Abstract
Clostridium perfringens enterotoxin (CPE) is responsible for the diarrheal and cramping symptoms of human C. perfringens type A food poisoning. CPE-producing C. perfringens isolates have also recently been associated with several non-food-borne human gastrointestinal (GI) illnesses, including antibiotic-associated diarrhea and sporadic diarrhea. The current study has used restriction fragment length polymorphism (RFLP) and pulsed-field gel electrophoresis (PFGE) analyses to compare the genotypes of 43 cpe-positive C. perfringens isolates obtained from diverse sources. All North American and European food-poisoning isolates examined in this study were found to carry a chromosomal cpe, while all non-food-borne human GI disease isolates characterized in this study were determined to carry their cpe on an episome. Collectively, these results provide the first evidence that distinct subpopulations of cpe-positive C. perfringens isolates may be responsible for C. perfringens type A food poisoning versus CPE-associated non-food-borne human GI diseases. If these putative associations are confirmed in additional surveys, cpe RFLP and PFGE genotyping assays may facilitate the differential diagnosis of food-borne versus non-food-borne CPE-associated human GI illnesses and may also be useful epidemiologic tools for identifying reservoirs or transmission mechanisms for the subpopulations of cpe-positive isolates specifically responsible for CPE-associated food-borne versus non-food-borne human GI diseases.
Clostridium perfringens type A food poisoning currently ranks as the second most common foodborne disease in the United States (2). The diarrhea and cramps that comprise the typical clinical symptoms of this human illness are induced by a single 35-kDa polypeptide named C. perfringens enterotoxin (CPE) (20, 23, 24). Although cpe-positive isolates represent only a very small fraction of the global C. perfringens population (16, 19, 31, 32), recent epidemiologic studies (5–8, 10, 17, 22, 26) indicate that these bacteria can also cause non-food-borne gastrointestinal (GI) illnesses in humans. In particular, some surveys (5, 10, 26) have suggested that CPE-producing C. perfringens may be responsible for ∼10% of all cases of antibiotic-associated diarrhea (AAD) and 5 to 20% of all cases of sporadic non-food-borne diarrhea (SPOR). Some evidence implicating CPE-producing C. perfringens as a cause of AAD and SPOR includes the following. (i) Feces from many humans suffering from AAD (5, 7, 22) or SPOR (10, 17, 26) contain CPE at levels comparable to those found in feces from C. perfringens type A food-poisoning victims, while CPE is rarely, if ever, detectable in feces from either healthy individuals or individuals suffering from gastroenteritis caused by other enteropathogens (1, 3, 27); (ii) in the absence of other known enteropathogens, unusually high levels of C. perfringens cells or spores are present in feces from many individuals suffering from AAD or SPOR (7, 10); and (iii) many C. perfringens strains isolated from the feces of individuals suffering from AAD or SPOR are able to express CPE (7, 9), in contrast to the lack of expression of CPE by nearly all C. perfringens isolates found in feces from either healthy individuals or individuals suffering from gastroenteritis caused by other pathogens (27, 28).
Genotypic differences have recently been identified among cpe-positive C. perfringens isolates. Results from two studies (13, 18) indicate that at least some C. perfringens type A food-poisoning isolates carry cpe on their chromosomes and that at least some cpe-positive C. perfringens veterinary isolates carry cpe on an episome (note that cpe-positive C. perfringens isolates are also considered important causes of enteric disease in domestic animals [30]). However, these putative source-related genotypic associations remain tentative due to the relatively small number and limited (European) geographic origin of enterotoxigenic C. perfringens isolates genotyped to date.
To better appreciate the role that genotypically distinct subpopulations of enterotoxigenic C. perfringens may play in CPE-associated human (and veterinary) diseases, the present study has genotypically characterized >40 cpe-positive C. perfringens isolates. Notably, the isolates used in our study originated from considerably more diverse host, geographic, and disease sources than the cpe-positive isolates genotyped to date and include a sizeable number of cpe-positive non-food-borne human GI disease isolates which had not yet been genotypically characterized. Results from the current study indicate that most (or all) cpe-positive food-poisoning isolates, regardless of their isolation date or geographic origin, carry a chromosomal cpe. Furthermore, our study also presents the first evidence that cpe is episomal in many (or all) CPE-associated non-food-borne human GI disease isolates. These new findings hold potential epidemiologic significance for suggesting that genotypically distinct subpopulations of cpe-positive C. perfringens isolates may be responsible for human C. perfringens type A food poisoning and CPE-associated non-food-borne human GI diseases.
MATERIALS AND METHODS
Bacterial strains.
The C. perfringens isolates used in this study are listed and described in Table 1.
TABLE 1.
Summary of genotypic characterization results obtained in this study for cpe-positive C. perfringens isolates
| Isolate group and source | Strain (reference) |
cpe RFLP pattern (kb) obtained with the follow- ing endonuclease:
|
PFGE-determined cpe locationa | |
|---|---|---|---|---|
| EcoRI | NruI | |||
| cpe-negative strain | ATCC 3624 (19) | Negative | Negative | Negative |
| Food-borne disease isolatesb | ||||
| 1950s European food-poisoning isolates | NCTC 8235 (19) | 10 | 5 | ND |
| NCTC 8238 (19) | 10 | 5 | ND | |
| NCTC 8239 (19) | 10 | 5 | S | |
| NCTC 8359 (19) | 10 | 5 | ND | |
| NCTC 8798 (19) | 10 | 5 | ND | |
| NCTC 8799 (19) | 10 | 5 | ND | |
| NCTC 10239 (19) | 10 | 5 | Chromosomal | |
| 1980s North American food-poisoning isolates | C-1841 (3) | 10 | 5 | Chromosomal |
| C-1849 (3) | 10 | 5 | ND | |
| C-1851 (3) | 10 | 5 | ND | |
| C-1869 (3) | 10 | 5 | ND | |
| C-1881 (3) | 10 | 5 | ND | |
| C-1887 (3) | 10 | 5 | ND | |
| FD 1041 (R. Labbe) | 10 | 5 | Chromosomal | |
| 1990s North American food-poisoning isolate | 5 (19) | 10 | 5 | ND |
| Non-food-borne disease isolates | ||||
| Stool isolates from 1980s European antibioticassociated diarrhea studyc | B2 (22) | >20 | >20 | Episomal |
| B11 (22) | >20 | >20 | Episomal | |
| B38 (22) | >20 | >20 | Episomal | |
| B41 (22) | >20 | >20 | ND | |
| B45 (22) | >20 | >20 | ND | |
| Newbury 16 (P. Borriello) | >20 | >20 | Episomal | |
| Stool isolates from 1990s European SPOR studyd | F4013 (M. Brett) | >20 | >20 | ND |
| F4129 (M. Brett) | >20 | >20 | Episomal | |
| F4393 (M. Brett) | >20 | >20 | ND | |
| F4396 (M. Brett) | >20 | >20 | ND | |
| F4406 (M. Brett) | >20 | >20 | ND | |
| F4591 (M. Brett) | >20 | >20 | Episomal | |
| F4859 (M. Brett) | >20 | >20 | ND | |
| F4969 (M. Brett) | >20 | >20 | Episomal | |
| F5537 (M. Brett) | >20 | >20 | ND | |
| F5599 (M. Brett) | >20 | >20 | ND | |
| F5603 (M. Brett) | >20 | >20 | Episomal | |
| Veterinary isolates | ||||
| 1990s European veterinary isolate from pig with enteritis | 29e (D. J. Taylor) | 10 | 5 | S |
| 1990s North American veterinary isolates | 153e (19) | >20 | >20 | ND |
| 157 (19) | >20 | >20 | ND | |
| 222e (19) | >20 | >20 | Episomal | |
| 382 (19) | >20 | >20 | ND | |
| 452e (19) | >20 | >20 | Episomal | |
| 455e (19) | >20 | >20 | ND | |
| 456e (19) | >20 | >20 | ND | |
| 457e (19) | >20 | >20 | ND | |
| 458e (19) | >20 | >20 | Episomal | |
ND, not determined; S, smearing of DNA bands observed (see Results).
Note that in reference 19, NCTC 8235 is called 182, NCTC 8238 is called 176, NCTC 8359 is called 175, and NCTC 8799 is called 183.
Note that these isolates, belonging to several different capsular serotypes, were all obtained from CPE-positive feces of diarrheic humans.
Note that these isolates were all obtained form CPE-positive feces of humans suffering from isolated cases of sporadic diarrhea.
Note that these isolates were obtained form diarrheic domestic animals.
Preparation of a DIG-labeled cpe-specific probe.
A 639-bp digoxigenin (DIG)-labeled, double-stranded DNA probe corresponding to internal cpe sequences was prepared by a two-step PCR amplification method, as described previously (14).
Restriction fragment length polymorphism (RFLP) analysis.
Starter vegetative C. perfringens cultures were grown overnight at 37°C in fluid thioglycolate broth (FTG) (Difco), as described previously (15). A 500-μl aliquot of each FTG starter culture was then used to inoculate 10 ml of TGY broth (3% Trypticase, 2% glucose, 1% yeast extract, 0.1% cysteine [14]), and these TGY cultures were incubated for 8 h at 37°C.
A previously described protocol (14) was used to isolate total C. perfringens DNA from each TGY culture. Samples of each isolated DNA were then digested to completion with either NruI (New England BioLabs) or EcoRI (Boehringer Mannheim), according to the manufacturer’s specifications. Eight micrograms of the digested DNA was then subjected to conventional electrophoresis on 0.8% agarose gels, transferred to positively charged nylon membranes (Nytran Maximum Strength; Schleicher & Schuell), and fixed with UV light (29). The 639-bp DIG-labeled cpe gene probe, prepared as indicated above, was hybridized to these blots, as described previously (14), and the blots were washed at high stringency (29). Hybridized probe was detected with a DIG chemiluminescence detection system with Lumi-Phos 530 substrate (Boehringer Mannheim).
PFGE studies.
C. perfringens cultures were grown to the mid-exponential phase (i.e., for approximately 5 h) at 37°C in TGY broth. Cells from these TGY broth cultures were used to prepare genomic C. perfringens DNA in agarose plugs, as described previously (12). DNA in 40 μl of an agarose plug was then incubated overnight at 37°C with or without 1.6 U of I-CeuI (New England Biolabs) in 200 μl of the buffer solution recommended by the enzyme manufacturer. Following these incubations, the DNA samples were analyzed by pulsed-field gel electrophoresis (PFGE) with 0.7% agarose gels prepared with PFGE-grade agarose (Bio-Rad). PFGE was performed with a Bio-Rad CHEF-DR II apparatus, with pulse times ramped from 20 to 120 s over 12 h, followed by ramping from 60 to 100 s over 12 h (21). After PFGE, the gels were subjected to Southern analysis by using the same procedure described above for DNA samples subjected to conventional electrophoresis, except that the PFGE blots were washed under standard stringency conditions (4).
The rationale for these PFGE analyses, which have been used previously to genotype cpe-positive C. perfringens isolates (13, 18), is that, without any restriction enzyme digestion, unsheared C. perfringens chromosomal DNA is too large to enter a pulsed-field gel. However, because of its smaller size, at least some episomal DNA should enter a pulsed-field gel, even without any restriction enzyme treatment. Furthermore, since all I-CeuI sites are chromosomal in C. perfringens DNA (13, 18), I-CeuI digestion of samples should produce chromosomal DNA fragments that can enter pulsed-field gels, but treatment with this restriction enzyme should not effect the migration of episomal DNA. Consequently, if isolates with a chromosomal cpe are analyzed by this PFGE protocol, their cpe-containing DNA should remain in the gel wells when samples are not treated with I-CeuI, but some cpe-containing DNA from these isolates should enter the gel when samples are treated with I-CeuI. In contrast, PFGE analysis of isolates carrying an episomal cpe should show similar migration of cpe-containing DNA into gels, whether or not a sample is treated with I-CeuI.
RESULTS
Comparative RFLP analysis of cpe-positive isolates.
A study (13) recently reported that cpe is localized to 5-kb NruI and 10-kb EcoRI DNA fragments in several European enterotoxigenic food-poisoning isolates but is present in several European veterinary isolates on DNA of >20 kb following digestion with either NruI or EcoRI. Considering that only a limited number of isolates, all with similar origins, were examined in that previous study (13), in our current study we initially tested these putative source-related cpe RFLP patterns using our laboratory’s collection of cpe-positive C. perfringens isolates.
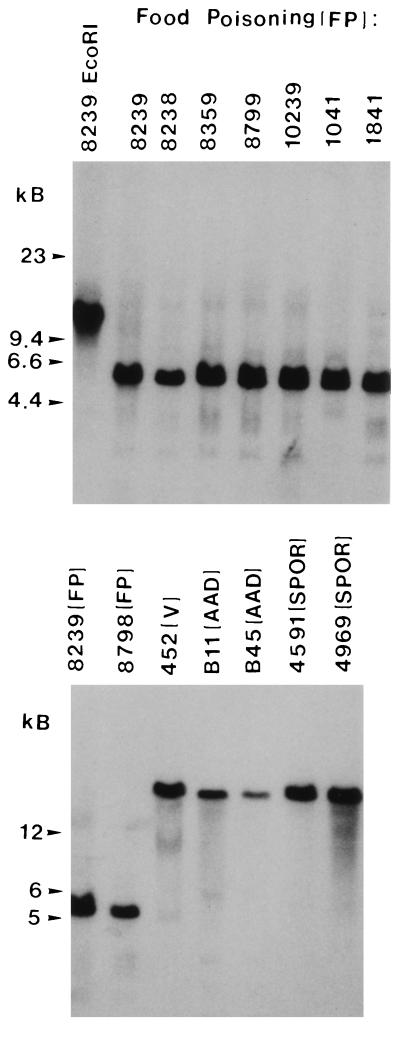
Our initial cpe RFLP experiments confirmed a previous report (13) that European food-poisoning isolates NCTC 8239, NCTC 8798, and NCTC 10239 all carry cpe on 5-kb NruI and 10-kb EcoRI fragments (see Fig. 1 for representative RFLP blot results for NCTC 8239 and NCTC 10239 and Table 1 for a summary of our results for all three of these strains). When similar RFLP analyses were extended to DNA from four European food-poisoning isolates and eight North American food-poisoning isolates that had not been previously genotyped, cpe was found to be similarly localized (see Fig. 1 for representative RFLP blot results for previously unexamined strains NCTC 8238, NCTC 8359, NCTC 8799, FD 1041, and C-1841 and Table 1 for a summary of our RFLP results for all 12 previously unexamined food-poisoning isolates) to 5-kb NruI and 10-kb EcoRI DNA fragments.
FIG. 1.
RFLP analysis of NruI-digested total DNA from selected C. perfringens disease isolates demonstrating presumptive evidence for episomal versus chromosomal distribution of cpe in isolates from different disease sources. Southern blots were probed with a 639-bp DIG-labeled cpe-specific fragment. VET, veterinary. All samples except 8239/EcoRI were cut with NruI; 8239/EcoRI was cut with EcoRI and the results are shown for comparison. Molecular sizes (in kilobase pairs) of the DNA markers are given to the left of each blot. See Table 1 for full strain designations.
The cpe RFLP profiles reported for food-poisoning isolates in Table 1 (and elsewhere [13]) differ from those reported previously (13) for several veterinary isolates, all of which were from Europe. To confirm that the cpe RFLP profiles of cpe-positive veterinary isolates consistently differ from those of food-poisoning isolates, we next performed cpe RFLP analyses with 10 cpe-positive veterinary isolates that had not been previously genotypically characterized (Table 1). These RFLP analyses revealed that all nine of the North American veterinary isolates in our collection share similar NruI or EcoRI cpe RFLP patterns (see Fig. 1 for representative RFLP blot results for veterinary isolate 452 and Table 1 for a summary of our RFLP results for all nine North American veterinary isolates examined), with cpe always localizing to DNA of >20 kb following either NruI or EcoRI digestion. While the RFLP patterns of these North American veterinary isolates match those reported previously (13) for European veterinary isolates, our study has also identified the first veterinary isolate with an atypical cpe RFLP pattern. European veterinary isolate 29, which was reputedly obtained from a diarrheic pig, was observed to carry its cpe on a 5-kb NruI and a 10-kb EcoRI fragment (Table 1).
Having confirmed, with the single exception of isolate 29, that food-poisoning and veterinary enterotoxigenic C. perfringens isolates exhibit consistent differences in their cpe RFLP patterns, we next performed the first cpe RFLP analyses of enterotoxigenic isolates originating from patients with CPE-associated non-food-borne human GI diseases. Interestingly, these RFLP analyses (see Fig. 1 for representative RFLP blot results for isolates B11, B45, F4591, and F4969 and Table 1 for a summary of our RFLP results for all non-food-borne human GI disease isolates examined) demonstrated that, following either NruI or EcoRI digestion, cpe is present on DNA fragments of >20 kb for all 7 AAD isolates and all 11 SPOR isolates in our collection. Therefore, these results demonstrate that the non-food-borne human GI isolates in our collection consistently exhibit cpe RFLP patterns different from those of human food-poisoning isolates.
Finally, the specificity of the cpe probe used in the current RFLP studies has been confirmed by control studies demonstrating (Table 1) that this probe does not hybridize to either NruI- or EcoRI-digested DNA from C. perfringens ATCC 3624, a strain previously shown to be cpe negative (14, 25).
PFGE confirmation of episomal versus chromosomal locations for cpe in selected isolates.
The RFLP results presented above supporting source-related genotypic differences between cpe-positive C. perfringens isolates are particularly interesting in light of recent studies (13, 18) showing that cpe is chromosomal in several European food-poisoning isolates carrying cpe on 5-kb NruI and 10-kb EcoRI fragments but is present on an episome in several European veterinary isolates carrying cpe on DNA fragments of >20 kb after NruI or EcoRI digestion. Therefore, the RFLP results shown in Fig. 1 and Table 1 apparently suggest that all food-poisoning isolates in our collection carry a chromosomal cpe and that all non-food-borne human GI disease and veterinary (except isolate 29) isolates in our collection carry cpe on an episome.
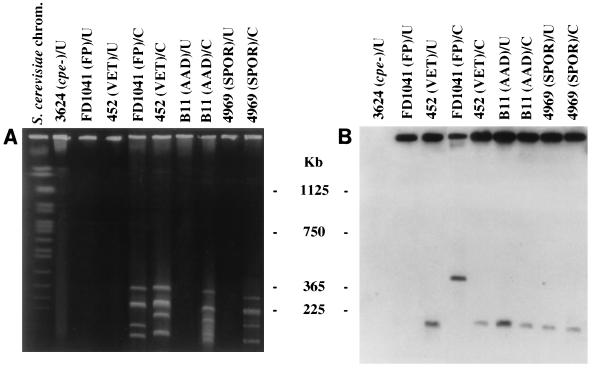
To test this hypothesis, selected isolates in our collection were analyzed by the same PFGE approach (see Materials and Methods) used in previous studies (13, 18) to establish chromosomal or episomal locations for cpe in selected European veterinary and food-poisoning isolates. When we performed these PFGE analyses with food-poisoning isolate NCTC 10239, no migration of cpe-containing DNA into pulsed-field gels was detected in the absence of I-CeuI treatment (data not shown). However, an ∼360-kb cpe-containing restriction fragment was observed when DNA from this food-poisoning strain was treated with I-CeuI prior to PFGE (data not shown). These results are in complete agreement with previous PFGE characterization of this strain (13, 18). When the same PFGE analyses were extended to North American food-poisoning isolates FD 1041 (see Fig. 2 and Table 1) and C-1841 (Table 1), which had not previously been examined by PFGE, the same migration patterns were observed for the cpe-containing DNAs of these two strains, as reported above for cpe-containing DNA from NCTC 10239. Therefore, our PFGE results confirm that all three of these food-poisoning isolates carry a chromosomal cpe.
FIG. 2.
PFGE evidence supporting the localization of cpe to episomes in isolates from some disease sources. PFGE and Southern hybridization analysis of undigested and I-CeuI-digested genomic DNA from select C. perfringens disease isolates. (A) pulsed-field gel stained with ethidium bromide. (B) Southern blot of the gel shown in panel A. The blot was probed with a 639-bp DIG-labeled cpe-specific fragment. FP, food poisoning; VET, veterinary; U, undigested, intact genomic DNA; C, I-CeuI-digested DNA. The gel was calibrated with Saccharomyces cerevisiae chromosomal DNA. Molecular sizes of the DNA markers are given in the center. See Table 1 for full strain designations.
The same PFGE analyses described above were also performed with DNAs from North American veterinary isolates 222, 452, and 458, which also have not been previously analyzed by PFGE, in order to confirm our RFLP results suggesting that these isolate carry an episomal cpe. PFGE analyses of DNAs from these three North American veterinary isolates showed that their cpe-containing DNA migrates into pulsed-field gels in the absence of I-CeuI treatment. Further supporting the fact that cpe is episomally localized (see Materials and Methods) in these three isolates, we observed no change in the migration of cpe-containing DNA from isolates 222, 452, and 458 with I-CeuI treatment (see Fig. 2 for representative PFGE blot results for isolate 452 and Table 2 for a summary of PFGE conclusions for all three veterinary isolates examined). During these studies we also attempted to perform similar PFGE analyses with DNA from isolate 29, the unusual veterinary isolate that exhibits an RFLP profile suggesting a chromosomal cpe gene. Unfortunately, this strain was not amenable to PFGE analyses, yielding DNA smears on PFGE gels even in the absence of treatment with I-CeuI (data not shown). Similar PFGE smearing has been observed (Table 1) (13) for food-poisoning strain C. perfringens NCTC 8239 and is believed to result from the fact that some C. perfringens strains produce unusually large amounts of a very stable endogenous nuclease(s) (12).
The final series of experiments conducted in the current study involved extending PFGE analyses to eight representative non-food-borne human GI disease isolates from our collection (including four AAD and four SPOR isolates, none of which have previously been examined by PFGE). Interestingly, DNAs from these eight non-food-borne human GI disease isolates were found to exhibit the same PFGE behavior described above for DNAs from veterinary isolates 222, 452, and 458, which carry an episomal cpe. That is, we observed the entrance of cpe-containing DNA species from all eight of these AAD or SPOR isolates into pulsed-field gels in the absence of I-CeuI treatment, and the migration of these cpe-containing species was unaffected by I-CeuI treatment (see Fig. 2 for representative PFGE blot results for isolates B11 and F4969 and Table 2 for a summary of PFGE results for all 8 non-food-borne disease isolates examined in this study).
Finally, the specificity of the cpe probe used in our PFGE studies was confirmed by demonstrating that this probe did not hybridize to either nondigested (Fig. 2) or I-CeuI-digested (data not shown) samples of DNA from cpe-negative strain ATCC 3624 that had been run on pulsed-field gels.
DISCUSSION
The current study offers several significant contributions to our understanding of the recently discovered (13, 18) subpopulations of cpe-positive C. perfringens. First, the RFLP results presented in this study for three previously examined European food-poisoning isolates (NCTC 8239, NCTC 8798, and NCTC 10239) and four previously unexamined European food-poisoning isolates (NCTC 8235, NCTC 8238, NCTC 8359, and NCTC 8799), which are supported by PFGE results for NCTC 10239, significantly strengthen the hypothesis (13, 18) that most, if not all, European food-poisoning isolates carry a chromosomal cpe. Our RFLP results confirming that NCTC 8235 is cpe positive are particularly interesting. Even though NCTC 8235 was originally isolated as an enterotoxigenic strain, a recent study (13) could not detect cpe in ATCC 12922, which is reputedly the same strain as NCTC 8235. Collectively, these results for NCTC 8235/ATCC 12922 could be indicating that the cpe gene disappeared from some subcultures of NCTC 8235. If so, these subcultures may have lost a mobile cpe-containing genetic element that had been integrated into the NCTC 8235 chromosome, since a putative cpe-carrying transposon has recently been identified in the chromosome of NCTC 8239, another European food-poisoning strain (11).
The current study also reports the first genotypic characterization of cpe-positive North American food-poisoning isolates. Our RFLP results for eight North American food-poisoning isolates, supported by PFGE results for North American food-poisoning isolates FD1041 and C-1841, indicate that all these isolates (which originated from three separate food-poisoning outbreaks) also carry a chromosomal cpe. When these results are coupled with similar results for European food-poisoning isolates ((13, 18); this study), it is now clear that cpe has a chromosomal location in most (or all) C. perfringens food-poisoning isolates, regardless of the isolate’s geographic origin or isolation date (note that the food-poisoning isolates examined in our current study and previous studies [13, 18] were isolated over a period extending from the 1950s to the 1990s).
Additionally, the PFGE and RFLP results presented in the current study provide the first evidence that most (or all) cpe-positive North American veterinary isolates carry an episomal cpe. When combined with previous genotyping results (13, 18) for European cpe-positive veterinary isolates, it now appears that most cpe-positive veterinary isolates, regardless of geographic origin or date of isolation, carry an episomal cpe. However, the current study has also identified the first anomalous cpe-positive veterinary isolate (isolate 29) whose DNA does not fit the standard genotypic pattern for these veterinary isolates, i.e., RFLP analyses suggest that isolate 29 is an unusual cpe-positive veterinary isolate carrying a chromosomal cpe. Since isolate 29 is a porcine isolate, this observation could provide an insight into the epidemiology of C. perfringens type A food poisoning by suggesting that some cases of C. perfringens type A food poisoning, which almost always results from consumption of a contaminated meat product (22), may be caused by ingesting meats originating from animals that had been contaminated with atypical C. perfringens veterinary isolates (like isolate 29) carrying the chromosomal cpe linked to food poisoning.
The current study’s most significant finding is presentation of the first evidence that cpe is episomal in many or all cpe-positive isolates originating from CPE-associated non-food-borne human GI diseases. While this association is based upon PFGE analysis of eight different non-food-borne human GI disease isolates (and is further implied by the similar RFLP patterns shared by these 8 isolates analyzed by PFGE and the 10 other non-food-borne human GI disease isolates examined in the current study), caution should still be exercised before extrapolating the overall involvement of isolates with episomal cpe in CPE-associated non-food-borne human GI diseases from the current data. Since the AAD and SPOR isolates used in our current study all share a similar (European) geographic origin, it will be important to conduct additional surveys with isolates from other geographic locations to confirm the putative association between episomal cpe isolates and CPE-associated non-food-borne human GI diseases suggested by our study. In this respect, it should be noted that reliably characterized North American enterotoxigenic AAD and SPOR C. perfringens isolates were unavailable for our current study, reflecting the limited research attention CPE-associated non-food-borne human GI illnesses have received to date in the United States.
Pending results from additional surveys, the fact that all 18 non-food-borne human GI disease isolates surveyed in the current study apparently carry an episomal cpe still holds considerable significance. First, this result provides important new evidence that enterotoxigenic C. perfringens isolates are, in fact, responsible for non-food-borne human GI diseases. Although this association has been strongly suggested by classical epidemiologic studies (see Introduction), these previous studies have not totally eliminated the possibility that individuals putatively identified as suffering from CPE-associated AAD or SPOR were, instead, victims of isolated, unrecognized cases of C. perfringens type A food poisoning. However, if these individuals had been sickened by C. perfringens type A food poisoning, then it would be expected that the “non-food-borne human GI disease” isolates in our collection should have exhibited the same genotypic patterns as isolates causing recognized cases of C. perfringens type A food poisoning.
Second, genotyping results for our non-food-borne disease isolates now strongly suggest that both episomal and chromosomal cpe-positive isolates can be enteropathogenic for humans. However, there may be differences in virulence between these subpopulations of cpe-positive C. perfringens isolates, based upon epidemiologic reports (5, 10, 17, 22, 26) indicating that the diarrheal symptoms of CPE-associated non-food-borne human GI diseases are generally more severe and of longer duration than the typical symptoms of C. perfringens type A food poisoning. Since the basis for these differences in symptomatology between CPE-associated human GI diseases remains unclear, future studies should compare the virulence phenotypes of chromosomal versus episomal cpe-positive isolates. Also, note that the identification of isolate 29 (which apparently carries a chromosomal cpe and which originated from a diarrheic pig) in the current study strongly suggests that both chromosomal (isolate 29) and episomal (13, 18; this study) cpe-positive isolates can be enteropathogenic for domestic animals.
Third, by implying that enterotoxigenic isolates from CPE-associated non-food-borne human GI diseases often (or always) differ genotypically from C. perfringens type A food-poisoning isolates, the current results also provide the first evidence that distinct subpopulations of cpe-positive C. perfringens isolates may be linked to particular types of CPE-associated human GI diseases. This insight could have potentially important diagnostic and epidemiologic implications. For example, if the associations suggested by this and previous genotyping studies are verified in further surveys, RFLP or PFGE assays for cpe would become useful for differentially diagnosing cases of CPE-associated food-borne versus non-food-borne diarrhea. In this regard, note that results with >20 isolates (13; this study) indicate that RFLP assays for cpe are very reliable predictors of whether an isolate carries an episomal versus chromosomal cpe. Therefore, RFLP appears to be a useful alternative for the presumptive identification of chromosomal versus episomal cpe isolates in laboratories lacking PFGE equipment (it also deserves mention that existing cpe detection-based assays other than PFGE and RFLP cannot distinguish chromosomal versus episomal cpe-positive isolates).
Similarly, cpe RFLP or PFGE analyses could prove to be useful for epidemiologic surveys for the identification of reservoirs for each genotypic subpopulation of cpe-positive isolates. Identification of these reservoirs, which are poorly understood at present, may allow for the introduction of measures specifically aimed at preventing the introduction of chromosomal cpe isolates into foods or preventing the spread of CPE-associated non-food-borne diseases, which may be exogenous infections (5, 7). These epidemiologic surveys should also better determine the relative prevalence of chromosomal versus episomal cpe-positive isolates, both globally and in specific ecologic niches. Although a previous study (18) claims that chromosomal cpe isolates are less common in nature than episomal cpe isolates, this conclusion appears premature since it was based upon surveys (13, 18) that predominantly examined veterinary isolates, which mostly carry an episomal cpe (13, 18; this study).
Finally, by strongly suggesting that many (or most) CPE-associated non-food-borne human GI disease isolates carry an episomal cpe, the current study also provides important additional support for the growing appreciation (18) of the important role that extrachromosomal genetic material plays in the virulence of clostridia involved in human infections. A future challenge will be to determine whether the cpe-carrying episome(s) present in most, or all, of our non-food-borne human GI disease (and most veterinary) isolates is a large plasmid or a phage capable of extrachromosomal replication.
ACKNOWLEDGMENTS
We thank Saleem Khan for assistance with PFGE experiments and helpful discussions. We also thank Peter Borriello, M. M. Brett, Ronald Labbe, David Mahony, J. Glenn Songer, and D. J. Taylor for generously providing us with many of the isolates used in this study.
This work was supported by Public Health Service grant AI 19844-15.
REFERENCES
- 1.Batholomew B A, Stringer M F, Watson G N, Gilbert R J. Development and application of an enzyme-linked immunosorbent assay for Clostridium perfringens type A enterotoxin. J Clin Pathol. 1985;38:222–228. doi: 10.1136/jcp.38.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bean N H, Goulding J S, Lao C, Angulo F J. Surveillance for foodborne-disease outbreaks—United States, 1988–1982. Morbid Mortal Weekly Rep. 1996;45:1–54. [PubMed] [Google Scholar]
- 3.Birkhead G, Vogt R L, Heun E M, Snyder J T, McClane B A. Characterization of an outbreak of Clostridium perfringens food poisoning by quantitative fecal culture and fecal enterotoxin measurement. J Clin Microbiol. 1988;26:471–474. doi: 10.1128/jcm.26.3.471-474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehringer Mannheim Biochemicals. The Genius system users guide for filter hybridization. Indianapolis, Ind: Boehringer Mannheim Biochemicals; 1992. [Google Scholar]
- 5.Borriello S P. Newly described clostridial diseases of the gastrointestinal tract: Clostridium perfringens enterotoxin-associated diarrhea and neutropenic enterocolitis due to Clostridium septicum. In: Borriello S P, editor. Clostridia in gastrointestinal disease. Boca Raton, Fla: CRC Press, Inc.; 1985. pp. 223–228. [Google Scholar]
- 6.Borriello, S. P. 1995. Clostridial diseases of the gut. Clin. Infect. Dis. 20(Suppl. 2):S242–S250. [DOI] [PubMed]
- 7.Borriello S P, Barclay F E, Welch A R, Stringer M F, Watson G N, Williams R K T, Seal D V, Sullens K. Epidemiology of diarrhea caused by enterotoxigenic Clostridium perfringens. J Med Microbiol. 1985;20:363–372. doi: 10.1099/00222615-20-3-363. [DOI] [PubMed] [Google Scholar]
- 8.Borriello S P, Welch A R, Larson H E, Barclay F, Stringer M F, Bartholomew B A. Enterotoxigenic Clostridium perfringens: a possible cause of antibiotic-associated diarrhea. Lancet. 1984;i:305–307. doi: 10.1016/s0140-6736(84)90359-3. [DOI] [PubMed] [Google Scholar]
- 9.Brett, M. M. 1996. Personal communication.
- 10.Brett M M, Rodhouse J C, Donovan T J, Tebbut G M, Hutchinson D N. Detection of Clostridium perfringens and its enterotoxin in cases of sporadic diarrhea. J Clin Pathol. 1992;45:609–611. doi: 10.1136/jcp.45.7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brynestad S, Synstad B, Granum P E. The Clostridium perfringens enterotoxin gene is on a transposable element in type A human food poisoning strains. Microbiology. 1997;143:2109–2115. doi: 10.1099/00221287-143-7-2109. [DOI] [PubMed] [Google Scholar]
- 12.Canard B, Saint-Joanis B, Cole S T. Genomic diversity and organization of virulence genes in the pathogenic anaerobe Clostridium perfringens. Mol Microbiol. 1992;6:1421–1429. doi: 10.1111/j.1365-2958.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 13.Cornillot E, Saint-Joanis B, Daube G, Katayama S, Granum P E, Carnard B, Cole S T. The enterotoxin gene (cpe) of Clostridium perfringens can be chromosomal or plasmid-borne. Mol Microbiol. 1995;15:639–647. doi: 10.1111/j.1365-2958.1995.tb02373.x. [DOI] [PubMed] [Google Scholar]
- 14.Czeczulin J R, Collie R E, McClane B A. Regulated expression of Clostridium perfringens enterotoxin in naturally cpe-negative type A, B, and C isolates of C. perfringens. Infect Immun. 1996;64:3301–3309. doi: 10.1128/iai.64.8.3301-3309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czeczulin J R, Hanna P C, McClane B A. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun. 1993;61:3429–3439. doi: 10.1128/iai.61.8.3429-3439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daube G, Simon P, Limbourg B, Manteca C, Mainil J, Kaeckenbeeck A. Hybridization of 2,659 Clostridium perfringens isolates with gene probes for seven toxins (α, β, ɛ, ι, τ, μ and enterotoxin) and for sialidase. Am J Vet Res. 1996;57:496–501. [PubMed] [Google Scholar]
- 17.Jackson S, Yip-Chuck D, Clark J, Brodsky M. Diagnostic importance of Clostridium perfringens enterotoxin analysis in recurring enteritis among the elderly, chronic care psychiatric patients. J Clin Microbiol. 1986;23:748–751. doi: 10.1128/jcm.23.4.748-751.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama S I, Dupuy B, Daube G, China B, Cole S T. Genome mapping of Clostridium perfringens strains with I-Ceu I shows many virulence genes to be plasmid-borne. Mol Gen Genet. 1996;251:720–726. doi: 10.1007/BF02174122. [DOI] [PubMed] [Google Scholar]
- 19.Kokai-Kun J F, Songer J G, Czeczulin J R, Chen F, McClane B A. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J Clin Microbiol. 1994;32:2533–2539. doi: 10.1128/jcm.32.10.2533-2539.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labbe R G. Clostridium perfringens. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 192–234. [Google Scholar]
- 21.Liu S L, Hessel A, Sanderson K A. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci USA. 1993;90:6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahony D E, Stringer M F, Borriello S P, Mader J A. Plasmid analysis as a means of strain differentiation in Clostridium perfringens. J Clin Microbiol. 1987;25:1333–1335. doi: 10.1128/jcm.25.7.1333-1335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClane B A. Clostridium perfringens. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C: ASM Press; 1997. pp. 305–326. [Google Scholar]
- 24.McDonel J L. Toxins of Clostridium perfringens types A, B, C, D, and E. In: Dorner F, Drews H, editors. Pharmacology of bacterial toxins. Oxford, United Kingdom: Pergamon Press; 1986. pp. 477–517. [Google Scholar]
- 25.Melville S B, Labbe R, Sonenshein A L. Expression from the Clostridium perfringens cpe promoter in C. perfringens and Bacillus subtilus. Infect Immun. 1994;62:5550–5558. doi: 10.1128/iai.62.12.5550-5558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mpamugo O, Donovan T, Brett M M. Enterotoxigenic Clostridium perfringens as a cause of sporadic cases of diarrhoea. J Med Microbiol. 1995;43:442–445. doi: 10.1099/00222615-43-6-442. [DOI] [PubMed] [Google Scholar]
- 27.Notermans S, Heuvelman C, Beckers H, Uemura T. Evaluation of the ELISA as a tool in diagnosing Clostridium perfringens enterotoxins. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig Reihe B. 1984;174:225–234. [PubMed] [Google Scholar]
- 28.Saito M. Production of enterotoxin by Clostridium perfringens derived from humans, animals and the natural environment in Japan. J Food Prot. 1990;53:115–118. doi: 10.4315/0362-028X-53.2.115. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J E, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Songer J G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Songer J G, Meer R M. Genotyping of Clostridium perfringens by polymerase chain reaction is a useful adjunct to diagnosis of clostridial enteric disease in animals. Anaerobe. 1996;2:197–203. [Google Scholar]
- 32.Van Damme-Jongsten M, Wernars K, Notermans S. Cloning and sequencing of the Clostridium perfringens enterotoxin gene. Antonie Leeuwenhoek J Microbiol. 1989;56:181–190. doi: 10.1007/BF00399981. [DOI] [PubMed] [Google Scholar]