Abstract
Background
Psychological stress can affect the incidence and mortality of non-small cell lung cancer. However, how stress influenced tumor immunity, especially tumor-infiltrating CD8+ T cells, is still unclear.
Methods
We constructed anxiety-like model with acute restraint stress and evaluated behaviors of mice through open-field test, light–dark box test and forced swimming test. Flow cytometry was performed to detect the proportion of immune cells in tumor tissues, immunofluorescence to explore the expression of related proteins, smart RNA sequence and qRT-PCR to monitor the relative genes in RNA levels, and ELISA to measure hormone concentrations in mouse serum.
Results
We observed that acute restraint stress can cause anxiety-like behaviors in mice and promote the progression of non-small cell lung cancer. Stress suppressed the expression of TNF-α, IFN-γ, granzyme B and Ki67, and induced an upregulation of PD-1+, LAG3+ and TIM-3+ CD8+ T cells. The surge on corticosterone in blood and its corresponding receptor in tumors consequent upon stress are positively correlated with tumor growth. Similarly, glucocorticoid receptor inhibitor RU486 restrained tumor growth in vivo. Specifically, RU486 reversed pro-tumor ability and CD8+ T cell exhaustion induced by corticosterone in vitro. Consistently, smart RNA sequence and enrichment analyses indicated that stress-induced pro-tumor effects were partially attributed to T cell receptor signaling pathway.
Conclusions
In brief, our study demonstrates that stress–corticosterone axis induces exhaustion of tumor-infiltrating CD8+ T cells, contributing to accelerated progression of non-small cell lung cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-025-04179-w.
Keywords: Stress, Corticosterone, NSCLC, CD8+ T cells, T cell exhaustion
Introduction
With the acceleration of social pace and the intensification of life pressure, the negative impact of psychological stress on people’s health is gradually becoming prominent, especially in cancer. On the one hand, researches revealed that people diagnosed with anxiety or depression are more likely to develop cancers [1]. On the other hand, cancer diagnosis tends to appear with depression and anxiety disorders, especially death anxiety [2]. A systematic review of 210 studies reported that the average prevalence of clinical depression was 21.2% across all types of cancer [3]. Compared to the general population, cancer patients have a higher prevalence of mental disorders [4], which has an undeniable impact on patients’ overall quality of life. Besides, anxiety and depression are known to highly associate with tumor incidence rate, tumor-specific mortality rate and all-cause mortality rate [1], contributing to the reduction in overall survival of cancer patients [5, 6]. What is more, anxiety and depression significantly weaken the effectiveness of anti-tumor treatments, including surgery [7], chemotherapy [8], radiation therapy [9], immunotherapy [5], etc. The negative impact of psychological pressure on cancer patients cannot be underestimated.
Non-small cell lung cancer (NSCLC) accounts for about 80–85% of all lung cancers, of which about 75% of patients are diagnosed to be in the middle to late stages, and the 5-year survival rate is extremely low [10, 11]. A clinical randomized controlled trial involving 151 NSCLC patients showed that depression predicts decreased survival rates for newly diagnosed metastatic NSCLC patients [6]. This effect is irreversible, and even if patients’ depressive symptoms improve significantly after treatment, their survival rates cannot be improved [6]. Immunotherapy, especially the application of immune checkpoint inhibitor PD-L1, has completely changed the treatment landscape of NSCLC, significantly prolonging the overall survival of advanced patients [12]. However, a recent clinical study involving 227 NSCLC patients indicated that the objective response rate of immune checkpoint inhibitors significantly declined in patients with anxiety or depression [5]. Therefore, it is necessary to clarify how stress affects immune monitoring and response in NSCLC.
The stress response consists of neuroendocrine cascades mediated by the sympathetic nervous system and the hypothalamic pituitary–adrenal (HPA) axis through the release of stress neurotransmitters and hormones, including catecholamines and glucocorticoids (GCs) [13]. Researchers have revealed the mysteries of stress and stress hormones in relation to the tumor immune microenvironment. Of T cells, chronic stress-induced high corticosterone (COR) levels increased Treg cell infiltration while they decreased the numbers of cytotoxic T lymphocytes and helper T cells in tumors [14]. Distress can also promote T cell exhaustion, characterized by reduced cytokine secretion, decreased effector function and elevated inhibitory receptor expression [15]. What is more, chronic stress-induced β2 adrenergic receptor (β2-AR) activation has been found to lead to an increase in myeloid-derived suppressor cells [16] and their accumulation in the spleen and tumor, promoting tumor growth, metastasis and vascularization [17]. Another research has found that stress impairs anti-tumor immunity by intervening in the glucocorticoid–Tsc22d3 axis of dendritic cells in the tumor microenvironment [8]. Stress increased M2 tumor-associated macrophage polarization through β2-AR signaling and promoted breast cancer growth and metastasis.
However, the effect and the mode of stress on T cell in tumor microenvironment of NSCLC, especially for CD8+ T cells, is not yet clear. Therefore, investigating how stress affects CD8+ T cell anti-tumor immunity and promotes NSCLC progression is critical.
Materials and Methods
Cell culture
LLC cells were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (SCSP-5252), and maintained in the Lab of Institute of Integrative Medicine, Fudan University. KLN205 cell was a gift from Institute of Integrative Medicine, Fudan University. Cells were cultured with DMEM (6,124,404, Gibco) supplemented with 10% fetal bovine serum (FBS) (40131ES76, Yeasen Biotechnology) and 1% penicillin–streptomycin solution (GNM15140-1, GENOM), kept at 37 °C in an incubator with humidity and 5% CO2.
Animals
Male C57BL/6 J and DBA/2 mice, 6–8 weeks old, weighing 20-22g, were purchased from Shanghai Jihui Experimental Animal Feeding Co., Ltd (Shanghai, China) (SCXK (沪) 2022–0009) and raised under specific pathogen-free conditions at Fudan University (Shanghai, China). Each set of five mice was kept in plastic cages (300 × 200 × 120 mm) with paper chips for bedding. Rooms were kept at a constant temperature (23 ± 3 °C) and humidity (50% ± 10%), with simulated day/night cycles (12 h each day, lights on at 8:00 am). Animal experimental protocols were approved by Fudan University’s Institutional Animal Care and Use Committee (Number: 202405FD0001). All authors are informed and agree.
After one-week adaption, the mice were randomly divided into five groups: the control group (Con), corticosterone treatment group (COR), RU486 treatment group (RU486), the acute restrain (AR) stress group and AR + RU486 treatment group (AR + RU486). In short, 5 × 105 LLC cells or KLN205 cells were subcutaneously implanted in the right flank of each mouse in 0.1 ml of PBS. Every two days, tumor volumes were measured with a caliper and computed as (length × width × width)/2. After one week, mice in the AR group received three hours of restraint stress treatment every day for ten days, from 10:00 to 13:00. During this period, each AR mouse was fixed with a plastic holder without food and water. At the same time, mice exposure to RU486 were treated glucocorticoid receptor (GR) inhibitors RU486 once a day (i.p. 10mg/kg) (HY-13683, MedChemExpress). The other two groups of mice were given the same dose of sterile PBS solution. What is more, mice of COR exposure group were given water containing 1.3μg/ml corticosterone (S2559, Selleck) which was diluted in 0.002% DMSO and balanced with 0.02% 2-hydroxypropyl-β-cyclodextrin as vehicle, according to Hong et al. [18].
Isolation and cultivation of primary CD8+ T cell
CD8a (Ly-2) MicroBeads (130–117-044, Miltenyi Biotec) was used to isolate CD8+ T cells from spleens of mice. After euthanizing the mice by cervical dislocation, the spleens of the mice were obtained. Grind the spleen by 5ml syringe tail in PBS solution and filter it through a 40μm cell filter (352,340, Falcon®). Determine cell number and resuspend cell pellet in 90 µL of buffer per 10⁷ total cells (130–091-221, Miltenyi Biotec). For every 10⁷ cells, add 10 µL of CD8a (Ly-2) MicroBeads. Stir thoroughly and place in the refrigerator (2–8 °C) for 10 min. Place MS columns (130–042-201, Miltenyi Biotec) in the magnetic field of a suitable MACS Separator and separate CD8+ T cell. Cells were cultured using RPMI 1640 Medium (6,124,417, Gibco) supplemented with 10% fetal bovine serum (FBS) (10,099-141C, Gibco) and additional 5 μg/ml anti-mouse CD3 (100,340, Biolegend), 1 μg/ml anti-mouse CD28 (102,116, Biolegend) and 10 ng/ml IL-2 (212–12, PeproTech) were added into the medium. Then place CD8+ T cells at 37 °C in an incubator with humidity and 5% CO2 for cultivation.
Open-field test
The open-field test (OFT) was performed in a box (80 cm × 80 cm × 80 cm). Each mouse was placed in a corner at the start of the test and recorded for 6 min by a camera located above the box. We cleaned the device with 75% alcohol after each trial. A tracking system with an automated analysis system recorded the number of entries into the center zone, the time spent in the center zone and the total distance traveled (Omnitech SuperFlex). The center area of the open-field apparatus was 25% of the total area (a square of approximately 40 cm × 40 cm).
Light–dark box test
For the light–dark box test (LDBT), each mouse was placed in the center of a light compartment (30 cm × 30 cm × 30 cm, white surfaces) connected with a dark compartment (20 cm × 30 cm × 30 cm, black surfaces) by a square aperture. Mouse activity was recorded during a 6-min test period. The time that the mouse spent in the light box, as well as the number of transitions from the light to the dark compartment, was analyzed by Noldus EthoVision XT.
Forced swimming test
The forced swimming test (FST) was used to estimate the depression-related behavior as described before [19]. The mice were put inside a Plexiglas cylinder that was 200 mm high by 140 mm in diameter and filled with water, which was 21°C or slightly warmer, to a height of 10 cm, preventing the mice from climbing out or touching the bottom. Use the automatic forced swimming test system (Bioseb BIO-FST-DSM) to record for 6 min, of which the immobility time of mice for the last 4 min was analyzed.
Flow cytometry
Mix tumor tissues or cultured CD8+ T cells to prepare a single-cell suspension, and centrifuge at 300 g 4 ℃ for 5 min. After discarding the supernatant, cells were added TruStain FcX™ PLUS (156,604, Biolegend) and 50 μL of staining buffer (00–4222-57, Thermo Fisher) to each sample, and the cells were stained with anti-mouse antibodies purchased from Biolegend, including Live-APC-Cy7, CD45-BV510, CD11b-FITC, F4/80-BV421, NK1.1-APC, CD11c-PE, MHC II-BV786, CD3-BUV396, CD4-PE-Cy7, CD8a-BV786, PD-1-PE, LAG3-BV421, TIM-3-APC, TNF-α-BV421, IFN-γ-BV605, Ki67-FITC, CD107a-FITC, granzyme B-PE-Cy7, IFN-γ-PE, PD-1-FITC, TIM-3-BV711 separately. For intracellular cytokine staining, T cells were stimulated with PMA (0.5 μg/mL) plus ionomycin (1 μg/mL) for 3 h and monensin for another 3 h (423,303, Biolegend), and then subjected to intracellular IFN-γ, TNF-α, granzyme B and CD107a by flow cytometry analysis. The data were collected using a BD LSR Fortessa flow cytometer (BD Biosciences) and analyzed using the FlowJo 10.9.0 software.
ELISA
The level of corticosterone (COR), adrenocorticotropic hormone (ACTH), norepinephrine and adrenaline in the mice serum was measured by COR kits (RK09054, ABclonal Biotech), ACTH kits (RK09105, ABclonal Biotech), norepinephrine kits (RK00694, ABclonal Biotech) and adrenaline kits (RK09110, ABclonal Biotech) following the manufacturers’ instructions, respectively.
Immunofluorescence
Immunofluorescent staining was conducted on tumor tissue sections embedded in paraffin, following established protocols. After standard procedures for deparaffinization, rehydration and blocking, the sections were subjected to overnight incubation at 4 ℃ with primary antibodies. The primary antibodies utilized in this investigation targeted CD8a (ab217344, Abcam, UK), PD-1(ab214421, Abcam, UK), TIM-3 (75743T, CST, USA) and GR (24,050–1-AP, Proteintech, China). Subsequent to PBS washing, the sections were incubated for 1 h at room temperature with fluorescence-conjugated anti-rabbit antibodies. Following an additional wash, the sections were mounted using a mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI). Ultimately, the stained sections were examined and documented through a confocal microscope. Fluorescence intensity of slices was analyzed by ImageJ software.
Cell viability assay
The assessment of cell viability in CD8+ T cells was conducted employing the CCK-8 assay, which relies on the conversion of water-soluble tetrazolium salt (WST-8) by dehydrogenases present in viable cells, resulting in the formation of an orange-colored product. CCK-8 assays were performed at different concentrations of corticosterone (ranging from 1µM to 100 µM), following the manufacturer’s protocols, at 24 h post-drug intervention. The CCK-8 reagent was introduced to the cellular samples, followed by an incubation period of 1 h at 37 ℃. Subsequently, the absorbance was measured at 450 nm using a microplate reader (Molecular Devices, CA, USA).
Smart RNA sequence
After anesthesia with 2% pentobarbital sodium, the mice were euthanized and fresh subcutaneous tumor tissue was taken to sort CD8+ T cells. CD8 (TIL) Micro Beads (130–116-478, Miltenyi Biotec) were used to sort CD8+ T cells from tumor tissues according to the instructions and verify their purity through flow cytometry. Total RNA was extracted from CD8+ T cells with the Universal RNA Extraction CZ Kit (RNC643, ONREW) according to the manufacturer’s instructions. RNA quantity was analyzed using Qubit 4.0 (Invitrogen) and RNA quality examined by electrophoresis on a denaturing agarose gel. Total RNA was used as input material. cDNA synthesis and amplification were using by Single Cell Full Length mRNA Amplification Kit (N712-03, Vazyme) and purification by VAHTS DNA Clean Beads (N411-02, Vazyme). Purified cDNA quality was examined by gel electrophoresis and with Qubit 4.0 (Invitrogen). Sequencing library was prepared by following the TruePrep® DNA Library Prep Kit V2 (TD502-02, Vazyme) user manual. Sequencing was carried out using the Illumina Nova seq 6000 platform with the 150 paired-end sequencing strategy.
qRT-PCR
Reverse transcription polymerase chain reaction and real-time PCR (qRT-PCR) were performed according to the manufacturers’ instruction. Briefly, total RNAs in CD8+ T cells were isolated by RNA Extraction Kit (AG21017, Accurate Biology). The cDNAs were obtained by the PrimeScript RT reagent Kit (RR037A, Takara) and subjected to qRT-PCR with SYBR® Premix Ex TaqTM (DRR041A, Takara). The relative expression levels of mRNAs compared with Gapdh were calculated by the 2-ΔΔCt method. The primers used in this study are shown in Supplementary Table 1.
NSCLC scRNA-seq Analysis of CD8Tex-Related Genes
The expression of CD8+ T cell exhaustion (CD8Tex)-related genes was explored and plotted in the NSCLC_EMTAB6149 and NSCLC_GSE149655 scRNA-seq dataset from the TISCH database (http://tisch.comp-genomics.org/home/, accessed on April 18, 2025). The expression of GRAP2 in NSCLC across a variety of cell types was analyzed and plotted in UMAP plots.
Data statistics
For smart RNA seq, the raw data were handled by Skewer v0.2.2 (https://sourceforge.net/projects/skewer/files/?source=navbar) and data quality was checked by FastQC v0.11.2. The read length was 2 × 150 bp (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The clean reads were aligned to the mouse genome (mm10) from ensembl using STAR (https://github.com/alexdobin/STAR), with one mismatch allowed. StringTie (v1.3.1c) was used to generate gene expression data and differential gene expression was analyzed by DESeq2 (v1.16.1) (https://bioconductor.org/packages/release/bioc/html/DESeq2.html). The thresholds for determining DEGs are p < 0.05 and absolute fold change ≥ 2. Then DEGs were chosen for function and signaling pathway enrichment analysis using TopGO (https://www.bioconductor.org/packages/release/bioc/html/topGO.html) and KEGG database (https://www.genome.jp/kegg/pathway.html). The significantly enriched pathways were determined when p < 0.05.
Other data were presented as the mean ± standard deviation (SD). Group-to-group comparisons were made utilizing the unpaired Student’s t-test, while multi-group analyses were conducted using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. Pearson correlation analysis was used to analyze the linear relationship between two variables or genes. Significance levels were designated as follows: *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 signifying statistical significance, while “ns” indicated the absence of a significant difference.
Results
Stress leads to accelerated proliferation of NSCLC
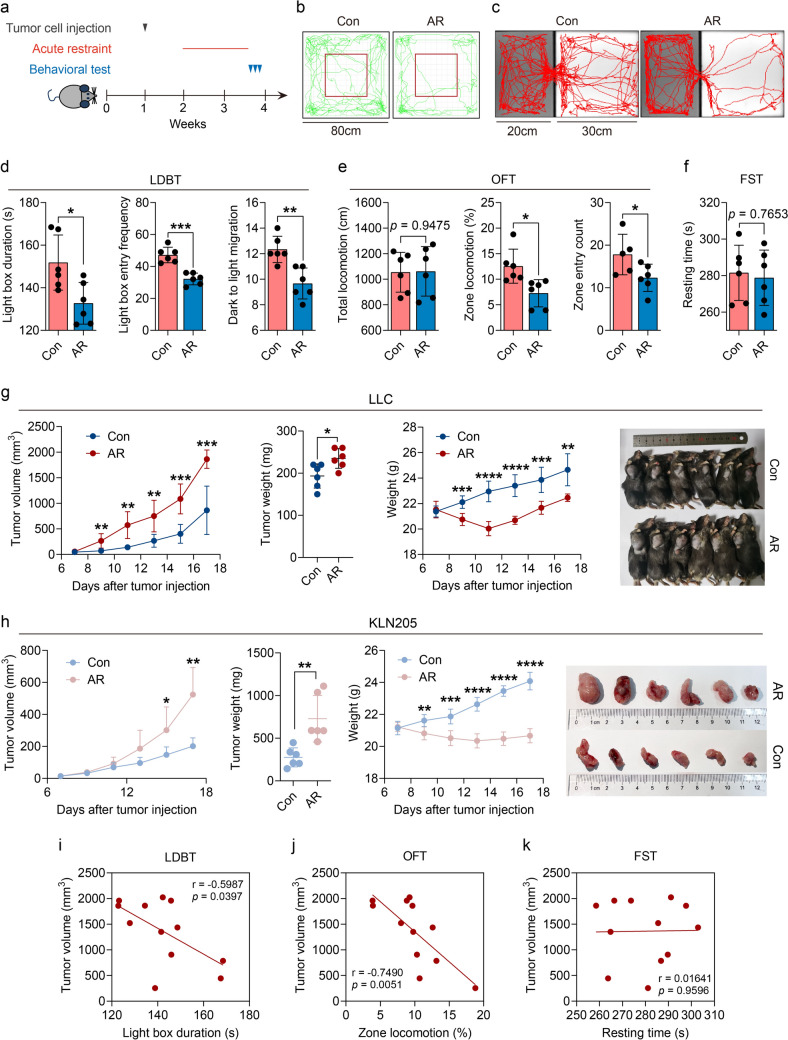
To investigate the potential impact of stress on NSCLC development, we took advantage of acute restraint stress (AR) models (Fig. 1a). Repeated exposure of male C57BL/6 mice to restraint stress treatment causes anxiety-like behavior rather than depression-like behavior, which manifests as reduced exploration of the light compartment in the light–dark box test (Fig. 1c-d), diminished locomotion and exploration in the central area of the open-field test (Fig. 1b + e) and no difference in immobility time during the forced swimming test (Fig. 1f). We determined the effect of AR on non-small cell lung cancer (NSCLC) in mice, in which we measured the volume and weight of subcutaneous tumors. AR caused an increase in tumor proliferation (tumor size in mm3) relative to non-stressed controls (Con) (Fig. 1g-h). Next, we attempted to explore the relationship between anxiety behavior and tumor growth in mice through correlation analysis, and found that the more obvious the anxiety behavior, the faster the tumor growth (Fig. 1i-j), while there is no significant correlation with depression-like behavior (Fig. 1k).
Fig. 1.
Stress promotes NSCLC progression. a Process of animal experiment; b–c representative locomotion tracks of the control (Con) and acute restraint (AR) mice in the open-field test (OFT) b and the light–dark box test (LDBT) c. d Total duration and migration to light box in LDBT were compared between the control and AR groups (n = 6). e Total locomotion and central zone activity in OFT were compared between the control and AR groups (n = 6). f Resting times in the forced swimming test (FST) were compared between the control and AR groups (n = 6). g The LLC tumor growth curves, the endpoint tumor weights, weight of mice and tumor sizes were represented (n = 6). h The KLN205 tumor growth curves, the endpoint tumor weights, weight of mice and tumor sizes were represented (n = 6). (i-k) Correlation analysis between tumor volumes and LDBT, OFT and FST (n = 12). Data are represented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001 by an unpaired Student’s t-test or Pearson analysis
Stress induces alterations in tumor immune microenvironment of NSCLC
To investigate the potential immunosuppressive impact of acute restrain stress, we analyzed the immune infiltrates of subcutaneous cancers in mice using flow cytometry (Fig. 2a). Flow cytometric analyses revealed that AR treatment significantly reduced the frequency of CD45+ cells (Fig. 2b), myeloid cells (Fig. 2c), macrophages (Fig. 2d), CD4+ T cells and CD8+ T cells (Fig. 2h), but had no impact on lymphoid cells (Fig. 2c), dendritic cells (Fig. 2f) and total T lymphocytes (Fig. 2g). Interestingly, the density of natural killing cells (NK) was increased after acute restrain stress (Fig. 2e).
Fig. 2.
Stress impairs NSCLC immunosurveillance. a Flow cytometric logic diagram of tumor tissue. Flow cytometric analysis of the proportions of b tumor CD45+, c lymphoid and myeloid, d macrophage, e NK cell, f dendritic cell, g CD3+ T cell, h CD4+ and CD8+ T cell at the tumor endpoint between the control (Con) and acute restraint (AR) mice (n = 4). Data are represented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 by an unpaired Student’s t-test
Stress promotes exhaustion of CD8+ T lymphocytes
Due to the crucial role of T lymphocytes in tumor immunity, we further analyzed the immune function changes of T cells in subcutaneous tumors of mice. Flow cytometric analyses showed that anxiety-like behavior caused by stress can inhibit the proliferation and tumor killing function of T cells and induce T cell exhaustion, specifically manifested in reduced secretion of TNF-α and IFN-γ (Fig. 3a-b), declined expression of Ki67 and CD107a (Fig. 3c-d), and increased expression of PD-1, LAG3 and TIM-3 of CD3+ T cells (Fig. 3e-g). Considering the predominant direct killing effect of CD8+ T cells on tumor, we delved deeper into the alteration of CD8+ T cells. Similar to above results, stress promoted the depletion of CD8+ T cells, which manifested by the significantly upregulation of the proportion of PD-1+ and TIM-3+ CD8+ T cells compared to the control mice (Fig. 4b-c), and weakened the proliferation function of CD8+ T cells, as evidenced by a sharp decrease in the proportion of Ki67+ CD8+ T cells (Fig. 4a). What is more, we also validated the effect of stress on CD8+ T cell depletion through immunofluorescence analysis of tumor tissue. The results revealed a significantly decreased proportion of CD8+ T cells in tumor tissue, but an obviously increased fluorescence intensity of PD-1+ and TIM-3+ CD8+ T cells (Fig. 4d-e).
Fig. 3.
Stress increases CD3+ T cell exhaustion. Flow cytometric analysis of the proportions of a tumor TNF-α+ CD3+, b IFN-γ+ CD3+, c Ki67+ CD3+, d CD107a+ CD3+, e PD-1+ CD3+, f LAG3+ CD3+ and g TIM-3+ CD3+ at the tumor endpoint between the control (Con) and acute restraint (AR) mice (n = 4). Data are represented as the mean ± SEM. *p < 0.05, **p < 0.01 by an unpaired Student’s t-test
Fig. 4.
Stress induces CD8+ T cell exhaustion. Flow cytometric analysis of the proportions of a tumor Ki67+ CD8+, b PD-1+ CD8+ and c TIM-3+ CD8+ at the tumor endpoint between the control (Con) and acute restraint (AR) mice (n = 4). d–e Immunofluorescence analysis of the expression levels of PD-1, TIM-3 and CD8a in tumor tissues (30x). Data are represented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by an unpaired Student’s t-test
HPA axis involves in tumor growth promoting effect induced by stress
Previous studies indicated that stress often causes disruption of the HPA axis. Thus, we further explored in the alternations of stress hormones in mice serum by ELISA after ten-day AR. After AR treatment in mice, a significant elevation of ACTH and COR in serum was observed (Fig. 5a-b), which was correlated with anxiety-like behaviors (Fig. 5e-f) and tumor volumes (Fig. 5g-h) in mice. Besides, we also detected a lower level of norepinephrine in AR mice (Fig. 5d), having an opposite development trend to tumor growth (Fig. 5i). However, there were neither significant changes of the level of adrenaline (Fig. 5c), nor observable correlation with tumor volumes in mice (Fig. 5j). Immunofluorescence revealed a fewer proportion CD8+ T cell in AR mice and a high expression of GR in tumor tissue (Fig. 5k). Importantly, stress intensified the expression of GR in CD8+ T cells (Fig. 5l).
Fig. 5.
Stress elevates corticosterone in blood and glucocorticoid receptor (GR) in tumor. The concentration of a adrenocorticotropic hormone (ACTH), b corticosterone (COR), c adrenaline and d norepinephrine were tested by ELISA at the tumor endpoint between the control (Con) and acute restraint (AR) mice (n = 6). (e-f) Correlation analysis between ACTH and COR with LDBT and OFT, respectively. (g-j) Correlation analysis between tumor volumes with concentration of ACTH, COR, norepinephrine and adrenaline, respectively. k Immunofluorescence analysis of the expression levels of GR and CD8a in tumor tissues and the white arrows point to CD8+ T cells with high expression of GR (50x). l Flow cytometric analysis of the expression of GR in CD8+ T cells of the control (Con) and corticosterone treatment (COR) mice (n = 3). Data are represented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001 by an unpaired Student’s t-test or Pearson analysis
Corticosterone facilitates tumor proliferation by promoting exhaustion of CD8+ T cells
Then, we further figured out the effect of COR on tumor growth in vivo and in vitro. The results showed that COR treatment intensified tumor progression (Fig. 6a), aggravated the exhaustion (Fig. 6b-c) and damaged the killing function (Fig. 6c-d) of CD8+ T cells in tumor tissues. Administering the GR inhibitor RU486 significantly inhibited the progression of non-small cell lung cancer in vivo (Fig. 6e-f). COR significantly inhibited the viability of CD8+ T cells, specifically manifested in a gradual decrease in CD8+ T cell viability with increasing COR concentration (Fig. 6g). In addition, COR can also weaken the anti-tumor ability of CD8+ T cells, such as reduced the secretion of IFN-γ and granzyme B (Fig. 6h-i), and induce immune suppression, which was manifested by increased the proportion of LAG3+, PD-1+ and TIM-3+ CD8+ T cells (Fig. 6j-l), all of what were reversed by RU486. The above results suggested that COR-GR may be a key pathway for stress-induced CD8+ T cell dysfunction.
Fig. 6.
Corticosterone (COR) promotes CD8+ T cell exhaustion and NSCLC growth. a The LLC tumor growth curves and the endpoint tumor weights were displayed of control (Con) and corticosterone treatment (COR) mice (n = 5). (b-d) Flow cytometric analysis of the proportions of PD-1+ and LAG3+, granzyme B+ and IFN-γ+ of CD8+ T cells of tumor tissues from Con and COR mice (n = 4). (e-f) The LLC tumor growth curves, the endpoint tumor weights and tumor sizes were showed of acute restraint (AR+PBS) and acute restraint+RU486 treatment (AR+RU486) mice (n = 6). g CCK-8 detection of CD8+ T cell viability after 24 hours of treatment with different concentrations of COR. Flow cytometric analysis of the proportions of h IFN-γ+, i granzyme B+, j LAG3+, k PD-1+ and l TIM-3+ of CD8+ T cells among DMSO, COR and COR+RU486 treatment groups (n = 3). Data are represented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by an unpaired Student’s T-test or one-way ANOVA
Smart RNA sequencing reveals the mechanism of stress regulated CD8+ T cell function
To further explore the mechanism of stress affecting CD8+ T cells, we used positive magnetic bead sorting to isolate CD8+ T cells from solid tumors of mice for smart RNA sequencing (Fig. 7a). Compared to the control mice, 1036 genes were significantly upregulated while 893 were downregulated in stress mice, a total of 1929 differentially expressed genes (Fig. 7b). As was shown, stress significantly increases the expression of exhaustion related genes in CD8+ T cells, such as Pdcd-1, Lag3, Cd200r1, Tox and Ctla4 (Fig. 7b), which were acknowledged to mediate tumor immune escape. KEGG and GESA functional enrichment analyses were then performed (Fig. 7c-d). The results indicated that T cell receptor signaling pathway (mmu04660), Th17 cell differentiation (mmu04659), osteoclast differentiation (mmu04380), natural killer cell-mediated cytotoxicity (mmu04650) and cytokine receptor interaction (mmu04060) were significantly enriched in both KEGG and GSEA. Among them, the T cell receptor signaling pathway is known to closely relate to the tumor killing ability of CD8+ T cells, which is similar to our above result that COR involves in stress-induced CD8+ T cell exhaustion (Fig. 7c-d). To identify the mechanism by which stress hormones COR affected CD8+ T cell function, we conducted qRT-PCR validation on the 8 most significant genes in this pathway, including Plcg1, Prkcq, Tec, Grap2, Rasgrp1, Ppp3cc, Mapk11 and Mapk14 (Fig. 7e-l). Among these genes, we found Tec, Grap2 and Ppp3cc were upregulated by COR and inhibited after RU486 treatment significantly (Fig. 7g + h + j).
Fig. 7.
Smart RNA sequencing reveals the mechanism of stress regulated CD8+ T cell function. a Isolation process of CD8+ T cells from mice tumors. b Differential genes of CD8+ T cells in tumors of the control (Con) and acute stress (AR) mice (n = 3) (|Log2(Fold change)| ≥ 1 and p < 0.05). c KEGG analysis of differential genes of CD8+ T cells and the most significant 20 enriched pathways are displayed (p < 0.05). d GSEA analysis of differential genes of CD8+ T cells and the most significant 20 enriched pathways are displayed (p < 0.05). Detect the mRNA expression levels of Plcg1 e, Prkcq f, Tec g, Grap2 h, Rasgrp1 i, Ppp3cc j, Mapk11 k and Mapk14 l in CD8+ T cells among DMSO, COR and COR+RU486 treatment groups using qRT-PCR (n = 3). Data are represented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by one-way ANOVA e–l
GRAP2 involves in CD8+ T cell exhaustion in NSCLC
The TISCH2 database is a scRNA-seq database that includes 190 datasets focusing on the TME. Model-based analyses of transcriptome and RegulOme were utilized in TISCH2 to perform quality control, normalization, clustering and cell-type annotation for each collected dataset [20]. In total, 2762 genes and 6353 genes associated with CD8Tex were obtained from the NSCLC_EMTAB6149 and NSCLC_GSE149655 scRNA-seq dataset respectively in the TISCH2 database (Fig. 8a-b; Supplementary Table 2). There was a typically high expression of GRAP2 in CD8Tex cells of NSCLC patients (Fig. 8a-b). Consistently, through multi-gene correlational analysis in TCGA database, it was found that the expression of GRAP2 was positively correlated with exhaustive genes PDCD1, LAG3, HAVCR2 and TIGIT in NSCLC patients (Fig. 8c-f). The high expression of GRAP in CD8Tex cells indicates an increasing risk of survival reduction in lung adenocarcinoma patients (Fig. 8g). The above results suggested that GRAP2 may be the key gene of stress–COR pathway-mediated CD8+ T cell exhaustion in NSCLC (Fig. 8h).
Fig. 8.
GRAP2 involves CD8+ T cell exhaustion in NSCLC. a UMAP plot in NSCLC_GSE149655 scRNA-seq dataset from TISCH2 database. The CD8Tex mainly consisted of cells from cluster 4 and cluster 13. GRAP2 expresses highly in both clusters CD8Tex. b UMAP plot in NSCLC_EMTAB6149 scRNA-seq dataset from TISCH2 database. The CD8Tex mainly consisted of cells from cluster 0, cluster 8 and cluster 12. GRAP2 expresses highly in all three cluster CD8Tex. (c-f) Correlation analysis of gene expression levels between GRAP2 and PDCD1 c, LAG3 d, HAVCR2 e and TIGIT f of NSCLC patients through TCGA database. g Hazard ratio (HR) of impact of GRAP2 expression levels in CD8Tex on the survival of different cancer patients. The high expression of GRAP2 in CD8Tex significantly increases the risk of shortened survival in lung adenocarcinoma patients (p < 0.05). h Summary of the mechanism of stress promoting CD8+ T cell exhaustion in NSCLC
Discussion
In this study, we found that acute stress facilitated CD8+ T cell exhaustion to promote NSCLC growth and progression. Stress and stress hormone COR can induce high expression of depletion markers such as PD-1, LAG3 and TIM-3 on the surface of CD8+ T cells in the tumor microenvironment, leading to a decline in anti-tumor immune, which is manifested by a decrease in the secretion of cytokines such as TNF-α, IFN-γ and granzyme B as well, thus facilitating the growth of NSCLC. Conversely, the use of GR inhibitor RU486 effectively inhibits tumor growth in vivo. Furthermore, RU486 can effectively reverse the depletion and dysfunction of CD8+ T cells caused by COR, which accounting for the involvement of COR and its receptor in the CD8+ T cell exhaustion induced by stress. Consistently, smart RNA sequencing with sorted CD8+ T cell from solid tumors confirmed that differentially expressed genes were significantly enriched in T cell receptor signaling pathways with KEGG and GSEA analyses.
Researches have revealed stress involves in the tumor immune microenvironment in multiple cancers [15, 21, 22]. A recent clinical study involving 227 NSCLC patients indicated that the objective response rate of immune checkpoint inhibitors significantly declined in patients with anxiety or depression [5]. Besides, chronic stress restrains the infiltration of CD8+ T cells by reducing the abundance of microorganism Blautia and its metabolite acetate in breast cancer [21]. Moreover, social disruption stress decreased dendritic cell maturation, which subsequently contributed to the impairment of CD8+ T cell responses in melanoma [22]. Blocking β-AR signaling in CD8+ tumor-infiltrating lymphocytes (TILs) isolated from stressed mice increased the secretion of IFN-γ, granzyme B and IL-12a, and decreased PD-1, LAG3, and Tim-3 expressed on CD8+ TILs, the mechanism of which is the promotion of glycolysis and mitochondrial oxidative phosphorylation in CD8+ TILs [15]. In addition, distress can also promote T cell exhaustion through kisspeptin–Gpr54 pathway [23]. Here, our study proposes that AR accounts for the exhaustion of CD8+ T cells in NSCLC.
As an indispensable hormone for living lives, GC play a crucial role in tumor immune microenvironment. For instance, stress hormone COR upregulate the expression of glucocorticoid inducible factor Tsc22d3 in dendritic cells, thereby blocking type I interferon response in dendritic cells and the activation of IFN-γ+ T cells [8]. What is more, the release of glucocorticoids during chronic stress can lead to the formation of neutrophil extracellular trap and establish a beneficial microenvironment for metastasis [24]. Furthermore, the increase of cortisol can inhibit the activation of NK cells, resulting in an immunosuppressive microenvironment in pancreatic cancer [25]. In addition, the flattening of the diurnal cortisol rhythm predicts poor prognosis in lung cancer [26]. Prior to our study, it is indicated that GCs affects the infiltration and function of CD8+ T cells. For example, it is found that GCs exposure in the perinatal period of female mice leaded to a long-term impairment in CD8+ T cell function of the offsprings by interfering with HPA axis [18]. GC derived from tumor-associated macrophage induces the expression of CD8+ T cell dysfunction related genes in melanoma [27]. Additionally, GCs can induce tumor immune escape in pancreatic cancer cells [28]. In our current study, we revealed that serum-derived corticosterone induced by stress contributed to CD8+ T cell exhaustion in NSCLC, thus activating T cell receptor signaling.
Regarding the mechanisms of GCs in T cell exhaustion, elevated GCs can inhibit T cell responses through GR-induced transcription of immunosuppressive genes. Liganded GRs induce transcription of immunosuppressive genes such as Tsc22d3, Dusp1 and Nfkbia [29, 30]. Glucocorticoid-induced leucine zipper associates with NF-κB and AP-1 to prevent their transactivation of inflammatory and cytokine genes [31, 32] and associates with RAS and RAF to prevent induction of AKT-induced and ERK-induced proliferation [33]. The GR also directly associates with NF-κB, AP-1 and Nur77 family proteins to inhibit their transcriptional activity [34–37]. To delve deeper into the mechanism of AR on CD8+ T cell exhaustion, we sorted out CD8+ T cells in the solid tumors and confirmed that T cell receptor signaling pathway was significantly enriched through smart RNA sequencing using KEGG and GSEA analyses. We then verified the results of RNA sequencing with qRT-PCR and ensured that Grap2 and Ppp3cc were significantly upregulated by COR and inhibited after RU486 treatment. Consistent with our results, the protein phosphatase encoded by PPP3CC dephosphorylates and activates transcription factor NFATC1, therefore transactivating PD-1 expression after infection and promoting exhaustion [38]. Besides, upregulation of PPP3CC in CD8+ T cells may also promote aging in the body [39]. Furthermore, previous study indicated that the interaction between Grap2 and SLP76 is involved in T cell receptor (TCR) signaling [40],which may contribute to the excessive activation or dysregulation of TCR signaling, thus promoting exhaustion. Similar to our results, NSCLC_EMTAB6149 and NSCLC_GSE149655 scRNA-seq dataset in the TISCH2 database showed a typically high expression of GRAP2 in CD8Tex cells of NSCLC patients. The expression of GRAP2 in CD8Tex indicated a significant reduction in the survival time of lung adenocarcinoma patients. Multi-gene correlational analysis using TCGA database revealed that the expression of GRAP2 was significantly positively correlated with exhaustive genes in NSCLC patients. Therefore, we propose that Grap2 is the potential molecular bridge for COR induced CD8+ T cell exhaustion under stress.
Our study is the first to clarify that stress and stress hormone COR can inhibit the function of CD8+ T cells and induce their exhaustion, forming a tumor immune suppressive microenvironment, thereby accelerating the progression of NSCLC. This study reveals the immune mechanism by which stress accelerates the growth of NSCLC, providing a new therapeutic target strategy It is suggested that in the future clinical treatment, immune checkpoint inhibitors combined with GR inhibitors can be considered to apply for immune therapy failure and tumor progression caused by psychological stress.
Last but not least, this study still has many limitations. Firstly, corresponding human evidences are needed to further support our findings. Secondly, the involvement and mechanism of the differentially expressed gene Grap2 screened by RNA sequencing in stress-induced CD8+ T cell exhaustion is required to be further investigated in our future work. Finally, this study did not exclude the synergistic effects of other stress-related factors, such as catecholamines and inflammatory factors. In our future research, we tend to validate the effects of Grap2 on CD8+ T cells and NSCLC through in vitro and in vivo experiments, and further analyze the heterogeneity of CD8+ T cell exhaustion through single-cell sequencing. What is more, a series of clinical trials are needed to be conducted on NSCLC patients diagnosed with or without anxiety or depression to clarify the anti-tumor efficacy of stress and stress hormones COR, and to determine whether the combination of immune checkpoint inhibitors and GR inhibitors is more effective in inhibiting tumor growth and prolonging the survival of NSCLC patients with anxiety and depression than using immune checkpoint inhibitors alone.
In conclusion, our study demonstrates the fact that stress promotes the progression of NSCLC through the COR–GRAP2–CD8+ T exhaustion network, filling the gap in the mechanism of stress-induced CD8+ T cell exhaustion in NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully thank all members of the Integrative Medicine Laboratory in Huashan Hospital, for their assistance in molecular biology experiments.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- AR
Acute restrain stress
- β2-AR
β2 Adrenergic receptor
- CD8Tex
CD8+ T cell exhaustion
- COR
Corticosterone
- Con
Control group
- FST
Forced swimming test
- GC
Glucocorticoid
- GR
Glucocorticoid receptor
- HPA
Hypothalamic pituitary–adrenal axis
- LLC
Lewis lung cancer cell
- LDBT
Light–dark box test
- NSCLC
Non-small cell lung cancer
- NK cells
Natural killing cells
- OFT
Open-field test
- TILs
Tumor-infiltrating lymphocytes
- TCR
T cell receptor
Author contributions
The Conception and design of study: Z-QQ, D-JC and D-ZQ. Implementation of the experiment and acquisition of data: Z-QQ, W-Q, Z-HJ, D-LL, ZGJ, Y-MM and F-P. Analysis and/or interpretation of data: Z-QQ and D-JC. Drafting the manuscript: Z-QQ. Revising the manuscript critically for important intellectual content: Z-QQ and D-JC. All authors contributed to the article and approved the submitted version and agree to be accountable for the content of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82174170 and No. 8220152749) and the Key research and development Project of Karamay Science and Technology Bureau (No. 2025BA0108).
Data availability
All data are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Animal experimental protocols were approved by Fudan University’s Institutional Animal Care and Use Committee (Number: 202405FD0001). All authors are informed and agree.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zaiquan Dong, Email: zaiquandong@wchscu.edu.cn.
Jingcheng Dong, Email: jcdong2004@126.com.
References
- 1.Wang YH, li JQ, shi JF et al (2020) Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry 25(7):1487–1499 [DOI] [PubMed] [Google Scholar]
- 2.Li Y, dong W, Tang H et al (2024) Correlates of death anxiety for patients with cancer: a systematic review and meta-analysis. J Clin Nurs 33(5):1933–1947 [DOI] [PubMed] [Google Scholar]
- 3.Riedl D, Schuessler G (2022) Prevalence of depression and cancer - a systematic review. Z Psychosom Med Psychother 68(1):74–86 [DOI] [PubMed] [Google Scholar]
- 4.Vehling S, Mehnert-Theuerkauf A, Philipp R et al (2022) Prevalence of mental disorders in patients with cancer compared to matched controls - secondary analysis of two nationally representative surveys. Acta Oncol 61(1):7–13 [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Hu CH, Li YZ et al (2024) Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer[J]. Nat Med 30(6):1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirl WF, Greer JA, Traeger L et al (2012) Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care[J]. J Clin Oncol 30(12):1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoni MH, Jacobs JM, Bouchard LC et al (2017) Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up[J]. Gen Hosp Psychiatry 44:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Xia L, Chen J et al (2019) Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity[J]. Nat Med 25(9):1428–1441 [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Qiao G, Hylander BL et al (2020) Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation[J]. Nat Commun 11(1):1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao W, Chen HD, Yu YW et al (2021) Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J 134(7):783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132 [DOI] [PubMed] [Google Scholar]
- 12.Garon EB, Hellmann MD, Rizvi NA et al (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I keynote-001 study[j]. J Clin Oncol 37(28):2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Zhou L, Zhang X et al (2022) Psychological distress and eustress in cancer and cancer treatment: advances and perspectives. Sci Adv 8(47):eabq7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhabhar FS, Saul AN, Holmes TH et al (2012) High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS ONE 7(4):e33069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao G, Chen M, Mohammadpour H et al (2021) Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol Res 9(6):651–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J, Wang X, Wang Q et al (2013) Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS ONE 8(9):e74497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadpour H, Macdonald CR, Qiao G et al (2019) Β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129(12):5537–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong JY, Lim J, Carvalho F et al (2020) Long-term programming of CD8 T cell immunity by perinatal exposure to glucocorticoids. Cell 180(5):847–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Q, Wang S, Tian X et al (2023) Fecal microbiota transplantation confirmed that 919 Syrup reduced the ratio of erucamide to 5-AVAB in hippocampus to alleviate postpartum depression by regulating gut microbes. Front Immunol 14:1203015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Sun D, Huang X et al (2020) Integrative analyses of single-cell transcriptome and regulome using MAESTRO. Genome Biol 21(1):198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye L, Hou Y, Hu W et al (2023) Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress[J]. Nat Commun 14(1):6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommershof A, Scheuermann L, Koerner J et al (2017) Chronic stress suppresses anti-tumor T(CD8+) responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav Immun 65:140–149 [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Yu F, Che A et al (2022) Neuroendocrine regulation of stress-induced T cell dysfunction during lung cancer immunosurveillance via the Kisspeptin/GPR54 signaling pathway. Adv Sci (Weinh) 9(13):e2104132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He XY, Gao Y, Ng D et al (2024) Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment[J]. Cancer Cell 42(3):474–86.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Liu A, Bo W et al (2024) Upregulation of HSD11B1 promotes cortisol production and inhibits NK cell activation in pancreatic adenocarcinoma. Mol Immunol 175:10–19 [DOI] [PubMed] [Google Scholar]
- 26.Sephton SE, Lush E, Dedert EA et al (2013) Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun 30:163–170 [Google Scholar]
- 27.Acharya N, Madi A, Zhang H et al (2020) Endogenous glucocorticoid signaling regulates CD8(+) T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity 53(3):658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Xia X, Zhao Y et al (2021) Glucocorticoid receptor regulates PD-L1 and MHC-I in pancreatic cancer cells to promote immune evasion and immunotherapy resistance. Nat Commun 12(1):7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maneechotesuwan K, Yao X, Ito K et al (2009) Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med 6(5):e1000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinman RI, Cogswell PC, Lofquist AK et al (1995) Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270(5234):283–286 [DOI] [PubMed] [Google Scholar]
- 31.Ayroldi E, Migliorati G, Bruscoli S et al (2001) Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood 98(3):743–753 [DOI] [PubMed] [Google Scholar]
- 32.Mittelstadt PR, Ashwell JD (2001) Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J Biol Chem 276(31):29603–29610 [DOI] [PubMed] [Google Scholar]
- 33.Ayroldi E, Zollo O, Bastianelli A et al (2007) GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling[J]. J Clin Invest 117(6):1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray A, Prefontaine KE (1994) Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A 91(2):752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens C, Bilodeau S, Maira M et al (2005) Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol 19(4):885–897 [DOI] [PubMed] [Google Scholar]
- 36.Heck S, Kullmann M, Gast A et al (1994) A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J 13(17):4087–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldenhoven E, Liden J, Wissink S et al (1995) Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol 9(4):401–412 [DOI] [PubMed] [Google Scholar]
- 38.Wei H, Xie A, Li J et al (2022) PD-1(+) CD4 T cell immune response is mediated by HIF-1α/NFATc1 pathway after P. yoelii infection. Front Immunol 13:942862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Pu P, Xiang Q et al (2025) Exploration of common molecular mechanisms of psoriatic arthritis and aging based on integrated bioinformatics and single-cell RNA-seq analysis. Biochimica et Biophysica Acta (BBA) 1871(4):167730 [Google Scholar]
- 40.Burack WR, Cheng AM, Shaw AS (2002) Scaffolds, adaptors and linkers of TCR signaling: theory and practice. Curr Opin Immunol 14(3):312–316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon reasonable request.