–8. doi: 10.1016/j.cub.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang T, Hauswirth AG, Tong A, Dickman DK, Davis GW. Endostatin is a trans-synaptic signal for homeostatic synaptic plasticity. Neuron. 2014;83(3):616–29. doi: 10.1016/j.neuron.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Younger MA, Müller M, Tong A, Pym EC, Davis GW. A presynaptic ENaC channel drives homeostatic plasticity. Neuron. 2013;79(6):1183–96. doi: 10.1016/j.neuron.2013.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris N, Braiser DJ, Dickman DK, Fetter RD, Tong A, Davis GW. The innate immune receptor PGRP-LC controls presynaptic homeostatic plasticity. Neuron. 2015;88(6):1157–64. doi: 10.1016/j.neuron.2015.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Müller M, Genç Ö, Davis GW. RIM-binding protein links synaptic homeostasis to the stabilization and replenishment of high release probability vesicles. Neuron. 2015;85(5):1056–69. doi: 10.1016/j.neuron.2015.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Müller M, Liu KSY, Sigrist SJ, Davis GW. RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool. J Neurosci. 2012;32(47):16574–85. doi: 10.1523/JNEUROSCI.0981-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J Neurosci. 2011;31(16):6041–52. doi: 10.1523/JNEUROSCI.6698-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goel P, Dufour Bergeron D, Böhme MA, Nunnelly L, Lehmann M, Buser C, et al. Homeostatic scaling of active zone scaffolds maintains global synaptic strength. J Cell Biol. 2019;218(5):1706–24. doi: 10.1083/jcb.201807165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oliva MK, Pérez-Moreno JJ, O’Shaughnessy J, Wardill TJ, O’Kane CJ. Endoplasmic reticulum lumenal indicators in Drosophila reveal effects of HSP-related mutations on endoplasmic reticulum calcium dynamics. Front Neurosci. 2020;14:816. doi: 10.3389/fnins.2020.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dhawan S, Myers P, Bailey DMD, Ostrovsky AD, Evers JF, Landgraf M. Reactive oxygen species mediate activity-regulated dendritic plasticity through NADPH oxidase and aquaporin regulation. Front Cell Neurosci. 2021;15:641802. doi: 10.3389/fncel.2021.641802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frank CA, James TD, Müller M. Homeostatic control of Drosophila neuromuscular junction function. Synapse. 2020;74(1):e22133. doi: 10.1002/syn.22133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56(1):109–23. doi: 10.1016/j.neuron.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39(2):255–67. doi: 10.1016/s0896-6273(03)00427-6 [DOI] [PubMed] [Google Scholar]
- 109.McCabe BD, Marqués G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39(2):241–54. doi: 10.1016/s0896-6273(03)00426-4 [DOI] [PubMed] [Google Scholar]
- 110.Kikuma K, Li X, Perry S, Li Q, Goel P, Chen C, et al. Cul3 and insomniac are required for rapid ubiquitination of postsynaptic targets and retrograde homeostatic signaling. Nat Commun. 2019;10(1):2998. doi: 10.1038/s41467-019-10992-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hauswirth AG, Ford KJ, Wang T, Fetter RD, Tong A, Davis GW. A postsynaptic PI3K-cII dependent signaling controller for presynaptic homeostatic plasticity. Elife. 2018;7:e31535. doi: 10.7554/eLife.31535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Orr BO, Fetter RD, Davis GW. Retrograde semaphorin-plexin signalling drives homeostatic synaptic plasticity. Nature. 2017;550(7674):109–13. doi: 10.1038/nature24017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goel P, Li X, Dickman D. Disparate postsynaptic induction mechanisms ultimately converge to drive the retrograde enhancement of presynaptic efficacy. Cell Rep. 2017;21(9):2339–47. doi: 10.1016/j.celrep.2017.10.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Penney J, Tsurudome K, Liao EH, Elazzouzi F, Livingstone M, Gonzalez M, et al. TOR is required for the retrograde regulation of synaptic homeostasis at the Drosophila neuromuscular junction. Neuron. 2012;74(1):166–78. doi: 10.1016/j.neuron.2012.01.030 [DOI] [PubMed] [Google Scholar]
- 115.Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15(10):918–28. doi: 10.1016/j.cub.2005.04.030 [DOI] [PubMed] [Google Scholar]
- 116.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58(2):195–209. doi: 10.1016/j.neuron.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stephan R, Goellner B, Moreno E, Frank CA, Hugenschmidt T, Genoud C, et al. Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev Cell. 2015;33(1):5–21. doi: 10.1016/j.devcel.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 119.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem. 2001;276(42):38388–93. doi: 10.1074/jbc.M105395200 [DOI] [PubMed] [Google Scholar]
- 120.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248(10):3582–92. [PubMed] [Google Scholar]
- 121.Baudhuin P, Beaufay H, De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965;26(1):219–43. doi: 10.1083/jcb.26.1.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966;46(2):323–57. doi: 10.1152/physrev.1966.46.2.323 [DOI] [PubMed] [Google Scholar]
- 123.Grinberg L, Fibach E, Amer J, Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med. 2005;38(1):136–45. doi: 10.1016/j.freeradbiomed.2004.09.025 [DOI] [PubMed] [Google Scholar]
- 124.Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, et al. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp Neurol. 2014;257:95–105. doi: 10.1016/j.expneurol.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, et al. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp Neurol. 2014;257:106–13. doi: 10.1016/j.expneurol.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.James TD, Zwiefelhofer DJ, Frank CA. Maintenance of homeostatic plasticity at the Drosophila neuromuscular synapse requires continuous IP3-directed signaling. Elife. 2019;8:e39643. doi: 10.7554/eLife.39643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hazelrigg T, Levis R, Rubin GM. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984;36(2):469–81. doi: 10.1016/0092-8674(84)90240-x [DOI] [PubMed] [Google Scholar]
- 128.Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13(3):507–23. doi: 10.1016/0896-6273(94)90022-1 [DOI] [PubMed] [Google Scholar]
- 129.Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci U S A. 1995;92(15):7036–40. doi: 10.1073/pnas.92.15.7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Usui-Aoki K, Matsumoto K, Koganezawa M, Kohatsu S, Isono K, Matsubayashi H, et al. Targeted expression of Ip3 sponge and Ip3 dsRNA impaires sugar taste sensation in Drosophila. J Neurogenet. 2005;19(3–4):123–41. doi: 10.1080/01677060600569713 [DOI] [PubMed] [Google Scholar]
- 131.Ha E-M, Oh C-T, Ryu J-H, Bae Y-S, Kang S-W, Jang I-H, et al. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8(1):125–32. doi: 10.1016/j.devcel.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 132.Missirlis F, Phillips JP, Jäckle H. Cooperative action of antioxidant defense systems in Drosophila. Curr Biol. 2001;11(16):1272–7. doi: 10.1016/s0960-9822(01)00393-1 [DOI] [PubMed] [Google Scholar]
- 133.Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem. 2008;283(36):24972–81. doi: 10.1074/jbc.M804817200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A. 2002;99(25):16162–7. doi: 10.1073/pnas.252342899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryder E, Ashburner M, Bautista-Llacer R, Drummond J, Webster J, Johnson G, et al. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics. 2007;177(1):615–29. doi: 10.1534/genetics.107.076216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167(2):761–81. doi: 10.1534/genetics.104.026427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rikhy R, Ramaswami M, Krishnan KS. A temperature-sensitive allele of Drosophila sesB reveals acute functions for the mitochondrial adenine nucleotide translocase in synaptic transmission and dynamin regulation. Genetics. 2003;165(3):1243–53. doi: 10.1093/genetics/165.3.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y, Liu X, Bai J, Tian X, Zhao X, Liu W, et al. Mitoguardin regulates mitochondrial fusion through MitoPLD and is required for neuronal homeostasis. Mol Cell. 2016;61(1):111–24. doi: 10.1016/j.molcel.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 139.Paglione M, Restivo L, Zakhia S, Llobet Rosell A, Terenzio M, Neukomm LJ. Local translatome sustains synaptic function in impaired Wallerian degeneration. EMBO Rep. 2025;26(1):61–83. doi: 10.1038/s44319-024-00301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee K-S, Huh S, Lee S, Wu Z, Kim A-K, Kang H-Y, et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A. 2018;115(38):E8844–53. doi: 10.1073/pnas.1721136115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodríguez LR, Calap-Quintana P, Lapeña-Luzón T, Pallardó FV, Schneuwly S, Navarro JA, et al. Oxidative stress modulates rearrangement of endoplasmic reticulum-mitochondria contacts and calcium dysregulation in a Friedreich’s ataxia model. Redox Biol. 2020;37:101762. doi: 10.1016/j.redox.2020.101762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dus M, Lai JS-Y, Gunapala KM, Min S, Tayler TD, Hergarden AC, et al. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 2015;87(1):139–51. doi: 10.1016/j.neuron.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.François CM, Pihl T, Dunoyer de Segonzac M, Hérault C, Hudry B. Metabolic regulation of proteome stability via N-terminal acetylation controls male germline stem cell differentiation and reproduction. Nat Commun. 2023;14(1):6737. doi: 10.1038/s41467-023-42496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hudry B, de Goeij E, Mineo A, Gaspar P, Hadjieconomou D, Studd C, et al. Sex differences in intestinal carbohydrate metabolism promote food intake and sperm maturation. Cell. 2019;178(4):901-918.e16. doi: 10.1016/j.cell.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barry WE, Thummel CS. The Drosophila HNF4 nuclear receptor promotes glucose-stimulated insulin secretion and mitochondrial function in adults. Elife. 2016;5:e11183. doi: 10.7554/eLife.11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raut S, Mallik B, Parichha A, Amrutha V, Sahi C, Kumar V. RNAi-mediated reverse genetic screen identified Drosophila chaperones regulating eye and neuromuscular junction morphology. G3 (Bethesda). 2017;7(7):2023–38. doi: 10.1534/g3.117.041632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175(2):179–91. doi: 10.1007/BF00215114 [DOI] [PubMed] [Google Scholar]
- 149.Groen CM, Windebank AJ. Isolation of intact mitochondria from Drosophila melanogaster and assessment of mitochondrial respiratory capacity using seahorse analyzer. Bio Protoc. 2025;15(3):e5180. doi: 10.21769/BioProtoc.5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terriente-Felix A, Wilson EL, Whitworth AJ. Drosophila phosphatidylinositol-4 kinase fwd promotes mitochondrial fission and can suppress Pink1/parkin phenotypes. PLoS Genet. 2020;16(10):e1008844. doi: 10.1371/journal.pgen.1008844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choudhury SD, Mushtaq Z, Reddy-Alla S, Balakrishnan SS, Thakur RS, Krishnan KS, et al. σ2-adaptin facilitates basal synaptic transmission and is required for regenerating endo-exo cycling pool under high-frequency nerve stimulation in Drosophila. Genetics. 2016;203(1):369–85. doi: 10.1534/genetics.115.183863 [DOI] [PMC free article] [PubMed] [Google Scholar]