Abstract
The p21-activated kinase 2 (PAK2), a serine/threonine kinase, directly participates in the regulation of various cellular signaling pathways and plays a critical role in cell motility, survival, and proliferation. Due to its crucial role in cell signaling pathways, cytoskeletal organization, and cell survival, PAK2 has emerged as a promising drug target, especially in cancer and cardiovascular diseases. However, systematic studies examining PAK2 inhibition are still limited, and an effective inhibitor has proven quite challenging to develop. Existing drug discovery methods are labor-intensive and expensive. Therefore, new approaches like drug repurposing are required. Here, we employed a systematic, structure-based drug repurposing strategy to identify potential repurposed inhibitors of PAK2 from a library of FDA-approved drug molecules. Structure-based virtual screening of 3648 FDA-approved compounds led to the identification of Midostaurin and Bagrosin as top-hit candidates with predicted potency against PAK2, due to their high binding affinity and specificity to the PAK2 active site. Additional interaction analysis obtained from molecular docking suggested that stable hydrogen bonds were formed between Midostaurin and Bagrosin with key PAK2 residues, leading us to propose an inhibitory role. To ensure stability and interaction dynamics, a molecular dynamics (MD) simulation was conducted for 300 ns, demonstrating good thermodynamic properties for the stable binding of Midostaurin and Bagrosin to PAK2, in comparison to a control inhibitor, IPA-3. Although these results are encouraging, the study only yielded in silico data, and further experimental evaluation will be necessary to validate the inhibition of PAK2 by Midostaurin and Bagrosin. However, our results provide valuable insights for the future development of PAK2 inhibitors and underscore the importance of repurposed drugs in cancer therapy. Comparative docking and selectivity profiling also suggest that these compounds preferentially target PAK2 over other isoforms such as PAK1 and PAK3, warranting further experimental validation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03516-w.
Keywords: PAK2, Anticancer, Drug repurpose, Virtual screening, Molecular dynamics simulation, Bagrosin, Midostaurin
Introduction
P21-activated kinases (PAKs) are a family of serine/threonine kinases that serve as a pivotal point in cellular signaling networks, regulating processes such as cytoskeletal dynamics, cell proliferation, apoptosis, and survival [1]. The PAK family consists of six members (PAK1–PAK6), having been classified into two classes based on their structural and functional features (Group I: PAK1-PAK3, Group II: PAK4-PAK6) [2]. PAK2 is expressed in most human tissues and has been involved in various human diseases, especially in cancer and cardiovascular diseases [3, 4]. PAK2 hyperactivation has been implicated in several malignant diseases and is associated with enhanced tumorigenesis, metastatic dissemination, and drug resistance [5]. Consequently, PAK2 has emerged as a promising therapeutic target for cancer therapy [6].
Nevertheless, the increasing recognition of the role of PAK2 in oncogenesis has yet to be matched with systematic efforts to discover potent and selective PAK2 inhibitors [7]. De novo design and screening of novel compounds, the mainstay of traditional drug discovery, is time-consuming, resource-intensive, and harbors a high failure rate [8]. Another promising strategy that has gained momentum in the past few years is drug repurposing, which aims to identify new therapeutic applications of molecules that have previously been approved for safety and efficacy by the FDA [9]. This method takes advantage of the known pharmacokinetics and safety profiles of approved compounds, which can greatly accelerate and reduce the cost of clinical translation [10].
Virtual screening approaches involving molecular docking, molecular dynamics (MD), and structure–activity relationship methods are state-of-the-art computational drug discovery protocols that are widely used to search for potential inhibitors targeting certain protein targets [11]. Molecular docking is widely used to predict the binding poses of small molecules in the active site of a target protein [12]. MD simulations are then used to examine the stability and conformation dynamics of the ligand during the lifetime of the complex, providing insight into the mechanism behind the observed interaction [13]. At the same time, structure-guided activity predictions are exploited to predict potential biological properties of small molecules for therapeutic development [14]. These computational approaches were used to screen a library of FDA-approved compounds for potential PAK2 inhibitors, aiming to expedite the drug discovery process.
Among the limited small-molecule PAK inhibitors, IPA-3 was selected as our reference compound due to its demonstrated specificity for Group I PAKs and reported inhibitory activity against PAK2 in cellular assays. Although other inhibitors such as G-5555 and FRAX486 exhibit low nanomolar IC₅₀s against PAK1 and PAK2, their PAK2-specific selectivity profiles remain less well characterized, supporting our choice of IPA-3 for comparative computational benchmarking.
Here, we conducted a systematic structure-based virtual screening of 3,648 FDA-approved drugs to discover potential repurposed PAK2 inhibitors. We performed molecular docking, drug profiling, pharmacokinetic evaluation, and interaction analyses to pinpoint hit molecules among the screened library of approved drugs. Then, we performed extensive 300 ns all-atom MD simulations, followed by essential dynamics to evaluate the structural stability and folding mechanism of PAK2 in the presence of the screened drugs. Although our study has some limitations, it demonstrates the potential of computational approaches in identifying promising therapeutic candidates and provides a basis for future experimental studies on the development of PAK2-targeted therapeutics. To benchmark our findings, we selected IPA-3 as the reference inhibitor due to its well-established specificity for Group I PAKs and its previously reported activity against PAK2 in cellular systems. While G-5555 and FRAX486 are potent pan-PAK inhibitors, their selectivity for PAK2 remains limited, justifying our use of IPA-3 for comparative analysis.
Materials and methods
Data preparation: PAK2 and drugs library
A bioinformatics workflow driven by various computational servers and tools was used in this study. Several state-of-the-art software such as AutoDock Tools [15], AutoDock Vina [16], PyMOL [17], LigPlus [18], and GROMACS 2020 beta [19] to perform virtual screening and MD simulations. The corresponding 3D model structure of the PAK2 protein (AlphaFold ID: AF-Q13177) was retrieved from AlphaFold for computational evaluations [20]. Before docking, the protein structure was preprocessed to remove any steric clashes via energy minimization. To evaluate the reliability of the PAK2 structure, we analyzed per-residue confidence using the Predicted Local Distance Difference Test (pLDDT) and Predicted Aligned Error (PAE). The selected PAK2 model exhibited an average pLDDT score of 94.08 and Qscore of 98.01, indicating a highly reliable structure suitable for computational studies. The PAE graph further validated the structural accuracy by showing minimal intra-domain and inter-domain errors (Supplementary Figure S1). ERRAT analysis yielded an overall quality factor of 98.7603, comparable to that of high-resolution crystal structures, which reinforces the structural integrity of the PAK2 model. Furthermore, the Ramachandran plot also showed that most of the amino acid residues were inside the allowed region. The Swiss-PDB Viewer tool was utilized for energy minimization to stabilize the protein conformation. A dataset of 3,648 FDA-approved compounds was obtained from DrugBank [21] and curated for docking studies. Each drug molecule was processed for structural refinement and preparation by AutoDock tools, and the appropriate ionization states and tautomeric forms were maintained for docking simulations.
Molecular docking screening
Molecular docking-based screening was executed to identify high-affinity binding partners of PAK2 using AutoDock Vina, which has been acknowledged for its efficiency and scoring power [16]. The docking procedure was performed using a blind docking method, where a grid box covering the whole PAK2 structure was constructed. The grid was centered at the coordinates X: − 4.62 Å, Y: 1.396 Å, Z: − 1.185 Å with dimensions of X-axis = 69 Å, Y-axis = 63 Å, and Z-axis = 73 Å, and a grid spacing of 1 Å, while all other docking parameters were set to their default values.
Drug profiling and PASS analysis
Drug profiling from the FDA database and a literature search, along with PASS (Prediction of Activity Spectra for Substances), was used to assess the biological activities of the screened compounds [14]. The PASS program (https://www.way2drug.com/passonline/) infers possible pharmacological activities based on the chemical structures of small molecules. The PASS algorithm uses molecular fragment descriptors to assess spatial characteristics and predict potential biological functions. Upon obtaining the biological activity labeling process, the classification program creates each compound with a biological activity score, pictured as the ratio between the described probability for active (Pa) against inactive (Pi), giving the following equation: The higher the Pa value, the more likely that a particular molecule exhibits the predicted biological effect.
Interaction analysis
The selected inhibitors were subjected to detailed interaction analysis after performing drug profiling and PASS analysis to investigate their binding features for PAK2. The best-docked candidates were analyzed using PyMOL [17] and LigPlus [18] to further evaluate their binding orientations and interaction profiles within the PAK2 active site. With this evaluation, the drug candidates that demonstrated significant molecular interactions with PAK2 were identified, highlighting their potential to serve as suitable inhibitors.
MD simulations
MD simulations provide insights into the structural stability and dynamic behavior of proteins and their docked complexes with small molecules [13]. All-atom MD simulations were carried out using the GROMACS 2020 β simulation suite at 300 K [19] and the GROMOS 54A7 force field [22]. Force-field parameters for the selected compound topologies were generated from the Auto Topology Builder (ATB) server [23]. The protein–ligand complexes were subsequently solvated in a cubic water box where counterions were introduced to neutralize the protein and protein–ligand systems. Energy minimization was done by the steepest method to reduce steric clashes and stabilize the system. The simulations were performed using a constant volume with periodic boundary conditions at a pressure of 1 bar. The final production run was an all-atom MD simulation of 300 ns, while the simulation trajectory was analyzed in the GROMACS toolset. Several structural parameters, such as protein–ligand stability, conformational alterations, compactness, and hydrogen bonding interactions, were analyzed. To validate the robustness and consistency of the binding interactions observed, both simulation-derived structural properties were compared quantitatively.
Essential dynamics
Principal component analysis (PCA) is a powerful technique for revealing the dominant motions of the system and for understanding the dynamics of protein–ligand interaction [24]. PCA was used with data extracted from the MD simulation trajectories to determine the conformational flexibility and atomic motions of PAK2 and docked complexes. At the same time, the free energy landscapes (FELs) analysis is an essential aspect of understanding the inherent plasticity of a protein and the conformational change induced by ligand binding [25]. It provides snapshots of these low-energy states, revealing how ligands interact with their targets to form energetically favorable configurations even in large and stable conformations. The FELs of PAK2 and its docked complexes were established to further analyze the energetic stability and conformational states of the protein in the context of its native conformation.
Results and discussion
Molecular docking screening
We developed a comprehensive virtual screening pipeline to systematically evaluate all compounds, with a focus on those exhibiting high binding affinities towards PAK2. First, a large-scale molecular docking-based virtual screening consisting of 3,648 FDA-approved small-molecule drugs was performed to discover high-affinity binding partners of PAK2 inhibitors. After the first pass of screening, ligand efficiency was assessed within the highest-ranking compounds to identify molecules with better binding profiles. Consequently, a set of molecules was found to exhibit promising binding affinity towards PAK2 in the molecular docking screening. The results showed that the top docked hits showed an affinity ranging from − 9.3 to − 10.7 kcal/mol (Table 1). As this is a computational study, we did not evaluate experimental IC₅₀ or Ki values. Instead, we report docking scores, predicted pKi values, and ligand efficiencies as comparative metrics of affinity. Importantly, all the identified compounds demonstrated a higher docking score than IPA-3 (the reference inhibitor) for PAK2 (− 8.1 kcal/mol). Notably, these newly discovered molecules appear to be better binding partners for PAK2, indicating that they have great potential as attractive drug candidates in PAK2-based drug repurposing after further evaluation.
Table 1.
List of screened hits and their binding affinities toward PAK2
| S. No | Drug | Binding Free Energy (kcal/mol) | pKi | Ligand Efficiency (kcal/mol/non-H atom) | Torsional Energy |
|---|---|---|---|---|---|
| 1. | Dactinomycin | − 10.7 | 7.85 | 0.1189 | 2.8017 |
| 2. | Midostaurin | − 10.6 | 7.77 | 0.2465 | 1.8678 |
| 3. | Paritaprevir | − 10.2 | 7.48 | 0.1855 | 2.1791 |
| 4. | Bagrosin | − 9.7 | 7.11 | 0.4409 | 0.3113 |
| 5. | Rifabutin | − 9.6 | 7.04 | 0.1574 | 2.4904 |
| 6. | Venetoclax | − 9.6 | 7.04 | 0.1574 | 4.0469 |
| 7. | Teniposide | − 9.5 | 6.97 | 0.2065 | 2.8017 |
| 8. | Eptifibatide | − 9.4 | 6.89 | 0.1649 | 3.4243 |
| 9. | Zorubicin | − 9.4 | 6.89 | 0.2 | 3.4243 |
| 10. | Mosapramine | − 9.3 | 6.82 | 0.2735 | 1.2452 |
| 11. | IPA-3 | − 8.1 | 5.94 | 0.3375 | 1.5565 |
Drug profiling and PASS analysis
Following molecular docking analysis, a comprehensive drug profiling study was conducted to assess the therapeutic significance and biological functions of the examined compounds. Here, out of the 10 screened compounds, Midostaurin and Bagrosin were identified as the most promising molecules with favorable drug profiles in the context of anticancer drug development and kinase inhibitory potential. To investigate their relevant biological activities based on their molecular structures, we used the PASS server [14]. The results showed that Midostaurin and Bagrosin had functional similarities to a reference inhibitor, IPA-3, which is used as a PAK2-targeted anticancer agent [26]. Midostaurin and Bagrosin demonstrated high antineoplastic, apoptosis agonist activity, antiinflammatory, and kinase inhibitory potential with probability scores ranging from 0.257 to 0.979 (Table 2). The high value of Pa indicates that these compounds have a high probability of possessing anticancer properties, as seen in Midostaurin and Bagrosin. These findings open up the possibility of Midostaurin and Bagrosin emerging as repurposable drug candidates for targeting PAK2-driven drug resistance, which needs to be further validated via experimental studies.
Table 2.
Elucidated drug molecules and their biological activities from the PASS server
| S. No | Molecule | Pa | Pi | Biological Activity |
|---|---|---|---|---|
| 1. | Midostaurin | 0,979 | 0,001 | Protein kinase A inhibitor |
| 0,973 | 0,001 | Protein kinase C inhibitor | ||
| 0,813 | 0,010 | Antineoplastic | ||
| 0,386 | 0,025 | Chemoprotective | ||
| 0,320 | 0,115 | Apoptosis agonist | ||
| 2. | Bagrosin | 0,499 | 0,053 | JAK2 inhibitor |
| 0,499 | 0,057 | Antiinflammatory | ||
| 0,284 | 0,034 | Antineoplastic enhancer | ||
| 0,359 | 0,113 | Pin1 inhibitor | ||
| 0,257 | 0,012 | Antineoplastic (glioma) | ||
| 3. | IPA-3 | 0,847 | 0,005 | JAK2 expression inhibitor |
| 0,689 | 0,008 | Pin1 inhibitor | ||
| 0,642 | 0,012 | Histidine kinase inhibitor | ||
| 0,569 | 0,024 | Kinase inhibitor | ||
| 0,280 | 0,138 | Apoptosis agonist |
Interaction analysis
The binding modes of the top-hit compounds Midostaurin and Bagrosin, along with the control inhibitor IPA-3, were analyzed using PyMOL and LigPlus. Detailed interaction analysis was performed, considering the binding conformations and interactions within the PAK2 active site (Fig. 1). The best-docked conformations for Midostaurin and Bagrosin, as well as the reference inhibitor IPA-3, were obtained from the docking output files for comparison (Fig. 1A). Targeting residues located in the PAK2 binding site, this analysis showed that both compounds, Midostaurin and Bagrosin, made strong hydrogen bonding interactions with critical amino acids located in the PAK2 binding pocket (Fig. 1B). The results showed that Midostaurin and Bagrosin interacted with PAK2 in several stable interactions with a binding pattern similar to IPA-3. The analysis showed that all the compounds directly interacted with the ATP-binding site of PAK2. Both compounds seemed to occupy into the deepest part of the PAK2 binding pocket, suggesting their potential as efficient inhibitors (Fig. 1C). In summary, the interaction analysis indicates that Midostaurin and Bagrosin are promising binding partners of PAK2, as they exhibit good binding characteristics. Such findings advocate the prospect of Midostaurin and Bagrosin as repurposed drug candidates, for stability evaluation and experimental validation to establish their inhibitory potential.
Fig. 1.
Docking profiles of Midostaurin and Bagrosin with PAK2. A Cartoon representation of PAK2 with Midostaurin (yellow) and Bagrosin (green) along with the reference inhibitor IPA-3 (cyan). B PAK2 binding site residues interacting with Midostaurin, Bagrosin, and IPA-3. C Surface potential view of PAK2 binding pocket occupied by Midostaurin, Bagrosin, and IPA-3
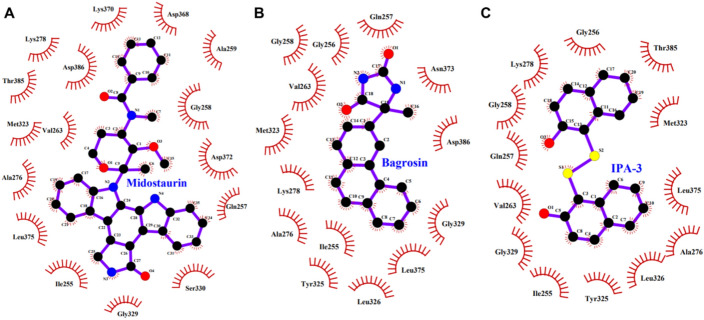
A detailed analysis of the interactions between PAK2 and selected compounds was conducted to investigate their binding coordinates. This assessment aimed to identify interactions with key functional amino acid residues crucial to the activity of PAK2. The binding interactions for both Midostaurin and Bagrosin, and with the reference inhibitor IPA-3 in the PAK2 binding pocket, are shown in Fig. 2. Midostaurin and Bagrosin effectively bind to the PAK2 binding site, stabilizing multiple close interactions with essential residues, as indicated by the results. Midostaurin was found to interact with Ile255, Gln257, Gly258, Ala259, Vals263, Ala276, Lys278, Met323, Gly329, Ser330, Asp368, Lys370, Asp372, Leu375, Thr385, and Asp386 amino acids in the binding pocket (Fig. 2A). Similarly, Bagrosin forms close interactions with Ile255, Gly256, Gln257, Gly258, Vals263, Ala276, Lys278, Met323, Tyr325, Leu326, Gly329, Asn373, Leu375, and Asp386, thus enhancing its binding affinity with PAK2 (Fig. 2B). Similarly, the reference PAK2 inhibitor IPA-3 interacted with Ile255, Gly256, Gln257, Gly258, Vals263, Ala276, Lys278, Met323, Tyr325, Leu326, Gly329, Leu375, and Thr385 (Fig. 2C). The interactions showed that all the compounds directly interact with the ATP-binding site of PAK2, i.e., Lys278. All compounds bound the ATP-binding pocket of PAK2, involving a binding cleft of residues Ile255-Val263. The identification of common binding interactions between Midostaurin and Bagrosin indicates that they may have strong potential as ATP-competitive inhibitors of PAK2. These findings support the idea that Midostaurin and Bagrosin may be effective therapeutic candidates after further validation.
Fig. 2.
LigPlot representation of PAK2 interactions with A Midostaurin, B Bagrosin, C IPA-3. The figure was generated using LigPlus to dock protein–ligand complexes
Selectivity profiling against other PAK isoforms
To assess whether Midostaurin and Bagrosin preferentially target PAK2 over other family members, we performed parallel docking of both compounds along with G-5555 and FRAX486 against PAK1 (PDB ID: 4O0T) and PAK3 (PDB ID: 6FD3). The calculated binding affinities (docking scores) are presented in Table 3. Midostaurin exhibits the strongest binding to PAK2 (–10.6 kcal/mol), which is 0.3 kcal/mol more favorable than its affinity for PAK1 (–10.3 kcal/mol) and 1.6 kcal/mol stronger than for PAK3 (–9.0 kcal/mol). Bagrosin similarly shows a preference for PAK2 (–9.7 kcal/mol) over PAK1 (–9.2 kcal/mol) and PAK3 (–9.6 kcal/mol), though with a narrower differential. By comparison, G-5555 and FRAX486 demonstrate their highest (but substantially weaker) affinities for PAK2 (–9.1 and –8.7 kcal/mol, respectively), while binding even less tightly to PAK1 and PAK3. These results indicate a modest yet consistent selectivity of Midostaurin and Bagrosin for PAK2 over the closely related PAK1 and PAK3 isoforms, bolstering their candidacy as isoform‐focused inhibitors in future experimental validation.
Table 3.
Docking scores (kcal/mol) of compounds against PAK isoforms
| Compound | PAK1 | PAK2 | PAK3 |
|---|---|---|---|
| Midostaurin | – 10.3 | –10.6 | –9.0 |
| Bagrosin | – 9.2 | –9.7 | –9.6 |
| G-5555 | –8.5 | –9.1 | –7.9 |
| FRAX486 | –8.2 | –8.7 | –7.7 |
MD simulations analysis
MD simulation is a widely exploited computational tool in the field of drug discovery, used to study biomolecular behaviors at the atomic scale [27]. This approach provides valuable insights into the kinetic characteristics of proteins, which are crucial for understanding their functional mechanisms and interactions with small-molecule inhibitors. To evaluate the extent of PAK2-ligand interactions, we performed a 300 ns all-atom MD simulation for PAK2-Midostaurin and PAK2-Bagrosin along with PAK2-IPA-3 complexes before examining the trajectories for their interactions. Multiple structural indices were used to evaluate the changes in the structural integrity and dynamic behavior of these complexes over time, as discussed in the subsequent sections (Table 4).
Table 4.
Average values of different parameters of MD simulations. #H-bonds, number of intramolecular hydrogen bonds
| System | RMSD (nm) | RMSF (nm) | Rg (nm) | SASA (nm2) | #H-bonds |
|---|---|---|---|---|---|
| PAK2 | 0.18 | 0.12 | 1.91 | 139.1 | 182 |
| PAK2-Midostaurin | 0.24 | 0.12 | 1.93 | 141.2 | 181 |
| PAK2-Bagrosin | 0.22 | 0.13 | 1.93 | 144.4 | 176 |
| PAK2-IPA-3 | 0.19 | 0.10 | 1.91 | 139.7 | 183 |
Structural deviations and compactness
Root-mean-square deviation (RMSD) analysis was performed to assess the structural stability and deviations of PAK2, as well as its bound ligand complexes. For free PAK2, PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3, the average RMSD values calculated from the time evolution of values were 0.18 nm, 0.24 nm, 0.22 nm, and 0.19 nm, respectively. The RMSD values of PAK2 were slightly increased after ligands binding but showed a stable binding during the simulation trajectory (Fig. 3A). Notably, both the PAK2-Midostaurin and PAK2-Bagrosin complexes exhibited an equilibrated RMSD distribution, indicating a stable conformation throughout the simulation duration (Fig. 3A, lower panel). Such consistently distributed RMSD values assured the general conformational stability of protein–ligand complexes.
Fig. 3.
Structural dynamics of PAK2 upon Midostaurin and Bagrosin binding. A RMSD plot of PAK2 in complex with Midostaurin, Bagrosin, and IPA-3. B Residual fluctuations of PAK2 before and after Midostaurin and Bagrosin binding. The lower panels showed the probability distribution function (PDF) of the values
To analyze residual flexibility in free and ligand-bound PAK2, root-mean-square fluctuation (RMSF) analysis was performed. RMSF profiles revealed different levels of atomic fluctuations in various regions of PAK2 (Fig. 3B). The rise and fall patterns for PAK2-Midostaurin and free PAK2 were quite similar, suggesting that the overall conformation of PAK2-Midostaurin was stable. However, there was a slight rise in the residual fluctuations in PAK2-Bagrosin, indicating minor conformational adjustments. Bagrosin and IPA-3 binding showed a minor increase in the regions of amino acids 390–410 and 415–425, which were found to be more flexible. Bagrosin binding also showed a significant increase in the region of amino acids 445–460. Apart from these increased fluctuations, all systems showed a similar RMSF pattern from N- to C-termini. Overall, the RMSF plots showed that PAK2 exhibited a stable structure after the ligand was bound, indicating structural integrity of the protein–ligand complexes (Fig. 3B, lower panel).
The radius of gyration (Rg) of PAK2 alone and bound to Midostaurin and Bagrosin were calculated to investigate the conformational stability of PAK2 during the simulations. The average Rg for free PAK2 was 1.91 nm, the average Rg of PAK2-Midostaurin and PAK2-Bagrosin was 1.93 nm, and the average Rg of PAK2-IPA-3 was 1.91 nm. These values indicate that PAK2 adopts a compact structural fold upon binding to the ligand and that the differences between each state are slight (Fig. 4A). The minor variation in Rg values during the entire simulation suggests that neither Midostaurin and Bagrosin nor IPA-3 produced any significant structural expansion or destabilization, verifying the stability and integrity of the complexes (Fig. 4A, lower panel).
Fig. 4.
Structural compactness of PAK2 upon Midostaurin, Bagrosin, and IPA-3 binding. A Time evolution of Rg and B SASA of PAK2 and its complexes with Midostaurin, Bagrosin, and IPA-3
Solvent-accessible surface area (SASA) analysis was performed to further evaluate the changes in compactness and solvent accessibility of the protein. The average SASA values were calculated for free PAK2, PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3 to be 139.1 nm2, 141.2 nm2, 144.4 nm2, and 139.7 nm2, respectively (Fig. 4B). The small increase in SASA after ligand binding indicates that PAK2 shows a slight expansion upon binding to Midostaurin and Bagrosin. Interestingly, the PAK2-Midostaurin complex showed a marginal decrease in SASA as compared to PAK2-Bagrosin, which suggests that the binding of both Midostaurin may constrain PAK2 in a compact conformation when compared to Bagrosin (Fig. 4B, lower panel). This corroborates the binding stability outcomes, as Midostaurin and Bagrosin appear to engage in more stable molecular interactions in the binding pocket, resulting in a closed binding pocket. The results from both Rg and SASA analyses demonstrate that Midostaurin and Bagrosin are promising stable PAK2 inhibitors.
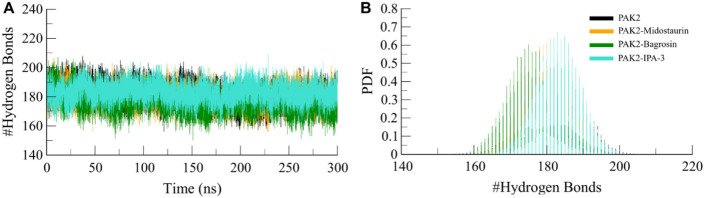
Dynamics of hydrogen bonds
Hydrogen bonds are a crucial stabilizing force in proteins, contributing to conformational rigidity and binding specificity with ligands [28]. To further investigate the structural stability of PAK2 before and after ligand binding, we analyzed the temporal trajectory of hydrogen bond formations throughout each simulation. The intramolecular hydrogen bonding analysis in PAK2 indicated that this protein formed an average of 182 hydrogen bonds per unbound protein (Fig. 5). This number fluctuated slightly after ligand binding; for example, the PAK2-Midostaurin complex had 181 hydrogen bonds, and the PAK2-Bagrosin complex had 176 (Fig. 5A). At the same time, the reference inhibitor complex PAK2-IPA-3 is formed of 183 intramolecular hydrogen bonds. Such a slight deviation indicates that, compared with the original hydrogen bonding network of PAK2, the binding of Midostaurin and Bagrosin hardly disturbed it and thus enables the structural integrity and stability of the protein to remain during the whole simulation. A PDF analysis was then performed to further investigate hydrogen bond stability (Fig. 5B). The PDF plot visually depicted hydrogen bond dynamics as statistical variations for each pair of hydrogen bond states; these graphs indicated an overall consistency for the distribution of hydrogen bonds throughout the time course of the simulation. Thus, PAK2 maintained its compact structure with minor variations in hydrogen bond formation upon ligand binding.
Fig. 5.
Intramolecular hydrogen bonding PAK2. A Hydrogen bond dynamics in PAK2 before and after Midostaurin, Bagrosin, and IPA-3 binding. B The probability distribution function (PDF) of hydrogen bonding in PAK2
At the same time, intermolecular hydrogen bonding can be used as a measure of protein–ligand complex stabilization, providing atomic-level insights into the binding affinity and dynamics of interactions, as well as the stability of the complex [29]. To assess these parameters, we examined the formation and stability of hydrogen bond interactions in the PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3 complexes (Fig. 6). The results indicated that PAK2-Midostaurin and PAK2-Bagrosin formed 1 to 4 hydrogen bonds with higher flexibility, while one bond remained stable throughout the simulations (Fig. 6A and 6B). At the same time, IPA-3 formed up to 6 hydrogen bonds with PAK2, with 2 hydrogen bonds remaining consistently stable throughout the trajectory, indicating a conserved set of interactions over time (Fig. 6C). This suggests that both compounds formed intense anchoring interactions within the PAK2 binding pocket, which contributed to the stabilization of the complexes. We also performed a PDF analysis to assess the stability of the hydrogen bonding between ligands and the protein (Fig. 6, lower panels). Results from the PDF indicated a high degree of order in the hydrogen bonds, with a precise concentration around a single hydrogen bond, supporting the notion that these interactions are structurally essential and serve to maintain the ligand in each of the bound conformations. These results indicate that Midostaurin and Bagrosin form stable intermolecular hydrogen bonds with PAK2, validating them as viable PAK2 drug candidates. However, their inhibitory effects in a biological setting need to be validated in experimental settings.
Fig. 6.
Intermolecular hydrogen bonds between PAK2 and elucidated drug molecules. A PAK2 and PAK2 and Midostaurin, B PAK2 and Bagrosin, and C IPA-3. The lower panels showed PDF plots of intermolecular hydrogen bonding
Secondary structure dynamics
To observe how PAK2 integrates its structure upon binding to the ligand, we performed secondary structure element analysis over time (Fig. 7). We sought to assess whether the binding of Midostaurin and Bagrosin caused any substantial conformational changes in the backbone structure of PAK2. The results showed that free PAK2 retained its secondary structure largely stable during the simulation (Fig. 7A). Furthermore, the general secondary structure composition of PAK2 was similar in both the PAK2-Midostaurin and PAK2-Bagrosin complexes as the reference complex of PAK2-IPA-3 when comparing changes before and after ligand binding (Fig. 7B–D). The analysis showed that key structural elements, such as α-helices, β-sheets, and loops, were well-conserved throughout the simulation, implying that neither Midostaurin and Bagrosin nor IPA-3 induced significant structural perturbations. PAK2 remained well-conserved, further indicating that ligand binding does not induce significant conformational shifts, but instead populates the native PAK2 fold with higher stability (Table 5). This characteristic implies that Midostaurin and Bagrosin maintain PAK2 in their native conformation without any distortion or folding effects. Such stability is especially important during drug design, where compounds preserve the structural integrity of the protein while also effectively inhibiting its function, thereby increasing their chances of specificity and reducing off-target effects. Collectively, these observations support the idea that Midostaurin and Bagrosin are both highly promising compounds for development as repurposed PAK2 inhibitors, warranting further experimental validation of their therapeutic efficacy.
Fig. 7.
Secondary structure elements in A free PAK2, B PAK2-Midostaurin, C PAK2-Bagrosin, and D PAK2-IPA-3. The structure is the sum of α-helix, β-sheet, β-bridge, and turns
Table 5.
The number of residues in different secondary structure components of PAK2 with Midostaurin, Bagrosin, and IPA-3 binding
| Element | PAK2 | PAK2-Midostaurin | PAK2-Bagrosin | PAK2-IPA-3 |
|---|---|---|---|---|
| Coil | 52 | 52 | 52 | 48 |
| β-sheet | 49 | 48 | 47 | 52 |
| β -bridge | 4 | 4 | 2 | 3 |
| Bend | 25 | 26 | 30 | 25 |
| Turn | 35 | 34 | 30 | 32 |
| α-helix | 81 | 81 | 83 | 84 |
| α-helix | 0 | 0 | 0 | 0 |
| 310-helix | 8 | 9 | 9 | 8 |
| κ-Helix | 5 | 5 | 6 | 7 |
Principal component analysis
A widely used method to elucidate dominant trends in protein motion is PCA, which is commonly employed to help visualize system dynamics by simplifying the complexity of conformational space [30]. PCA identifies key degrees of freedom in molecular models, allowing researchers to model large protein conformational changes and evaluate their flexibility and stability under various conditions [24]. We used PCA to investigate the displacement in the conformation of unbound PAK2 and PAK2 in a complex with Midostaurin and Bagrosin. The normalized essential dynamics method was employed to examine the global motion modes of these systems. We conceptually view one of the characteristics of the protein as its conformational behavior, based on Cα atom fluctuations, which is observed in eigen vectors, EV-1 and EV-2 (Fig. 8). The PCA revealed that all four systems had slightly distinct patterns of motion. The free PAK2 conformation exhibited a wide range of native conformations that were prone to forming a stable structure (Fig. 8A). The PAK2-Midostaurin complex had comparably higher stability, showing minor deviations from its conformational state relative to free PAK2. In comparison, the PAK2-Bagrosin complex exhibited even higher backbone fluctuations than either PAK2-Midostaurin or PAK2-Bagrosin along the EV2 axis of motion, indicating a somewhat more flexible interaction with PAK2 than IPA-3 (Fig. 8B). These ligand-induced changes, however, did not elicit any pertinent conformational changes within any of the systems, implying that both Midostaurin and Bagrosin bind to PAK2 with minimal global conformational tailoring.
Fig. 8.
Principal component analysis. A 2D projections of trajectories of PAK2, PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3. Time-evolution projection of PCA trajectories of PAK2, PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3
Free energy landscape analysis
The analysis of the FEL provides a comprehensive, qualitative view of protein–ligand interaction stability and folding dynamics, highlighting possible metastable conformations [31]. This method has made significant contributions to drug discovery, primarily because it determines whether a potential conformation is energetically favorable or not; hence, it provides valuable insight into structure-based drug design. The free and ligand-bound conformational stability and global minima of PAK2 were analyzed by generating FELs using the first two principal components (PCs). The contoured FELs for PAK2, PAK2-Midostaurin, PAK2-Bagrosin, and PAK2-IPA-3 are shown in Fig. 9. Color in the plots indicates energy, with regions of blue representing lower energy and more thermodynamically stable states closer to the native conformation. The FELs of PAK2 and PAK2-Midostaurin showed a single dominant, global minimum, surrounded by two significant, localized minima (Fig. 9A–B), suggesting that the protein exists in its native conformation and possesses restricted conformational variability. FEL exhibited two to three well-defined basins, producing one to two global minima upon interaction with Bagrosin (Fig. 9C). This indicates that the PAK2-Bagrosin complex can occupy multiple stable conformations, as it samples a larger set of energy-favorable states than the free PAK2 and the PAK2-Midostaurin complex. Likewise, the binding of IPA-3 to PAK2 yielded two to three clear basins, each containing one or two global minima (Fig. 9D). This pattern supports the idea that the PAK2-IPA-3 complex exhibits considerable conformational diversity, which is highly suggestive of multiple stable modes of IPA-3 binding in the active site. The ability of PAK2 to adopt different low-energy conformations depending on the binding ligand provides essential corroboration of the data presented here, as shown by the new FEL finding for the corresponding interacting molecule post-ligand binding. The number of basins and global minima indicates that PAK2 adopts different conformations in the presence of both Midostaurin and Bagrosin, which is an essential property of an effective inhibitor. Thus, although similar energy landscapes are shown for both complexes, the greater diversity in stable conformations for the PAK2-Midostaurin system indicates that Midostaurin may promote a more adaptable and potentially more advantageous binding orientation than Bagrosin. This, in addition to the previously discussed potential of Midostaurin as a viable inhibitor of PAK2, is further validated through functional experiments.
Fig. 9.
The free energy landscapes of A PAK2, B PAK2-Midostaurin, C PAK2-Bagrosin, and D PAK2-IPA-3
Conclusions
Using a systematic, structure-guided drug repurposing approach, we screened a pool of FDA-approved drugs for promising inhibitors of PAK2. The study identified Midostaurin and Bagrosin as promising candidates with high binding affinity toward the PAK2 active site and suitable drug profiles. A set of 300 ns all-atom MD simulations was performed to validate that the protein–ligand complexes were stable and robust. The results of the RMSD, RMSF, Rg, SASA, hydrogen bonding, PCA, and FEL-based analyses collectively confirmed that Midostaurin and Bagrosin exhibit sturdy interactions with PAK2 at the same time as maintaining the native protein structural integrity. Midostaurin exhibits higher potential as a repurposed PAK2 inhibitor and displays more favorable binding characteristics compared to Bagrosin and the reference inhibitor IPA-3. Although these computational predictions show promise, this study is constrained by its in silico nature. Additional experimental validation is essential to confirm the inhibitory potential and efficacy of Midostaurin and Bagrosin towards PAK2. We therefore propose as future work: (1) in vitro kinase inhibition assays to determine IC₅₀ and Ki values for Midostaurin and Bagrosin against PAK2, (2) cell-based viability and phosphorylation assays to evaluate on-target activity and selectivity across PAK isoforms, and (3) in vivo efficacy studies in relevant cancer models. These experiments will bridge our in silico findings to therapeutic validation. This paves the way for future experimental studies and takes Midostaurin and Bagrosin one step closer to therapeutic application in targeting PAK2-driven cancers.
Supplementary Information
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/389/46.
Author contributions
Conceptualization, S.C and S.W.; software, S.C and M.A.; validation, M.A.A and W.A; formal analysis, S.W. and T.A.M; investigation, S.C.; resources, A.A. and M.A.A; data curation, S.W. and R.A.A; writing—original draft preparation, T.A.M and R.A.A; writing—review and editing, W.A and S.C; visualization, S.C and W.A; supervision, A.A and S.C; project administration, A.A; funding acquisition, S.W and A.A All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/389/46.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. The FDA-approved drugs and protein structures analyzed are publicly available from DrugBank (https://go.drugbank.com/) and the AlphaFold database (https://alphafold.ebi.ac.uk/; AlphaFold ID: Q13177), with the corresponding references cited in this study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu A, Jiang X. P21-activated kinases as promising therapeutic targets in hematological malignancies. Leukemia. 2022;36:315–26. [DOI] [PubMed] [Google Scholar]
- 2.Lei K, Luo M, Tu Z, Lv S, Liu J, Gong C, et al. Comprehensive analysis of the prognostic implications and functional exploration of PAK gene family in human cancer. Cancer Cell Int. 2022;22:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder P, Wang S, Radu M, Zin M, Collins L, Khan S, et al. Pak2 as a novel therapeutic target for cardioprotective endoplasmic reticulum stress response. Circ Res. 2019;124:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu M, Sarkar C, Guo B. Regulation of cancer metastasis by PAK2. Int J Mol Sci. 2024;25:13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Gururaj AE, Barnes CJ. P21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–71. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Liu K, Dong Z. The role of p21-activated kinases in cancer and beyond: where are we heading? Front Cell Dev Biol. 2021;9:641381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grebeňová D, Holoubek A, Röselová P, Obr A, Brodská B, Kuželová K. PAK1, PAK1Δ15, and PAK2: similarities, differences and mutual interactions. Sci Rep. 2019;9:17171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blass BE. Basic principles of drug discovery and development. Londan: Elsevier; 2015. [Google Scholar]
- 9.Parvathaneni V, Kulkarni NS, Muth A, Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today. 2019;24:2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. [DOI] [PubMed] [Google Scholar]
- 11.Khan S, Punnoose K, Bishara NZA, Ali R, Khan S, Ahmad S, et al. Identification of potential inhibitor molecule against MabA protein of Mycobacterium leprae by integrated in silico approach. J Biomol Struct Dyn. 2023;41:11231–46. [DOI] [PubMed] [Google Scholar]
- 12.Muhammed MT, Aki-Yalcin E. Molecular docking: principles, advances, and its applications in drug discovery. Lett Drug Des Discov. 2024;21:480–95. [Google Scholar]
- 13.Singh S, Singh VK. Molecular dynamics simulation: methods and application. Front Protein Struct Function Dynam. 2020;12:213–38. [Google Scholar]
- 14.Filimonov D, Lagunin A, Gloriozova T, Rudik A, Druzhilovskii D, Pogodin P, et al. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem Heterocycl Comp. 2014;50:444–57. [Google Scholar]
- 15.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. Autodock4 and Autodocktools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trott O, Olson AJ. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLano WL. An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002;40:82–92. [Google Scholar]
- 18.Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng Des Sel. 1995;8:127–34. [DOI] [PubMed] [Google Scholar]
- 19.Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–18. [DOI] [PubMed] [Google Scholar]
- 20.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, et al. Alphafold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knox C, Wilson M, Klinger CM, Franklin M, Oler E, Wilson A, et al. DrugBank 6.0: the DrugBank knowledgebase for 2024. Nucleic Acids Res. 2024;52:D1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuler LD, Daura X, Van Gunsteren WF. An improved GROMOS96 force field for aliphatic hydrocarbons in the condensed phase. J Comput Chem. 2001;22:1205–18. [Google Scholar]
- 23.Malde AK, Zuo L, Breeze M, Stroet M, Poger D, Nair PC, et al. An automated force field topology builder (ATB) and repository: version 1.0. J Chem Theory Comput. 2011;7:4026–37. [DOI] [PubMed] [Google Scholar]
- 24.Sittel F, Jain A, Stock G. Principal component analysis of molecular dynamics: on the use of Cartesian vs. internal coordinates. J Chem Phys. 2014. 10.1063/1.4885338. [DOI] [PubMed] [Google Scholar]
- 25.Papaleo E, Mereghetti P, Fantucci P, Grandori R, De Gioia L. Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: the myoglobin case. J Mol Graph Model. 2009;27:889–99. [DOI] [PubMed] [Google Scholar]
- 26.Kuželová K, Grebeňová D, Holoubek A, Röselová P, Obr A. Group I PAK inhibitor IPA-3 induces cell death and affects cell adhesivity to fibronectin in human hematopoietic cells. PLoS ONE. 2014;9:e92560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdullah A, Biswas P, Sahabuddin M, Mubasharah A, Khan DA, Hossain A, et al. Molecular dynamics simulation and pharmacoinformatic integrated analysis of bioactive phytochemicals from Azadirachtaindica (Neem) to treat diabetes mellitus. J Chem. 2023;1:23. [Google Scholar]
- 28.R.E. Hubbard, and M.K. Haider, Hydrogen bonds in proteins: role and strength. eLS. 2010.
- 29.Bitencourt-Ferreira G, Veit-Acosta M, de Azevedo WF. Hydrogen bonds in protein-ligand complexes. Docking Screens Drug Disc. 2019;12:93–107. [DOI] [PubMed] [Google Scholar]
- 30.Tharwat A. Principal component analysis-a tutorial. Int J Appl Pattern Recognit. 2016;3:197–240. [Google Scholar]
- 31.Maisuradze GG, Liwo A, Scheraga HA. Relation between free energy landscapes of proteins and dynamics. J Chem Theory Comput. 2010;6:583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information. The FDA-approved drugs and protein structures analyzed are publicly available from DrugBank (https://go.drugbank.com/) and the AlphaFold database (https://alphafold.ebi.ac.uk/; AlphaFold ID: Q13177), with the corresponding references cited in this study.