Abstract
With the use of Weber’s modified trichrome and Uvitex 2B techniques, spores of microsporidia were detected in the stools of four travelers presenting clinically with chronic diarrhea. The general health of these patients was not impaired, and human immunodeficiency virus screening was negative. Immune evaluation, including the study of lymphocytic subpopulations, assay of serum immunoglobulins, and an intradermal multitest, showed normal results. Molecular identification of microsporidian species was based on the PCR amplification of a small-subunit rRNA sequence followed by HinfI endonuclease restriction. Encephalitozoon intestinalis microsporidiosis was thus shown in two of the four patients examined. In two patients, therapy based on albendazole made stools devoid of microsporidian spores without influence on the intestinal disorders. The pathogenic role of E. intestinalis in immunocompetent individuals remains to be demonstrated.
Microsporidia are ubiquitous intracellular parasitic protozoa affecting the whole animal kingdom (4). Seven genera are pathogenic in humans: Encephalitozoon, Enterocytozoon, Nosema, Pleistophora, Vittaforma, Trachypleistophora, and Microsporidium, the latter including all species with undetermined status. Some of them are known to be opportunistic pathogens in immunodepressed patients (8). They may cause chronic diarrhea with food malabsorption, as well as disseminated impairments (2, 3, 7). Ocular and gastrointestinal failures related to microsporidia have also been described in immunocompetent ones (1, 5, 10, 14, 17, 18). Staining methods (19, 22) and immunodiagnostic tests (15, 20, 21, 23, 24) can differentiate microsporidia from bacteria and yeasts in clinical samples such as stool samples, but precise identification of the species involved is not always successful. Recently, PCR amplification of conserved ribosomal DNA (rDNA) sequences was used to detect intestinal microsporidia in biopsy and stool specimens (6, 9, 11, 13, 16).
We screened four human immunodeficiency virus (HIV)-negative travelers with chronic diarrhea for microsporidia and gave them a complete immunologic evaluation. After detection of spores in stool samples by light microscopy, we tried to identify the corresponding parasite species. However, electron microscopy failed to provide evidence of microsporidia and no significant results were obtained for the detection of Encephalitozoon cuniculi by Western blotting and enzyme-linked immunosorbent assay. Thus, we decided to develop a new PCR procedure ensuring the differentiation of any known microsporidian species pathogenic to humans. This procedure enabled us to identify Encephalitozoon intestinalis in two immunocompetent patients.
MATERIALS AND METHODS
Patients.
The patients were travelers presenting with chronic diarrhea. When microsporidian spores were detected in stool samples as judged by Weber’s modified trichrome (22) and Uvitex 2B (19) techniques, an immunologic evaluation was performed including HIV tests, assay of immunoglobulins, and a study of lymphocytic subpopulations by flow cytometry after triple labeling with anti-CD3, -CD4, -CD8, -CD56 (NK cells), and -DC19 (B lymphocytes) antibodies, and an intradermal multitest (Bio-Mérieux, Marcy l’Etoile, France). Treatment with albendazole 400 mg twice daily was prescribed for 20 days, and the patients were re-examined 1 month after the end of the treatment.
Clinical samples from two homosexual AIDS patients (<20 CD4+ cells per μl) with intestinal microsporidiosis were used as a positive control.
Stool samples and parasite cultures.
Formalin-fixed stool samples were washed several times in phosphate-buffered saline (PBS) and stored at 4°C. Septata intestinalis Cali, Kotler, and Orenstein 1993 (2), subsequently reclassified as Encephalitozoon intestinalis by Hartskeerl et al. (12); E. cuniculi; and E. hellem were grown in vitro in MRC-5 human lung fibroblasts (Bio-Mérieux) or Madin-Darby canine kidney (MDCK) cells (Bio-Whittaker) in 75-cm2 tissue culture flasks (Polylabo) containing minimum essential medium supplemented with l-glutamine, 5% fetal calf serum, and diverse antibiotics (ampicillin, penicillin, and streptomycin). The cell cultures were incubated at 37°C with 5% CO2 in an air atmosphere. Supernatants containing mature spores were collected; spores were then sedimented by centrifugation, washed, and stored in 0.1 M PBS (pH 7.4) at 4°C.
DNA extraction.
Stool specimens were mixed with 1 volume of PBS buffer and then centrifuged at 18,000 × g for 2 min. The pellets were washed in PBS and resuspended in 1 ml of 1% sodium dodecyl sulfate–300 mM Tris (pH 9.0)–100 mM EDTA. After incubation at 65°C for 30 min, suspensions were centrifuged and resuspended in 500 μl of lysis buffer (10 mM Tris, 100 mM NaCl, 1 mg of proteinase K [Sigma] per ml, 200 U of Lyticase [Sigma]). Mechanical disruption was performed with zirconium beads (0.1-mm diameter; Biospec Products Inc., Bartlesville, Okla.). Following addition of 2% sodium dodecyl sulfate and 1 mg of proteinase K per ml, extracts were incubated at 55°C for 3 h and then proteins were precipitated with 1 M potassium acetate for 1 h at 4°C. DNA was phenol-chloroform extracted, precipitated with ethanol for 1 h, and resuspended in 50 μl of sterile water.
E. cuniculi, E. hellem, and E. intestinalis spores collected from MRC-5 or MDCK cell cultures were boiled at 100°C for 10 min to release DNA.
PCR amplification.
Primers for PCR were chosen to amplify a conserved region of the small-subunit (SSU) rRNA gene of four microsporidia reported in AIDS patients: E. cuniculi, E. hellem, E. intestinalis, and Enterocytozoon bieneusi. Forward primer C1 (5′-CACCAGGTTGATTCTGCC-3′) and reverse primer C2 (5′-GTGACGGGCGGTGTGTAC-3′) were determined by GenBank sequence analysis of these species. C1 was complementary to bases 1 to 18 of each one, C2 was complementary to bases 1169 to 1186 of E. intestinalis (accession no. U09929), bases 1173 to 1190 of E. cuniculi (accession no. L17072), bases 1188 to 1205 of E. hellem (accession no. L19070), and bases 1152 to 1170 of E. bieneusi (accession no. L16868).
Amplification was done in a 50-μl reaction mixture including 12.5 pmol of each primer, 200 μM each deoxynucleoside triphosphate, 2 mM MgCl2, and 1 U of Taq DNA polymerase (Goldstar, Eurogentec Belgium). Two different volumes of each DNA preparation were regularly tested: 1 μl of the initial extract and 1 μl of the extract diluted with 9 volumes of sterile water.
After denaturation of the DNA at 94°C for 5 min, 30 cycles were run with a Techne PHC-3 apparatus as follows: denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and elongation at 72°C for 1 min, with 5 min of extension at 72°C after the 30 cycles. Amplified products were electrophoretically analyzed on agarose gel and stained with ethidium bromide.
Digestion of PCR products.
Restriction endonucleases HindIII and HinfI (Eurogentec) were used to digest amplified fragments obtained from culture and stool DNA extracts. After amplification in a 50-μl reaction mixture, DNA was precipitated in 100 μl of ethanol with 0.1 M NaCl and then resuspended in 10 μl of sterile water. Three microliters was digested with 3 U of HindIII or 5 U of HinfI in a final volume of 10 μl. Digest fragments were electrophoretically analyzed on agarose gel and stained with ethidium bromide.
RESULTS
Development of a PCR protocol.
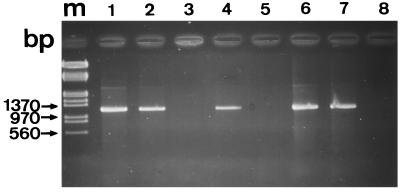
A PCR amplification procedure has been developed to facilitate the detection of microsporidia in human samples. The primers were designed to amplify a large part of the 16S rDNA from four microsporidian species, including the two major species involved in intestinal diseases: E. bieneusi (1,170-bp amplicon) and E. intestinalis (1,186-bp amplicon). When DNAs extracted from spores of E. cuniculi, E. hellem, and E. intestinalis cultures were used, agarose gel electrophoresis revealed the amplification of a DNA fragment with a size of about 1,200 bp (Fig. 1, lane 1). As also expected, the 1,200-bp PCR product was found in fecal samples from two AIDS patients, SID1 and SID2 (Fig. 1, lanes 6 and 7).
FIG. 1.
Analysis of PCR products by 1.5% agarose gel electrophoresis. A 1,200-bp DNA fragment of the SSU rRNA gene of microsporidia was amplified by using primers C1 and C2. Molecular size markers (lane m) were λ phage DNA digested with EcoRI and HindIII. DNAs were extracted from E. intestinalis in MRC-5 cell cultures (lane 1, positive control), immunocompetent patient (IC1, IC2, IC3, and IC4) stools (lanes 2, 3, 4, and 5), HIV-infected patient (SID1 and SID2) stools (lanes 6 and 7), and a negative control (lane 8).
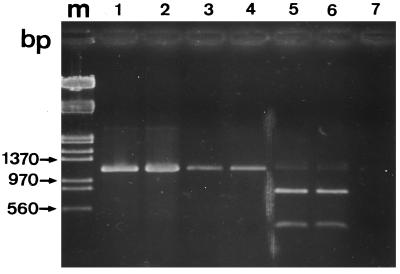
The amplified rDNA sequence displays one HindIII restriction site in E. bieneusi and none in any Encephalitozoon species. Thus, digestion of amplified products with the restriction endonuclease HindIII may be used to distinguish between the two candidate genera for intestinal infection. Results of digestion experiments are shown in Fig. 2. The PCR product from each of the two AIDS patients was cleaved by HindIII, giving two fragments with sizes close to those expected: 784 and 386 bp (Fig. 2, lanes 5 and 6). This indicated that these patients were infected with E. bieneusi, which is in agreement with some electron microscopy observations (data not shown).
FIG. 2.
Digestion of PCR products (1,200-bp DNA fragment) by HindIII restriction endonuclease. Shown is a 1.5% agarose gel containing E. intestinalis rDNA amplified before (lane 1) and after (lane 2) HindIII digestion and digested, amplified fragments from patients IC1 and IC3 (lanes 3 and 4) and patients SID1 and SID2 (lanes 5 and 6). Lane 7 contained a negative control. Two bands were obtained from E. bieneusi-infected patients (800 and 400 bp). Amplified DNA fragments of E. intestinalis, E. cuniculi, and E. hellem were not cleaved by HindIII. Lane m contained the same molecular size markers as lane m in Fig. 1.
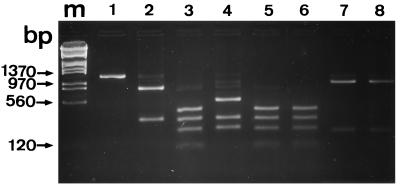
To identify Encephalitozoon species, HinfI digestion of the 1,200-bp amplicons was performed. From the examination of sequences deposited in GenBank, it could be predicted that these species may be differentiated on the basis of the number of HinfI restriction sites: one for E. cuniculi, two for E. hellem, and three for E. intestinalis, excluding one site at position 10 within each amplicon. Species-specific restriction patterns were indeed observed, the sizes of the different fragments being 350 and 830 bp for E. cuniculi (Fig. 3, lane 2); 120, 250, 350, and 460 for E. intestinalis (lane 3); and 260, 350, and 580 for E. hellem (lane 4). Digestion with the same enzyme of 1,200-bp amplicons from the two AIDS patients produced two distinctive DNA fragments with sizes (230 and 940 bp; Fig. 3, lanes 7 and 8) different from those of E. cuniculi (350 and 830 bp). It was confirmed that the two immunocompromised patients were infected with E. bieneusi, the amplified rDNA sequence of which displays one HinfI restriction site, at position 238.
FIG. 3.
Digestion of a 1,200-bp amplicon by restriction endonuclease HinfI. Shown is a 2% agarose gel. Lanes: 1, 1,200-bp PCR product before HinfI digestion; 2, E. cuniculi; 3, E. intestinalis; lane 4, E. hellem; 5 and 6, patients IC1 and IC3; 7 and 8, patients SID1 and SID2. Lane m contained the same molecular size markers as lane m in Fig. 1.
Diagnosis of diarrheic travelers.
For this study, four travelers were selected. They were males with a mean age of 29 years and had had diarrhea for 1 to 71 months. They had traveled in Africa, Nepal, or Southeast Asia. The mean daily number of stools was four, they contained neither blood nor mucus, and the patients’ general health was good. Only one patient complained of nonsystematic abdominal pain. The four travelers (IC1, IC2, IC3, and IC4) were HIV negative. The test for lymphocytic subpopulations and the cutaneous multitest yielded normal results (Table 1). Immunoglobulin levels in serum were normal, except in the patient with protracted diarrhea (6 years), whose immunoglobulin A (IgA) concentration was 0.86 g/liter (normal, 1 to 3 g/liter). An assay of the IgA in the saliva of this patient yielded 100 mg/liter (normal range, 87 to 500 mg/liter). Stool examinations using Weber’s modified trichrome and Uvitex 2B techniques revealed the presence of microsporidian spores. The patients were also screened for parasites by direct examination, culture of stools, and a Ziehl-Neelsen test for cryptosporidia, and no evidence of eukaryotic pathogens other than microsporidia was obtained.
TABLE 1.
Immunological characteristics of patients
| Patient | No. of cells/μl of blood (%)
|
CD4/CD8 cell ratio | Concn (g/liter of blood) of:
|
|||||
|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | NK | BLa | IgA | IgG | IgM | ||
| IC1 | 849 (42) | 667 (33) | 202 (10) | 141 (7) | 1.27 | 0.86 | 9.5 | 0.45 |
| IC2 | 968 (53) | 383 (21) | 201 (11) | 183 (10) | 2.52 | 1.89 | 8.6 | 1.94 |
| IC3 | 858 (39) | 440 (20) | 308 (14) | 220 (10) | 1.95 | 2.7 | 13.4 | 0.82 |
| IC4 | 538 (47) | 217 (19) | 103 (9) | 194 (17) | 2.47 | 2.54 | 10 | 1.33 |
| Normal range | 500–1,700 (32–62) | 250–1,200 (14–41) | <600 (<25) | 100–400 (10–15) | 0.5–2 | 1–3 | 7–13 | 0.6–2 |
BL, B lymphocyte.
The above-described PCR amplification procedure was applied to stool samples from four nonimmunocompromised patients. A positive response was obtained with samples from only patients IC1 and IC3 (Fig. 1, lanes 2 and 4), suggesting a microsporidial infection. HinfI digestion showed the four bands which are relatable to the presence of E. intestinalis (Fig. 3, lanes 5 and 6). The stool samples from patients IC2 and IC4 were spiked with cultured E. cuniculi spores. The expected amplified products were obtained, the detection limit of particular samples varying between 20 and 100 spores per 0.1 g of stool. Repeated sampling provided the same results.
DISCUSSION
Our PCR method involves the digestion of an amplified product as described by Fedorko et al. (9), except that the product corresponds to a much larger fragment of the SSU rRNA gene (more than 90% of the whole length). Ombrouck et al. (16) have detected E. intestinalis in stool samples from AIDS patients by amplifying a 380-bp DNA fragment with a specific reverse primer. Since it has been recently shown that E. cuniculi and E. hellem spores could be present in stools of AIDS patients (6), the differentiation of Encephalitozoon species is essential. This has been achieved through HinfI digestion of the 1,200-bp amplicon, which provides specific banding patterns. E. bieneusi and the three Encephalitozoon species can be specifically identified. The data support an E. intestinalis infection in two nonimmunocompromised humans (IC1 and IC3). Our DNA extraction and analysis protocol for stool samples is realizable in 1 day. This time could be shortened if a heating procedure were used for cell lysis (16).
Microsporidial pathogenicity in immunocompetent patients is still poorly understood, and difficulties in diagnosis persist. In the patients examined here, the Uvitex 2B and Weber staining methods (19, 22) provided evidence of microsporidian infection independent of the HIV syndrome. An IgA deficit can be ruled out in our patients. This deficit occurs frequently, and it may be asymptomatic and detected at a late stage of disease. IgA plays an important role in mucosal immunity, especially in the intestine, so that a partial secretion deficit might promote the development of microsporidia. In the literature, we found several reported cases of intestinal microsporidia in the absence of immunodeficiency proven on the basis of negative HIV tests (1, 10, 14, 17, 18). E. bieneusi was the predominant species, causing diarrhea that subsided spontaneously in less than 6 weeks. One single case of E. intestinalis infection concerned a homosexual patient whose mate was also a carrier of the parasite (10).
Two diarrheic travelers (IC1 and IC3) were found to be microsporidium positive by staining methods and PCR amplification. Albendazole treatment led to the elimination of E. intestinalis spores in stools (checked on three occasions in a 4-month period). However, as the clinical signs persisted, the pathogenic role of E. intestinalis is debatable.
Amplification failed to detect microsporidia in patients IC2 and IC4, while staining methods gave positive responses. Two interpretations may be considered: (i) the stool samples contained some DNA polymerase inhibitors, or (ii) the quantity of spores was below the limit of detection. As shown by Katzwinkel-Wladarsch et al. (13) with another PCR method applied to stools, the detection limit varies between 3 and 100 spores per 0.1 g of stool, depending on the particular samples, which may have qualitative and quantitative differences in DNA polymerase inhibitors. Results of our experiments involving the addition of cultured parasites to negative stool samples suggest that the concentration of microscopically identified microsporidian spores was probably below 20 spores per 0.1 g of stool. Patient IC4 was treated with albendazole. The clinical signs subsided, and microsporidium-like spores were still detected with Uvitex 2B. Thus, the hypothesis that another pathogenic organism was responsible for the chronic diarrhea cannot be excluded.
In conclusion, although microsporidia in stool samples can be easily detected by staining procedures, our PCR protocol should be of interest for the rapid determination of any particular species.
ACKNOWLEDGMENTS
L. Raynaud and F. Delbac contributed equally to this study.
We thank Elizabeth U. Canning for providing reference isolates of E. cuniculi and E. hellem and T. Van Gool for providing a reference isolate of E. intestinalis. We are grateful to Guy Métenier for helpful discussions and comments on the manuscript.
This work was supported by a grant from SIDACTION 1995–96. Véronique Broussolle was supported by a SIDACTION postdoctoral scholarship.
REFERENCES
- 1.Bretagne S, Foulet F, Alkassoum W, Fleury-Feith J, Develoux M. Prévalence des spores d’Enterocytozoon bieneusi dans les selles de patients sidéens et d’enfants africains non infectés par le VIH. Bull Soc Pathol Exot. 1993;86:351–357. [PubMed] [Google Scholar]
- 2.Cali A, Kotler D P, Orenstein J M. Septata intestinalis, n.g., n.sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol. 1993;40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 3.Cali A, Weiss L, Takvorian P, Tanowitz H, Wittner M. Ultrastructural identification of AIDS associated microsporidiosis. J Eukaryot Microbiol. 1994;41:24S. [PubMed] [Google Scholar]
- 4.Canning E U, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 5.Deluol A M, Poirot J L, Heyer F, Roux P, Levy D, Rozembaun W. Intestinal microsporidiosis: about clinical characteristics and laboratory diagnosis. J Eukaryot Microbiol. 1994;41:33S. [PubMed] [Google Scholar]
- 6.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 7.Desportes I, Le Charpentier Y, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. Occurrence of a new microsporidian: Enterocytozoon bieneusi n.g., n.sp., in the enterocytes of a human patient with AIDS. J Protozool. 1985;32:250–254. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 8.Desportes-Livage I. Human microsporidiosis and AIDS: recent advances. Parasite. 1996;3:107–113. doi: 10.1051/parasite/1996032107. [DOI] [PubMed] [Google Scholar]
- 9.Fedorko D P, Nelson N A, Cartwright C P. Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol. 1995;33:1739–1741. doi: 10.1128/jcm.33.7.1739-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flepp M, Sauer B, Lüthy R, Weber R. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Human microsporidiosis in HIV-seronegative, immunocompetent patients, abstr. LM25; p. 331. [Google Scholar]
- 11.Franzen C, Müller A, Hegener P, Salzberger B, Hartmann P, Fätkenheuer G, Diehl V, Schrappe M. Detection of microsporidia (Enterocytozoon bieneusi) in intestinal biopsy specimens from human immunodeficiency virus-infected patients by PCR. J Clin Microbiol. 1995;33:2294–2296. doi: 10.1128/jcm.33.9.2294-2296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartskeerl R A, van Gool T, Schuitema A R, Didier E S, Terpstra W J. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology. 1995;110:277–285. doi: 10.1017/s0031182000080860. [DOI] [PubMed] [Google Scholar]
- 13.Katzwinkel-Wladarsch S, Lieb M, Heise W, Löscher T, Rinder H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop Med Int Health. 1996;1:373–378. doi: 10.1046/j.1365-3156.1996.d01-51.x. [DOI] [PubMed] [Google Scholar]
- 14.Monneret G, Rabodonirina M, Desportes-Livage I, Paulus S, Bastien O, Troncy J, Lachaux A, Boibieux A, Roumanet-Dubois F, Piens M A, Mojon M. Détection de spores de microsporidies intestinales dans une population non infectée par le virus de l’immunodéficience humaine. Ann Biol Clin. 1995;53:563–564. [PubMed] [Google Scholar]
- 15.Ombrouck C, Desportes-Livage I, Achbarou A, Gentilini M. Specific detection of the microsporidia Encephalitozoon intestinalis in AIDS patients. C R Acad Sci Sci Vie. 1996;319:39–43. [PubMed] [Google Scholar]
- 16.Ombrouck C, Ciceron L, Desportes-Livage I. Specific and rapid detection of microsporidia in stool specimens from AIDS patients by PCR. Parasite. 1996;3:85–86. doi: 10.1051/parasite/1996031085. [DOI] [PubMed] [Google Scholar]
- 17.Sandfort J, Hannemann A, Gelderblom H, Stark K, Owen R L, Ruf B. Enterocytozoon bieneusi infection in an immunocompetent patient who had acute diarrhea and who was not infected with the human immunodeficiency virus. Clin Infect Dis. 1994;19:514–516. doi: 10.1093/clinids/19.3.514. [DOI] [PubMed] [Google Scholar]
- 18.Sobottka I, Albrecht H, Schottelius J, Schmetz C, Bentfeld M, Laufs R, Schwartz D A. Self-limited diarrhea due to a dual infection with Enterocytozoon bieneusi and Cryptosporidium parvum in an immunocompetent HIV-negative child. Eur J Clin Infect Dis. 1995;14:919–920. doi: 10.1007/BF01691502. [DOI] [PubMed] [Google Scholar]
- 19.Van Gool T, Snijders F, Reiss P, Eeftinck Schattenkerk J K M, van den Bergh Weerman M A, Bartelsman J F W M, Bruins J J M, Canning E U, Dankert J. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol. 1993;46:694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visvesvara G S, Leitch G J, Da Silva A J, Croppo G P, De Moura H, Wallace S, Slemenda S B, Schwartz D A, Moss D, Bryan R T, Pieniazek N J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visvesvara G S, Da Silva A J, Croppo G P, Pieniazek N J, Leitch G J, Fergusson D, De Moura H, Wallace S, Slemenda S B, Tyrrell I, Moore D F, Meador J. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 23.Weiss L M, Cali A, Levee E, Laplace D, Tanowitz H, Simon D, Wittner M. Diagnosis of Encephalitozoon cuniculi infection by Western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am J Trop Med Hyg. 1992;47:456–462. doi: 10.4269/ajtmh.1992.47.456. [DOI] [PubMed] [Google Scholar]
- 24.Zierdt C H, Gill V J, Zierdt W S. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J Clin Microbiol. 1993;31:3071–3074. doi: 10.1128/jcm.31.11.3071-3074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]